Abstract
Bone morphogenetic proteins (BMPs), members of the transforming growth factor (TGF)–β superfamily, are a group of related proteins that are capable of inducing the formation of cartilage and bone but are now regarded as multifunctional cytokines. We show in this report a novel function of BMPs in hematopoietic cells: BMP-2 induces apoptosis not only in human myeloma cell lines (U266, RPMI 8226, HS-Sultan, IM-9, OPM-2, and KMS-12 cells), but also in primary samples from patients with multiple myeloma. The mechanism of BMP-2–induced apoptosis was investigated with the use of U266 cells, which are dependent on the interleukin-6 autocrine loop. We showed that BMP-2 caused cell-cycle arrest in the G1 phase and the subsequent apoptosis of myeloma cells. BMP-2 up-regulated the expression of cyclin-dependent kinase inhibitors (p21CIP1/WAF1 and p27KIP1) and caused hypophosphorylation of retinoblastoma (Rb) protein. In studies of apoptosis-associated proteins, BMP-2 was seen to down-regulate the expression of Bcl-xL; however, BMP-2 had no effects on the expression of Bcl-2, Bax, or Bad. Therefore, BMP-2 induces apoptosis in various human myeloma cells by means of the down-regulation of Bcl-xL and by cell-cycle arrest through the up-regulation of p21CIP1/WAF1 and p27KIP1 and by the hypophosphorylation of Rb. Further analysis showed that the signal transducer and activator of transcription 3 (STAT3) was inactivated immediately after BMP-2 treatment. We conclude that BMP-2 would be useful as a novel therapeutic agent in the treatment of multiple myeloma both by means of its antitumor effect of inducing apoptotis and through its original bone-inducing activity, because bone lesions are frequently seen in myeloma patients.
Introduction
Multiple myeloma is a B-cell neoplasia characterized by the slow proliferation and accumulation of malignant plasma cells in the bone marrow. Since the introduction of melphalan and prednisolone, various combination chemotherapies have been attempted to improve the survival rate of patients with this disease; however, most myeloma patients ultimately responded poorly to conventional chemotherapy.1 Moreover, multiple myeloma is invariably incurable even with hematopoietic stem cell transplantations after high-dose chemotherapies, which are limited to a few selected young patients, with a median survival within several years.1
Bone morphogenetic proteins (BMPs), members of the transforming growth factor (TGF)–β superfamily, were originally identified as molecules that induce bone and cartilage formation and are now considered multifunctional cytokines.2,3 To date, about 20 BMPs have been identified in mammals, and BMP-2 exhibits potent activity in inducing bone and cartilage formation in vivo.4,5 In addition to bone formation, BMP-2 plays pivotal roles in cell proliferation, apoptosis, and differentiation.3,6-8 Recently, Ishisaki et al9 have reported that BMP-2 induces growth arrest in mouse B-cell hybridoma HS-72 cells; however, very little is known about the roles of BMPs in hematopoietic cells,8 10 much less in human myeloma cells.
The intracellular signaling pathways of the TGF-β superfamily from the membrane to the nucleus have recently begun to be revealed.11,12 Smad proteins have been identified as mediators of the TGF-β superfamily signal transduction pathways.13 By binding BMPs to their 2 different types of serine-threonine kinase receptors (type I and type II),14Smad1 and Smad5 are phosphorylated by BMP receptors, thereby forming hetero-oligomeric complexes with Smad4, and translocate into the nucleus and modulate the transcription of a variety of target genes.
Interleukin-6 (IL-6) is a pleiotropic cytokine well known to be a major growth factor for multiple myeloma in either autocrine or paracrine fashion and is also known to act as an antiapoptotic factor in multiple myeloma.15-18 IL-6 induces intracellular signaling through the signal transducers and activators of transcription (STAT) proteins.19,20 Once phosphorylated by Janus kinases (JAKs), STAT3 dimerizes, translocates into the nucleus, and activates the transcription of target genes. Subsequent studies have shown that STAT3 activation contributes to oncogenesis and/or the inhibition of apoptosis.21-24 Recently, Catlett-Falcone et al24 have reported that constitutively activated STAT3 is expressed in U266 myeloma cells and in the bone marrow cells of most patients with multiple myeloma, and they presented evidence that IL-6–mediated JAK/STAT signaling induces Bcl-xL expression and confers resistance to Fas-induced apoptosis in U266 cells.
In the present study, we report for the first time that BMP-2 inhibits the viability and growth of most myeloma cells via cell-cycle arrest in the G1 phase and the subsequent induction of apoptosis. To develop a novel therapeutic approach to patients with multiple myeloma, we further investigated the molecular mechanisms of BMP-2–induced apoptosis in myeloma cells.
Materials and methods
Cell culture and cytokines
U266, RPMI 8226, HS-Sultan, and IM-9 human multiple-myeloma–derived cell lines were purchased from American Type Culture Collection (Rockville, MD). KMS-12 cell line was obtained from Health Science Research Resources Bank (Osaka, Japan), and OPM-2 cell line was kindly provided by Dr Katagiri (Osaka University, Osaka, Japan).25 All cell lines except for HS-Sultan were maintained in RPMI 1640 medium (Gibco-BRL, Gaithersburg, MD) with 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, MT), 100 U/mL penicillin, and 100 mg/mL streptomycin in a humidified atmosphere with 5% CO2. HS-Sultan was cultured in Iscove's modified Dulbecco's medium (IMDM, Gibco) in the same conditions as other cell lines. Fresh myeloma cells were obtained from bone marrow or pleural effusion in 6 patients with multiple myeloma after obtaining informed consent. Mononuclear cells were separated by the Lymphoprep (Nycomed Pharma As, Oslo, Norway) sedimentation procedure and resuspended in RPMI 1640 medium with 15% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin. The morphology was evaluated from cytospin slide preparations with Giemsa staining, and the viability was assessed by trypan blue dye exclusion. Recombinant human BMP-2 was kindly provided by Yamanouchi Pharmaceutical Co, Ltd (Tokyo, Japan). The agent was dissolved in RPMI 1640 medium at a concentration of 50 μg/mL.
Assays for cellular proliferation
Cellular proliferation was measured by a viability and nonradioactive cell proliferation assay system (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromid) [MTT] assay; Boehringer-Mannheim, Indianapolis, IN). Cells were incubated with BMP-2 for 48 hours in 96-well plates (Flow Laboratories, Irvine, CA), and 10 μL of MTT (5 μg/mL) was added to each well. The reaction was stopped after 4 hours incubation by adding 100 μL of 0.04 N HCl in isopropanol, and the OD570 level was determined.
Cell-cycle analysis
Cells (1 × 106) were suspended in hypotonic (0.1% Triton X-100, 1 mmol/L Tris-HCL [pH 8.0], 3.4 mmol/L sodium citrate, and 0.1 mmol/L EDTA) and stained with 50 μg/mL of propidium iodide. DNA content was analyzed by a FACScan (Becton Dickinson, San Jose, CA). The population of cells in each cell-cycle phase was determined with the use of Cell FIT software (Becton Dickinson).
DNA fragmentation
Cells (1 × 106) were washed twice with ice-cold phosphate-buffered saline and incubated in a lysis buffer (10 mmol/L Tris-HCl [pH 7.4], 10 mmol/L EDTA, 0.5% Triton X-100) at 4°C for 10 minutes. After centrifugation at 12 000g for 5 minutes at 4°C, supernatants were collected. RNase A (Sigma, St. Louis, MO) was added to a final concentration at 50 μg/mL, which was incubated for 1 hour at 37°C. The cell lysate was then incubated for 1 hour at 37°C with proteinase K (Sigma) at 100 μg/mL. The DNA was extracted with the use of phenol-chloroform, precipitated in ethanol, and centrifuged for 20 minutes at 12 000g. The DNA was then dissolved in TE buffer (10 mmol/L Tris-HCl [pH 7.4], 10 mmol/L EDTA) and separated on a 2% agarose gel. DNA fragmentation was visualized by ethidium bromide staining of the gel.
Cell lysate preparation and Western immunoblotting
Cells were collected by centrifugation at 700g for 10 minutes, and the pellets were then resuspended in lysis buffer (1% NP-40, 1 mmol/L phenylmethylsulfonyl fluoride, 40 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 100 mmol/L Na3VO4, 1 mmol/L NaF) at 4°C for 15 minutes. Protein concentrations were determined by means of a Bio-Rad protein assay system (Bio-Rad, Richmond, CA). Cell lysates (15 μg of protein per lane) were fractionated in 12.5% sodium dodecyl sulfate (SDS)–polyacrylamide gels prior to transfer to the membrane (Immobilon-P membranes) (Millipore, Bedford, MA) with the use of standard protocol. For the analysis of retinoblastoma (Rb) and STAT3, 7.5% SDS-polyacrylamide gels were used. Antibody binding was detected by use of the enhanced chemiluminescence kit for Western blotting detection with hyper-ECL film (Amersham, Buckinghamshire, UK). Blots were stained with Coomassie brilliant blue and confirmed to contain a similar amount of protein extract on each lane. Antibodies used in this study were as follows: anti-p21CIP1/WAF1 (C-19-G), anti-Bax (H-20), anti-CDK4 (H-22-G), anti–cyclin D1 (HD1), anti–cyclin D2 (34B1-3), anti–cyclin E (HE12), anti-p15INK4 (M20-G,-STAT3 [C-20]) (Santa Cruz Biotech, Santa Cruz, CA); anti-p27KIP1, anti-CDK2, anti–cyclin D3, anti-Bad, anti–Bcl-xL(Transduction Laboratories, Lexington, KY); anti–Bcl-2 (DAKO), anti-Rb (G3-245) (PharMingen, San Diego, CA); and anti–phospho-STAT3 (Tyr705) (New England Biolabs, Beverly, MA).
Nuclear extracts and electrophoretic mobility shift assays
For use in standard electrophoretic mobility shift assays (EMSAs), nuclear extracts were prepared from U266 cells treated with BMP-2 for 30 minutes or 120 minutes, with the use of the modified Dignam method.26 The following complementary single-stranded oligonucleotides were synthesized and used in EMSAs: STAT3 wild-type oligonucleotide containing STAT binding sequence, TTCCCNNAA, 5′-GATCCGACATTTCCCGTAAATCG-3′, and STAT3 mutant oligonucleotide containing 6 nucleotide substitutions in the STAT binding site, 5′-GATCCGACATTctaataAAATCG-3′. One strand of these oligonucleotides was annealed at 100°C for 3 minutes to its complementary oligonucleotide, and then labeled with the use of T4 polynucleotide kinase and [γ-32P]adenosine triphosphate. We incubated 1 ng of probe with either 5 μg of nuclear extract for 25 minutes at room temperature in a 20 μL reaction containing 20 mmol/L Hepes (pH 7.9), 0.5 mmol/L EDTA, 2 mmol/L dithiothreitol, 10% glycerol, 50 mmol/L KCl, 1 μg poly dI:dC (Pharmacia LKB Biotechnology, Uppsala, Sweden) or an unlabeled competitor. Supershift experiments were performed with the use of the anti-STAT3 polyclonal immunoglobulin G (IgG) (Santa Cruz Biotech). Following incubation, the samples were loaded onto a 5% polyacrylamide gel and run in 0.5 × Tris-borate/EDTA electrophoresis buffer at room temperature. Gels were dried and exposed to XAR Kodak film (Eastman Kodak, Rochester, NY) overnight, with the use of intensifying screens at −80°C.
Results
Effects of BMP-2 on cellular proliferation of myeloma cells
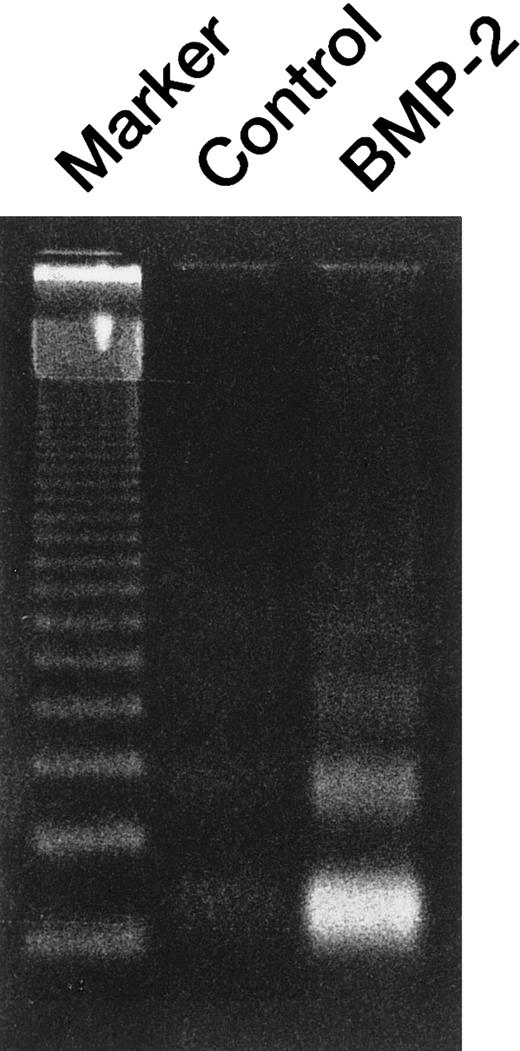
We examined the effects of BMP-2 on proliferation and apoptosis of myeloma cell lines by means of morphology and MTT assay. U266 and HS-Sultan human myeloma cells were cultured with various concentrations (0 to 100 ng/mL) of BMP-2 for 48 hours and 72 hours, respectively. Cultivation with BMP-2 suppressed the cell growth in both U266 and HS-Sultan cells in a time- and dose-dependent manner (Figure1 and data not shown). The cell growth was suppressed as low as 5 ng/mL, and the maximal effect was observed at 50 ng/mL of BMP-2 (data not shown). The effect of BMP-2 on the growth of other myeloma cell lines was also studied with the use of RPMI 8226, IM-9, KMS-12, and OPM-2 cells. Treatment of myeloma cell lines with 50 ng/mL of BMP-2 for 48 hours induced a marked inhibition of U266 cell growth and, to a lesser but significant extent, led to an inhibition of cellular growth in all other myeloma cells (Figures1 and 2). Exposure of all examined myeloma cell lines with 50 ng/mL of BMP-2 for 48 hours resulted in the typical morphological appearance of apoptosis, including condensed chromatin and fragmented nuclei with apoptotic bodies (Figure 1). Apoptosis was assessed in terms of both morphological changes and DNA ladder formation. DNA fragmentation was confirmed by electrophoresis of genomic DNA extracted from myeloma cells treated with 50 ng/mL of BMP-2 for 48 hours, which showed typical DNA ladders (Figure3).
Morphological changes characteristic of apoptosis, and time-dependent effects of BMP-2 on cellular proliferation.
U266 cells and HS-Sultan cells were treated with BMP-2 (50 ng/mL) for 48 hours and 72 hours, respectively. Cytospin slides were prepared and stained with Giemsa. Original magnification, ×1000. Cell viability was measured by MTT assay as compared with control cells cultured without BMP-2.
Morphological changes characteristic of apoptosis, and time-dependent effects of BMP-2 on cellular proliferation.
U266 cells and HS-Sultan cells were treated with BMP-2 (50 ng/mL) for 48 hours and 72 hours, respectively. Cytospin slides were prepared and stained with Giemsa. Original magnification, ×1000. Cell viability was measured by MTT assay as compared with control cells cultured without BMP-2.
Effects of BMP-2 on cellular proliferation of various human myeloma cell lines.
Myeloma cells were cultured with 50 ng/mL of BMP-2 for 48 hours, and cell viability was measured by MTT assay as compared with control cells cultured without BMP-2. Results are expressed as the mean ± SD of at least 3 different experiments. *HS-Sultan cells were cultured for 72 hours.
Effects of BMP-2 on cellular proliferation of various human myeloma cell lines.
Myeloma cells were cultured with 50 ng/mL of BMP-2 for 48 hours, and cell viability was measured by MTT assay as compared with control cells cultured without BMP-2. Results are expressed as the mean ± SD of at least 3 different experiments. *HS-Sultan cells were cultured for 72 hours.
BMP-2–induced apoptosis of U266 cells.
Agarose gel electrophoresis of DNA extracted from U266 cells treated with 50 ng/mL BMP-2 for 48 hours. A 123–base pair DNA ladder was used as a molecular marker. DNA was detected as ultraviolet fluorescence after ethidium bromide staining.
BMP-2–induced apoptosis of U266 cells.
Agarose gel electrophoresis of DNA extracted from U266 cells treated with 50 ng/mL BMP-2 for 48 hours. A 123–base pair DNA ladder was used as a molecular marker. DNA was detected as ultraviolet fluorescence after ethidium bromide staining.
Inhibition of primary myeloma cell growth by BMP-2
We also examined the effects of BMP-2 on primary myeloma cells from patient samples. To evaluate the inhibitory effects of BMP-2 on primary myeloma cells, mononuclear cells from bone marrow or pleural effusion from 6 patients were cultured for 48 hours with or without BMP-2. Clinical data are summarized in Table1. Inhibition of cell growth by BMP-2 was measured by MTT assay and expressed as the percentage of inhibition of cellular proliferation compared with cultures without BMP-2. Although the percentage of inhibition was diverse among the patients, BMP-2 also inhibited the proliferation of freshly isolated myeloma cells (Table 1).
Clinical Data and Effects of BMP-2 on the Patients' Samples
| Patient . | Age/Sex . | M Isotype . | Clinical Stage* . | Source of Samples . | % Myeloma Cells . | Patients' Treatment . | % Inhibition by BMP-2† . |
|---|---|---|---|---|---|---|---|
| 1 | 56/F | BJPλ | III A | BM | 79 | — | 63 |
| 2 | 67/M | IgGλ | III A | BM | 34 | MP | 77 |
| 3 | 65/F | IgAλ | III B | PE | 100 | VAD | 76 |
| 4 | 57/M | IgAλ | III A | BM | 39 | MP | 83 |
| 5 | 37/M | IgGλ | III A | BM | 65 | — | 81 |
| 6 | 55/F | IgAλ | III A | BM | 56 | MP | 87 |
| Patient . | Age/Sex . | M Isotype . | Clinical Stage* . | Source of Samples . | % Myeloma Cells . | Patients' Treatment . | % Inhibition by BMP-2† . |
|---|---|---|---|---|---|---|---|
| 1 | 56/F | BJPλ | III A | BM | 79 | — | 63 |
| 2 | 67/M | IgGλ | III A | BM | 34 | MP | 77 |
| 3 | 65/F | IgAλ | III B | PE | 100 | VAD | 76 |
| 4 | 57/M | IgAλ | III A | BM | 39 | MP | 83 |
| 5 | 37/M | IgGλ | III A | BM | 65 | — | 81 |
| 6 | 55/F | IgAλ | III A | BM | 56 | MP | 87 |
Ig indicates immunoglobulin; BM, bone marrow; PE, pleural effusion; MP, melphalan + prednisolone; VAD, vincristine + doxorubicin hydrochloride (ADR) + dexamethazone; M, M-protein; BJP, Bence Jones protein.
According to the Durie Salmon staging.
Mononuclear cells from patients were cultured for 48 hours with BMP-2 (50 ng/mL). Inhibition of cell growth was measured by MTT assay and expressed as percentage of inhibition of cellular proliferation compared with cells without treatment of BMP-2.
BMP-2–induced G1 cell-cycle arrest and subsequent apoptosis of U266 cells
The effect of BMP-2 on the cell-cycle progression was investigated in U266 cells. The cells were incubated with BMP-2 (50 ng/mL) for various periods (0 to 48 hours) and analyzed for cell-cycle distribution by means of flow cytometry (Figure4). Cultivation with BMP-2 for 24 hours increased the population of the cells in the G1 phase from 76% to 87% with a reduction of cells in the S phase from 24% to 9%. At 48 hours after treatment, a strong induction of apoptosis was shown by the appearance of a hypodiploid DNA peak with the proportion of apoptotic cells reaching 47% (Figure 4). These results were consistent with the cellular proliferation assay performed after 48 hours of treatment with BMP-2, indicating that BMP-2 led to cell-cycle arrest in the G1 phase followed by apoptosis.
Cell-cycle analysis of U266 cells cultured with BMP-2.
U266 cells were cultured in the absence or presence of 50 ng/mL of BMP-2 for 0, 4, 24, and 48 hours and then stained with propidium iodide. DNA content was analyzed by means of flow cytometry. G1, S, and G2/M indicate cell phases. Apo indicates apoptotic cells.
Cell-cycle analysis of U266 cells cultured with BMP-2.
U266 cells were cultured in the absence or presence of 50 ng/mL of BMP-2 for 0, 4, 24, and 48 hours and then stained with propidium iodide. DNA content was analyzed by means of flow cytometry. G1, S, and G2/M indicate cell phases. Apo indicates apoptotic cells.
Expression of p21CIP1/WAF1, p27KIP1, and Rb hypophosphorylation in U266 cells
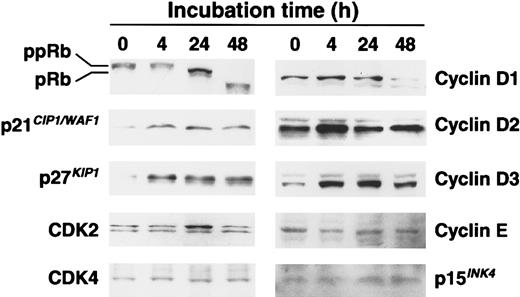
To clarify the mechanisms of BMP-2 effects on the cell-cycle progression, which represent G1 arrest in U266 cells, we examined the expression of cell-cycle–associated proteins, including cyclin-dependent kinase (CDK) inhibitors, Rb, cyclins, and CDKs, by Western blot analysis. As shown in Figure5, low levels of p21CIP1/WAF1 and p27KIP1were detected in exponentially growing U266 cells, but expressions of p21CIP1/WAF1 and p27KIP1were increased at 4 hours and sustained as late as 48 hours. Especially, the levels of p27KIP1 were markedly up-regulated after 4 hours' exposure with BMP-2 (Figure 5). We also examined the ankyrin family of CDK inhibitor, p15INK4, which is often associated with the signaling pathway of TGF-β27; however, BMP-2 had no effect on the expression of p15INK4 (Figure 5). We then examined the phosphorylation state of Rb. The control U266 cells contained high levels of the hyperphosphorylated (ppRb) form and low levels of the hypophosphorylated (pRb) form of Rb (Figure 5). Treatment with BMP-2 (50 ng/mL) in U266 cells for 24 hours led to down-regulation of ppRb and up-regulation of pRb. The lower bands of Rb at 48 hours indicated Rb degradation during apoptosis, because Rb can be cleaved during cell death.28 We also examined the expression of cyclin D–type cyclins, cyclin E, and CDKs. Levels of CDK2, CDK4, cyclin D2, and cyclin E were not altered by BMP-2. Interestingly, expression of cyclin D3 increased after 4 hours' exposure to BMP-2, and levels of cyclin D1 decreased at 48 hours' exposure of BMP-2 (Figure 5).
Western blot analysis of the cell-cycle–associated proteins Rb, p21 CIP1/WAF1, p27KIP1, CDK2, CDK4, cyclin D1, cyclin D2, cyclin D3, cyclin E, and p15INK4.
Total cellular proteins (15 μg/lane) were separated on 12.5% SDS-polyacrylamide gels and transformed to the membrane. For the analysis of Rb, 7.5% gels were used. Protein levels were detected by means of Western blotting with antibodies directed against each protein. pRb indicates hypophosphorylated Rb; ppRb, hyperphosphorylated Rb. Blots were stained with Coomassie brilliant blue to confirm that equal amounts of protein were present in each lane.
Western blot analysis of the cell-cycle–associated proteins Rb, p21 CIP1/WAF1, p27KIP1, CDK2, CDK4, cyclin D1, cyclin D2, cyclin D3, cyclin E, and p15INK4.
Total cellular proteins (15 μg/lane) were separated on 12.5% SDS-polyacrylamide gels and transformed to the membrane. For the analysis of Rb, 7.5% gels were used. Protein levels were detected by means of Western blotting with antibodies directed against each protein. pRb indicates hypophosphorylated Rb; ppRb, hyperphosphorylated Rb. Blots were stained with Coomassie brilliant blue to confirm that equal amounts of protein were present in each lane.
Expression of apoptosis-associated proteins in BMP-2–treated U266 cells
To investigate the molecular mechanism by which BMP-2 induces apoptosis in myeloma cells, the expression of several apoptosis-associated proteins were examined by means of Western blot analysis. A high level of Bcl-xL, an antiapoptotic member of the Bcl-2 family, was detected in the control U266 cells, and BMP-2 down-regulated the expression of Bcl-xL (Figure6). BMP-2 did not affect the expression of the proapoptotic proteins, Bax and Bad (Figure 6), which are the heterodimerizing partners of Bcl-xL and Bcl-2, respectively. Phosphorylated Bad and other antiapoptotic protein Bcl-2 were slightly detected in the control cells and were not modulated by BMP-2 (data not shown).
Western blot analysis of the apoptosis-associated proteins Bcl-xL, Bad, and Bax.
Cell lysates (15 μg protein per lane) were fractionated on 12.5% SDS-polyacrylamide gels and analyzed by Western blotting with antibodies directed against each protein.
Western blot analysis of the apoptosis-associated proteins Bcl-xL, Bad, and Bax.
Cell lysates (15 μg protein per lane) were fractionated on 12.5% SDS-polyacrylamide gels and analyzed by Western blotting with antibodies directed against each protein.
Modulation of activated STAT3 by BMP-2
Because STAT3 is a transducer of the IL-6 signaling pathway and activated STAT3 serves as an antiapoptotic factor,21 27 we examined whether BMP-2 could inhibit the STAT pathway. Expression of activated STAT3 was studied by Western blot analysis with the use of anti-STAT3 antibody that recognized tyrosine-phosphorylated STAT3. Control cells expressed activated STAT3, but BMP-2 triggered the down-regulation of phosphorylated STAT3 as early as within 10 minutes after treatment (Figure 7). Reprobing of the same blot with anti-STAT3 antibody showed it to contain equal amounts of protein extract on each lane (Figure 7). The down-regulation of STAT3 showed in a time-dependent manner after BMP-2 treatment for 24 hours (data not shown), and in addition to the tyrosine phosphorylation, the down-regulation of serine phosphorylation was revealed by Western blot analysis (data not shown).
Activated STAT3 in U266 cells is decreased by treatment with BMP-2.
U266 cells were cultured with 50 ng/mL BMP-2 for the indicated times and examined for levels of tyrosine-phosphorylated (P-tyr) STAT3 by means of immunoblotting with antiphosphotyrosine antibody (upper panel). The same blot was reprobed with anti-STAT3 antibody and confirmed to contain an equal amount of protein extract on each lane (lower panel).
Activated STAT3 in U266 cells is decreased by treatment with BMP-2.
U266 cells were cultured with 50 ng/mL BMP-2 for the indicated times and examined for levels of tyrosine-phosphorylated (P-tyr) STAT3 by means of immunoblotting with antiphosphotyrosine antibody (upper panel). The same blot was reprobed with anti-STAT3 antibody and confirmed to contain an equal amount of protein extract on each lane (lower panel).
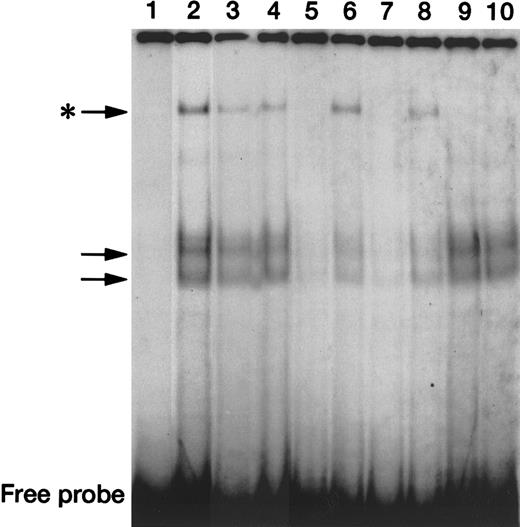
To study the DNA-binding activity of STAT3, we performed EMSAs and supershift experiments with the use of a radiolabeled, double-stranded, STAT3 wild-type oligonucleotide and nuclear extracts from untreated or BMP-2–treated U266 cells. STAT3 oligonucleotide probe with nuclear extracts from untreated U266 cells generated 3 major DNA-protein gel shift complexes (Figure 8, lane 2, indicated by arrows). By adding a 100-fold molar excess of unlabeled STAT3 wild-type oligonucleotide (lanes 5 and 7), we showed that all of these complexes were due to specific binding of nuclear proteins to the TTCCCNNAA sequences, with these sequences competing completely for binding, whereas a 100-fold excess of a STAT3 mutant oligonucleotide that lacked TTCCCNNAA (lanes 6 and 8) did not compete for protein binding. We also performed supershift experiments using an anti-STAT3 polyclonal IgG. In lanes 9 and 10, the slowly migrating complex (the uppermost one, indicated by the asterisk) was eliminated by the addition of anti-STAT3 antibody, indicating that this complex contains STAT3 proteins. Finally, as shown in lanes 3 and 4, nuclear extracts prepared from U266 cells treated with BMP-2 for either 30 minutes or 120 minutes, respectively, demonstrated a decrease in the intensity of the STAT3-containing gel shift complex, which suggests that BMP-2 down-regulates the DNA-binding activity of STAT3.
Down-regulation of in vitro DNA-binding activity of STAT3 by BMP-2.
EMSA using untreated U266 nuclear extracts (lane 1) and a radiolabeled STAT3 wild-type probe (GATCCGACATTTCCCGTAAATCG) generated DNA-protein complexes (arrows, lane 2), which were eliminated by a 100-fold molar excess of self-competitor (lane 5), but not by the same molar excess of the mutant STAT3 oligonucleotide that lacks the STAT binding site (lane 6). A similar sequence-specific DNA-binding activity was seen with the use of nuclear extracts from U266 cells treated with BMP-2 for 120 minutes (lanes 7-8). Supershift assays using the radiolabeled STAT3 probe, untreated or BMP-2–treated nuclear extract, and an anti-STAT3 polyclonal antibody eliminated cells that had been treated with a slowly migrating complex (indicated by the asterisk) (lanes 9-10). The uppermost DNA-protein complexes generated by nuclear extract prepared with BMP-2 for either 30 minutes or 120 minutes (lanes 3 and 4, respectively) were lower in intensity compared with those from untreated nuclear extract. The position of the free probe is indicated at the bottom of the gels.
Down-regulation of in vitro DNA-binding activity of STAT3 by BMP-2.
EMSA using untreated U266 nuclear extracts (lane 1) and a radiolabeled STAT3 wild-type probe (GATCCGACATTTCCCGTAAATCG) generated DNA-protein complexes (arrows, lane 2), which were eliminated by a 100-fold molar excess of self-competitor (lane 5), but not by the same molar excess of the mutant STAT3 oligonucleotide that lacks the STAT binding site (lane 6). A similar sequence-specific DNA-binding activity was seen with the use of nuclear extracts from U266 cells treated with BMP-2 for 120 minutes (lanes 7-8). Supershift assays using the radiolabeled STAT3 probe, untreated or BMP-2–treated nuclear extract, and an anti-STAT3 polyclonal antibody eliminated cells that had been treated with a slowly migrating complex (indicated by the asterisk) (lanes 9-10). The uppermost DNA-protein complexes generated by nuclear extract prepared with BMP-2 for either 30 minutes or 120 minutes (lanes 3 and 4, respectively) were lower in intensity compared with those from untreated nuclear extract. The position of the free probe is indicated at the bottom of the gels.
Discussion
Multiple myeloma follows an invariably fatal clinical course, although various chemotherapeutic agents have been introduced.1 Currently, high-dose chemotherapy followed by hematopoietic stem cell transplantations frequently produces higher remission rates; however, it often causes serious clinical side effects and entails the risk of early mortality in elderly patients. Most patients ultimately relapse; therefore, a novel therapeutic approach based on new insights into the pathogenesis of multiple myeloma is strongly desired. We demonstrated in this report that BMP-2 induces growth arrest via introduction of apoptosis both in myeloma cells of various cell lines and in freshly isolated myeloma cells from patients. BMP-2 inhibited cellular growth in primary myeloma cells less effectively than in myeloma cell lines. Because each of the samples with advanced multiple myeloma contained more than 30% of myeloma cells, the evaluation of the effect of BMP-2 was performed with only the use of these clinical materials. For a more accurate evaluation, it will be necessary to use purifying myeloma cells from clinical samples. In addition, primary myeloma cells in the culture medium are susceptible to spontaneous apoptosis, because bone marrow cells are strictly regulated by microenviromental factors, including IL-6.29,30 The severe combined immunodeficient (SCID)–hu host provides a hospitable environment for primary human acute leukemia cells.31 As well, the SCID-hu host reproducibly supports the growth of myeloma cells that developed out of typical myeloma manifestations when inoculated with fresh clinical materials.32 Therefore, further studies using an in vivo myeloma model in SCID-hu mice will also be necessary.
BMP-2 leads to cell-cycle arrest in the G1 phase and subsequently induces apoptosis in myeloma cells. BMP-2 up-regulated p21CIP1/WAF1 and p27KIP1expression and caused hypophosphorylation of Rb. Our previous studies have reported that BMP-2 and activin A, both members of the TGF-β superfamily, induce cell-cycle arrest in the G1 phase and apoptosis in mouse B-cell hybridoma HS-72 cells.9 33 Consistent with these studies, up-regulation of p21CIP1/WAF1 and the consequent hypophosphorylation of Rb may be an important mechanism of BMP-2 effect in the G1 arrest and growth inhibition of human myeloma cells. Interestingly, the expression of p27KIP1is greatly enhanced by BMP-2 in U266 myeloma cells. Distinct from mouse B-cell hybridoma HS-72 cells, the up-regulation of p27KIP1 may also play a pivotal role in inducing cell-cycle arrest and apoptosis in BMP-2–treated U266 cells.
The Bcl-2 family of proteins has been identified as an important regulator of apoptotic cell death in many cell types, and the aberrant expression of Bcl-2 family members is frequently found in diverse malignancies. Several genes and pathways have been suggested as playing possible roles in the prevention of apoptosis in multiple myeloma,18,34,35 and the importance of Bcl-xL in the protection against apoptosis in myeloma has been reported.36-38 More recently, Catlett- Falcone et al24 have reported that constitutive STAT3 signaling confers resistance to Fas-mediated apoptosis in U266 cells because STAT3 directly binds and activates the transcription of Bcl-xL gene promoter, resulting in the induction of the expression of Bcl-xL. Consistent with this report, we observed that in U266 cells, activated STAT3 was decreased and the expression of Bcl-xL was down-regulated by the treatment of BMP-2. These results suggest that the inactivation of STAT3 and the down-regulation of Bcl-xL may contribute to BMP-2–induced apoptosis in myeloma cells and that blocking STAT3 signaling may be associated at least in part with the down-regulation of Bcl-xL.
At present, 2 different signal transduction pathways of TGF-β have been identified: one is the Smad pathway and the other is mitogen-activated protein (MAP) kinase cascades mediated by TGF-β–activated kinase 1 (TAK1), a member of the MAP kinase kinase kinase family. Recent reports have shown that TAK1 and p38 MAP kinase participate in BMP signaling and that activated TAK1 induces apoptosis through activation of the JNK or p38 MAP kinase pathway.39-42 Therefore, it may be possible that BMP-2–induced apoptosis in myeloma cells is also mediated by the activation of TAK1/p38 MAP kinase pathway. This evidence suggests that cross-talk occurs between Smad signaling and the MAP kinase pathway, which has the possibility of playing an important role in BMP-induced apoptosis. More recently, it has been shown that signaling cross-talk also occurrs between STAT3 and Smads43; thus, further examinations are required to clarifiy the more detailed molecular mechanisms of BMP-induced apoptosis in myeloma cells.
In a clinical setting, multiple myeloma is often associated with bone disease, including bone pain, osteolysis, and pathological fractures that lead to a lessening of the quality of life of the patients.1,44 BMPs play a crucial role in fracture healing and bone regeneration in vivo.5,6 IL-6 not only is a major growth factor of multiple myeloma, but also plays a role in the pathogenesis of bone lesions by activating osteoclasts.1 44 BMP may prevent bone resorption by inactivating STAT3, resulting in the disruption of the IL-6 signaling pathway. Therefore, based on these 2 different biological functions, BMPs could provide a new treatment for multiple myeloma with both antitumor and bone regeneration effects.
In conclusion, we report for the first time that BMP-2 can induce G1 arrest of cell cycle and of the subsequent apoptosis in various human myeloma cell lines and primary myeloma cells. The up-regulation of p21CIP1/WAF and p27KIP1 and the hypophosphorylation of Rb might be responsible for the G1 arrest of the cell cycle, whereas the down-regulation of Bcl-xL with modulation of STAT3 might play an important role in BMP-2–induced apoptosis in myeloma cells. Finally, considering the 2 major effects of BMP-2 of both inducing apoptosis of myeloma cells and preventing the progression of bone diseases, we propose the use of BMP-2 as a novel therapeutic approach to multiple myeloma.
Supported by grants from the Ministry of Education, Science and Culture of Japan, and the National Grant-in-Aid for the Establishment of a High-Tech Research Center in a Private University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masahiro Kizaki, Division of Hematology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: makizaki@mc.med.keio.ac.jp.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal