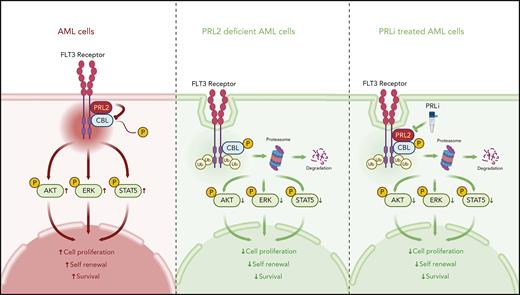
Key Points
Genetic and pharmacological inhibition of PRL2 significantly reduce FLT3-ITD-driven leukemia burden and extend leukemic mice survival.
PRL2 dephosphorylates CBL at tyrosine 371 and blocks CBL-mediated FLT3 ubiquitination and degradation in leukemia cells.
Abstract
Acute myeloid leukemia (AML) is an aggressive blood cancer with poor prognosis. FMS-like tyrosine kinase receptor-3 (FLT3) is one of the major oncogenic receptor tyrosine kinases aberrantly activated in AML. Although protein tyrosine phosphatase PRL2 is highly expressed in some subtypes of AML compared with normal human hematopoietic stem and progenitor cells, the mechanisms by which PRL2 promotes leukemogenesis are largely unknown. We discovered that genetic and pharmacological inhibition of PRL2 significantly reduce the burden of FLT3-internal tandem duplications–driven leukemia and extend the survival of leukemic mice. Furthermore, we found that PRL2 enhances oncogenic FLT3 signaling in leukemia cells, promoting their proliferation and survival. Mechanistically, PRL2 dephosphorylates the E3 ubiquitin ligase CBL at tyrosine 371 and attenuates CBL-mediated ubiquitination and degradation of FLT3, leading to enhanced FLT3 signaling in leukemia cells. Thus, our study reveals that PRL2 enhances oncogenic FLT3 signaling in leukemia cells through dephosphorylation of CBL and will likely establish PRL2 as a novel druggable target for AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive blood cancer with poor prognosis.1-3 Some human leukemia cells depend on aberrant receptor tyrosine kinase activation and the downstream effectors for proliferation and survival.4,5 FMS-like tyrosine kinase receptor-3 (FLT3) is one of the major oncogenic receptor tyrosine kinases aberrantly activated in AML.6,7 Activating FLT3 mutations, including internal tandem duplications in FLT3 (FLT3-ITD), are seen in approximately 30% of patients with AML and confer a poor prognosis.6,7 Despite substantial efforts devoted to the development of FLT3 inhibitors, the effectiveness of these inhibitors as a single agent in AML has been limited, and the development of drug resistance in patients with leukemia is always a concern.6-9 The resistance to targeted therapies seen in patients with AML may be associated with a rare population of leukemia-initiating cells (LICs) or leukemia stem cells (LSCs) that are capable of self-renewal and initiating leukemia.10-14
The CBL family E3 ubiquitin ligases, including CBL and CBL-b, are responsible for the ubiquitination and degradation of FLT3 in hematopoietic cells.15 CBL is a tumor suppressor in hematological malignancies. Indeed, loss of both CBL and CBL-b results in fetal myeloproliferative neoplasms (MPN) in mice.16-18 Both somatic and germ line CBL mutations are frequently found in myeloid malignancies, including juvenile myelomonocytic leukemia, myelodysplastic syndromes (MDS), MPN, and AML.19-22 In response to cytokine stimulation, CBL is phosphorylated and activated.15 However, how CBL phosphorylation is downregulated in leukemia cells is largely unknown.
The phosphatases of regenerating liver (PRL1, 2, and 3) are members of the protein tyrosine phosphatase (PTP) family that are pursued as biomarkers and therapeutic targets in human cancers.23-26 PRL2, also known as PTP4A2, is essential for hematopoietic stem and progenitor cell (HSPC) proliferation and promotes AML1-ETO–induced leukemia.27,28 In addition, PRL2 regulates T cell development and promotes oncogenic NOTCH1-induced T-cell leukemia.29,30 Although PRL2 is highly expressed in some subtypes of AML compared with normal human HSPCs,28 the mechanisms by which PRL2 promotes leukemogenesis are unclear. Here, we discovered that PRL2 dephosphorylates CBL at tyrosine 371 and inhibits its E3 ubiquitin ligase activity toward FLT3, leading to decreased ubiquitination of FLT3, and activation of FLT3-induced downstream signaling pathways in leukemia cells.
Methods
Detailed methodology is provided in the supplemental Information (available on the Blood website).
Mice
Wild-type (WT) C57BL/6 (CD45.2+), B6.SJL (CD45.1+), NSG, NSGS, C3H/HeJ, and Flt3+/ITD mice were purchased from the Jackson Laboratories. Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− mice were maintained in the Indiana and Northwestern University Animal Facility and kept in Thorensten units with filtered germ-free air. Embryonic day 14.5 (E14.5) fetal liver cells (Prl2+/+ and Prl2−/−) were isolated from pregnant Prl2+/− female mice that were mated with Prl2+/− male mice. The Institutional Animal Care and Use Committee of Indiana University School of Medicine and Northwestern University Feinberg School of Medicine approved all experimental procedures.
Statistical analysis
The animal sample size was based on previous studies evaluating the roles of PRL2 in leukemia and POWER analysis.26,27 Using chi-square analysis, 7 mice per group provided 80% POWER in detecting difference with 95% difference. Gehan-Breslow-Wilcoxon test was used for Kaplan-Meier survival curves. Other data were analyzed by paired or unpaired t test or analysis of variance for nonlinear distributions using GraphPad Prism 9. Results are expressed as the mean ± standard error of the mean (SEM) for at least triplicate experiments. P values of < .05 were regarded as statistically significant, which were calculated by GraphPad Prism 9. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. Further details about methods are available in supplemental Information.
Results
FLT3 mutated AML patients with high PRL2 expression have reduced overall survival
To determine the role of PRL2 (PTP4A2) in the pathogenesis of human AML, we first analyzed the published TCGA (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga) data set and found that PRL2 expression is higher in intermediate and poor risk AML compared with favorable risk AML (Figure 1A). PRL2 expression is also higher in dead patients with AML compared with those who are alive (Figure 1B). We then analyzed the data set from cBioPortal (https://www.cbioportal.org) and found that PRL2 levels are higher in patients with cytogenetic and central nervous system relapse (Figure 1C and supplemental Figure 1A). We defined PRL2 expression above the median as a high PRL2 expression group and below the median as a low PRL2 expression group. Notably, AML patients with high PRL2 expression have reduced overall survival compared with those with low PRL2 expression (supplemental Figure 1B). In AML bearing poor cytogenetic risk, patients with high PRL2 expression have reduced overall survival compared with patients with low PRL2 expression (Figure 1D and supplemental Figure 1C-D). Next, we performed differentially expressed gene analysis to compare gene expression in a subset of AML patients with high or low PRL2 expression. There are 790 genes upregulated and 948 genes downregulated in AML patients with high PRL2 expression (Figure 1E). GSEA revealed that AML, AML prognosis, LSC, and hematopoietic stem cell (HSC) gene signatures are enriched in AML patients with high PRL2 expression (Figure 1F). In addition, pathways associated with FLT3 and its downstream effectors, including STAT5A, PI3K/AKT, and ERK1/ERK2/MAPK, are enriched in the PRL2 high group (Figure 1G-H).
We then analyzed PRL2 expression in AML patients with or without FLT3 mutations using GSE15434 and cBioPortal data set and found that AML patients with FLT3 mutations have higher PRL2 expression compared with those negative for FLT3 mutations (Figure 1I and supplemental Figure 1E). In AML patients without FLT3 mutations, PRL2 expression did not appear to affect overall survival (supplemental Figure 1F). However, in FLT3 mutation–positive AML, patients with high PRL2 expression have reduced overall survival compared with patients with low PRL2 expression (Figure 1J). Taken together, these clinical data suggest that high PRL2 expression may be a prognostic marker in FLT3-mutated AML.
Prl2 deficiency alters FLT3-mediated gene transcription in murine HSPCs
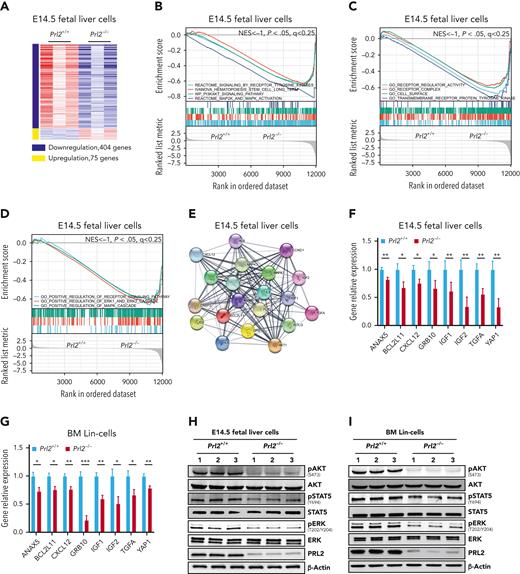
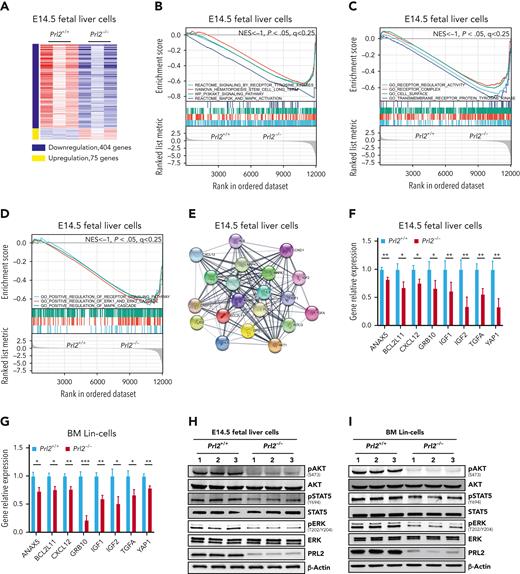
To gain insights into the molecular mechanisms underlying the role of PRL2 in HSPCs, we performed RNA-seq analysis to compare gene expression in Prl2+/+ and Prl2−/− E14.5 (embryonic day 14.5) fetal liver cells, which are enriched with HSPCs. Approximately 400 genes were significantly downregulated, and 75 genes were significantly upregulated in Prl2−/− fetal liver cells, respectively (Figure 2A). We then employed GSEA analysis to group potential PRL2 target genes into specific pathways important for HSPC behavior. Notably, long-term HSCs (LT-HSCs), receptor tyrosine kinase signaling, PI3K/AKT signaling, and ERK signaling gene signatures were significantly downregulated in Prl2 null fetal liver cells (Figure 2B). In addition, receptor regulator activity, receptor complex, positive regulation of receptor tyrosine kinase signaling, and positive regulation of ERK signaling gene signatures were significantly downregulated in Prl2 null fetal liver cells (Figure 2C-D). We used STRING 11.5 to perform protein association network analysis on genes downregulated in Prl2 null fetal liver cells and observed strong interconnection between downregulated genes with FLT3 and its downstream proteins in Prl2 null fetal liver cells (Figure 2E). We confirmed that the expression of genes interacting with the FLT3 signaling pathway was downregulated in Prl2 null fetal liver cells (Figure 2F), Prl2 null fetal liver cKit+ cells (supplemental Figure 2A), and Prl2 null BM Lin− cells (Figure 2G). Loss of Prl2 significantly decreased AKT, STAT5, and ERK phosphorylation in fetal liver cells (Figure 2H and supplemental Figure 2B) and BM Lin− cells (Figure 2I and supplemental Figure 2C).
Prl2 deficiency alters gene transcription in murine HSPCs. (A) Heat map of Prl2-regulated genes that are upregulated (red) or downregulated (blue) (log2FC < −1, FDR < 0.05, P < .05) in Prl2 null E14.5 (embryonic day 14.5) fetal liver cells compared with WT fetal liver cells. (B) GSEA analysis of gene transcription between WT and Prl2 null E14.5 fetal liver cells. HSC, receptor tyrosine kinases, PI3KAKT signaling pathway, and MAPK pathway gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (C) GSEA showed that receptor regulator activity, receptor complex, cell surface, and receptor protein tyrosine kinase gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (D) GSEA showed that regulation of receptor signaling pathway, positive regulation of ERK1 and ERK2 cascade, and positive regulation of MAPK cascade gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (E) STRING protein-protein interaction network between downregulated genes (log2FC > 1, FDR < 0.5, P < .05) related to FLT3 signaling in Prl2 null E14.5 fetal liver cells. (F) Quantitative RT-PCR analysis of gene expression in WT and Prl2 null E14.5 fetal liver cells (n = 4). (G) Quantitative RT-PCR analysis of gene expression in WT and Prl2 null BM Lin− cells (n = 4). (H) Immunoblot analysis of AKT, STAT5, and ERK phosphorylation in WT and Prl2 null E14.5 fetal liver cells (n = 3). (I) Immunoblot analysis of AKT, STAT5, and ERK phosphorylation in WT and Prl2 null BM Lin− cells (n = 3). Mean values (±SEM) are shown (∗P < .05, ∗∗P < .01, and ∗∗∗P < .001). BM, bone marrow; FDR, false discovery rate; RT-PCR, reverse transcription polymerase chain reaction.
Prl2 deficiency alters gene transcription in murine HSPCs. (A) Heat map of Prl2-regulated genes that are upregulated (red) or downregulated (blue) (log2FC < −1, FDR < 0.05, P < .05) in Prl2 null E14.5 (embryonic day 14.5) fetal liver cells compared with WT fetal liver cells. (B) GSEA analysis of gene transcription between WT and Prl2 null E14.5 fetal liver cells. HSC, receptor tyrosine kinases, PI3KAKT signaling pathway, and MAPK pathway gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (C) GSEA showed that receptor regulator activity, receptor complex, cell surface, and receptor protein tyrosine kinase gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (D) GSEA showed that regulation of receptor signaling pathway, positive regulation of ERK1 and ERK2 cascade, and positive regulation of MAPK cascade gene signatures were significantly downregulated in Prl2 null E14.5 fetal liver cells. (E) STRING protein-protein interaction network between downregulated genes (log2FC > 1, FDR < 0.5, P < .05) related to FLT3 signaling in Prl2 null E14.5 fetal liver cells. (F) Quantitative RT-PCR analysis of gene expression in WT and Prl2 null E14.5 fetal liver cells (n = 4). (G) Quantitative RT-PCR analysis of gene expression in WT and Prl2 null BM Lin− cells (n = 4). (H) Immunoblot analysis of AKT, STAT5, and ERK phosphorylation in WT and Prl2 null E14.5 fetal liver cells (n = 3). (I) Immunoblot analysis of AKT, STAT5, and ERK phosphorylation in WT and Prl2 null BM Lin− cells (n = 3). Mean values (±SEM) are shown (∗P < .05, ∗∗P < .01, and ∗∗∗P < .001). BM, bone marrow; FDR, false discovery rate; RT-PCR, reverse transcription polymerase chain reaction.
Loss of Prl2 decreases the self-renewal capability of FLT3-ITD–positive HSPCs
To determine the role of PRL2 in FLT3-ITD–mediated hematopoietic cell proliferation, we introduced WT FLT3 or FLT3-ITD mutant into BM Lin− cells purified from WT and Prl2 null mice and found that Prl2 null Lin− cells expressing FLT3-ITD exhibit decreased proliferation compared with those of the WT cells both in the absence of cytokines and in the presence of FLT3 ligand (supplemental Figure 2D). As expected, ectopic expression of FLT3-ITD increased the colony formation of WT HSPCs (supplemental Figure 2E). Although Prl2 deficiency did not affect the colony formation of HSPCs expressing WT FLT3, loss of Prl2 decreased the colony formation of HSPCs expressing FLT3-ITD (supplemental Figure 2E). These findings suggest that PRL2 is important for FLT3-ITD–mediated hematopoietic cell proliferation.
To further determine the impact of PRL2 on oncogenic FLT3 signaling, we have generated Flt3+/ITDPrl2−/− and Flt3ITD/ITDPrl2−/− mice.31Prl2−/− mice show decreased body size as we previously reported28,32; however, expression of FLT3-ITD did not rescue the body size defect seen in the Prl2−/− mice (supplemental Figure 3A). To determine the impact of Prl2 on hematopoiesis, we first analyzed the peripheral blood (PB) and BM of 8- to 12-week-old Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− mice. Flt3ITD/ITD mice showed increased white blood cell (WBC) counts as reported,31 whereas loss of Prl2 brought WBC counts back to normal (supplemental Figure 3B). Both Flt3ITD/ITD and Flt3ITD/ITDPrl2−/− mice were anemic, manifested by decreased red blood cell (RBC) counts and reduced hemoglobin (HGB) levels in PB (supplemental Figure 3C-D). In addition, Flt3ITD/ITD mice displayed decreased levels of platelets but increased levels of basophil and monocyte counts (supplemental Figure 3E-H). There was an increased number of myeloid cells in the PB of Flt3ITD/ITD mice; however, loss of Prl2 mitigated this effect (supplemental Figure 3I). Both Flt3+/ITD and Flt3ITD/ITD mice displayed increased BM cellularity compared with WT mice, whereas loss of Prl2 brought BM cellularity back to normal (supplemental Figure 3J). There was a decreased number of B cells but an increased number of myeloid cells in the BM of Flt3ITD/ITD mice (supplemental Figure 3K-M). Loss of Prl2 significantly reduced the number of myeloid cells in the FLT3-ITD background (supplemental Figure 3M).
We next examined the number of primitive HSPCs in the BM of Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− mice. Flt3ITD/ITD mice had an increased number of LT-HSCs, multipotent progenitor cells (MPPs), and Lin−Sca1+Kit+ cells (LSKs) in their BM, whereas loss of Prl2 brought the numbers of HSPCs back to WT level (Figure 3A,C-D and supplemental Figure 3N). Although the loss of Prl2 decreased the number of ST-HSCs, Prl2 deficiency had a modest impact on ST-HSCs in the FLT3-ITD background (Figure 3B). We then performed methylcellulose colony-forming unit assays to quantify myeloid progenitor cells. Although Flt3ITD/ITD BM cells showed increased colony formation, loss of Prl2 significantly decreased their ability to form colonies in vitro (Figure 3E), suggesting that PRL2 is important for FLT3-ITD–mediated enhanced hematopoietic cell proliferation.
To examine whether Prl2 deficiency affects Flt3+/ITD HSPC function in vivo, we performed serial competitive BM transplantation assays using Prl2+/+, Prl2−/−, Flt3+/ITD, and Flt3+/ITDPrl2−/− BM cells (CD45.2+). Equal numbers of donor and competitor BM cells were transplanted into lethally irradiated recipient mice (supplemental Figure 4A). Sixteen weeks after primary transplantation, we found that loss of Prl2 significantly decreases the engraftment of Flt3+/ITD BM cells (Figure 3F). Recipient mice repopulated with Flt3+/ITD BM cells showed increased levels of WBC counts, whereas loss of Prl2 in the Flt3+/ITD background brought WBC counts back to normal (supplemental Figure 4B-C).
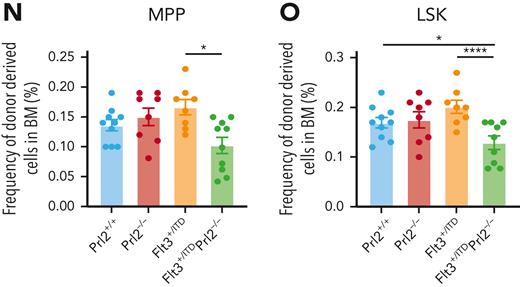
Analysis of the BM revealed a striking increase in the number of phenotypically defined MPPs and LSKs in the recipients repopulated with Flt3+/ITD BM cells, whereas the number of LT-HSCs and ST-HSCs was normal (Figure 3G-H). Loss of Prl2 significantly reduced the number of MPPs and LSKs in the Flt3+/ITD background (Figure 3I-J and supplemental Figure 4D). We then transplanted 3 × 106 BM cells isolated from the primary recipient mice repopulated with Prl2+/+, Prl2−/−, Flt3+/ITD, and Flt3+/ITDPrl2−/− BM cells into lethally irradiated secondary recipients (supplemental Figure 4E). Sixteen weeks after transplantation, Flt3+/ITDPrl2−/− cells continued to show decreased repopulating ability (Figure 3K). Recipient mice repopulated with Flt3+/ITD BM cells showed increased levels of WBC counts, whereas loss of Prl2 in the Flt3+/ITD background brought WBC counts back to normal (supplemental Figure 4F). Interestingly, we observed increased lymphocyte counts in the secondary recipients repopulated with Flt3+/ITD BM cells, and loss of Prl2 mitigated the effect (supplemental Figure 4G). Strikingly, loss of Prl2 significantly decreased the number of Flt3+/ITD ST-HSCs, MPPs, and LSKs but not LT-HSCs in the BM of secondary recipient mice (Figure 3L-O and supplemental Figure 4H). Recipient mice repopulated with Flt3+/ITD BM cells showed an enlarged spleen and loss of Prl2 rescued the defect (supplemental Figure 4I-J).
Prl2 is important for FLT3-ITD–induced myeloid proliferative neoplasm in mice
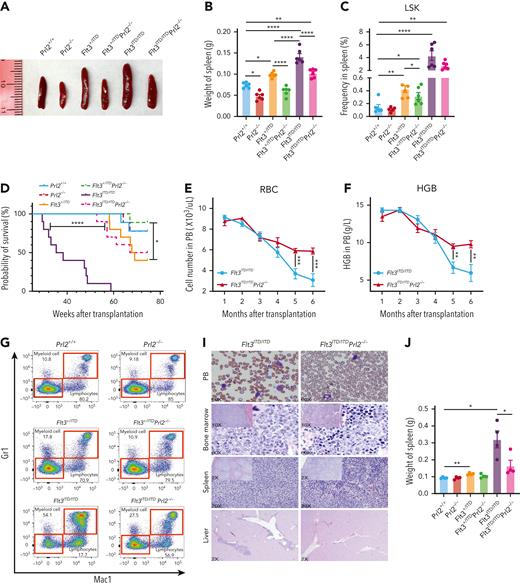
Both Flt3+/ITD and Flt3ITD/ITD mice develop MPN with monocytic features.31Flt3+/ITD and Flt3ITD/ITD mice displayed dose-dependent development of progressive splenomegaly, whereas loss of Prl2 significantly reduced splenomegaly seen in Flt3+/ITD and Flt3ITD/ITD mice (Figure 4A-B). Although there was an increased number of LSKs in the spleen of Flt3+/ITD and Flt3ITD/ITD mice, loss of Prl2 mitigated the effect (Figure 4C).
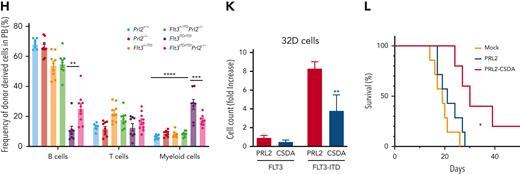
To determine the hematopoietic cell intrinsic effect of PRL2 on FLT3-ITD–induced MPN, we transplanted 3 × 106 BM cells (CD45.2+) isolated from Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− mice into lethally irradiated recipient mice (CD45.1+). All recipient mice repopulated with Flt3ITD/ITD BM cells developed MPN and died within 60 weeks after transplantation; however, loss of Prl2 significantly extended the survival of Flt3ITD/ITD mice, with 50% of mice still alive at 73 weeks following transplantation (Figure 4D). Prl2 deficiency rescued anemia seen in recipient mice repopulated with Flt3ITD/ITD BM cells, manifested by increased RBC counts and HGB levels in PB (Figure 4E-F and supplemental Figure 5A-B). In addition, loss of Prl2 rescued myeloid expansion seen in the PB of Flt3ITD/ITD mice (supplemental Figure 5C-G). Flow cytometric analysis further confirmed the expansion of Mac1+Gr1+ myeloid cells in the PB of recipient mice repopulated with Flt3+/ITD or Flt3ITD/ITD BM cells, and loss of Prl2 rescued the defect observed in the Flt3ITD/ITD group (Figure 4G-H). Recipient mice repopulated with Flt3+/ITD and Flt3ITD/ITD BM cells developed MPN, manifested by splenomegaly and infiltration of maturing myeloid hyperplasia in BM, spleen, and liver, along with the accumulation of myeloid blast cells in PB; however, these abnormalities were significantly reduced in Flt3+/ITDPrl2−/− and Flt3ITD/ITDPrl2−/− mice (Figure 4I and supplemental Figure 5H). Recipient mice repopulated with Flt3ITD/ITD BM cells displayed splenomegaly, whereas loss of Prl2 significantly reduced the splenomegaly seen in Flt3ITD/ITD mice (Figure 4J).
To complement our murine studies, we ectopically expressed WT PRL2 or a catalytically inactive mutant (PRL2-CSDA, where the active sites C101 and D69 were mutated to S and A, respectively) in a murine hematopoietic progenitor cell line, 32D, and performed in vitro and in vivo experiments.30,33 We found that ectopic expression of PRL2-CSDA decreases the proliferation of 32D cells expressing FLT3-ITD (Figure 4K). We also transplanted transduced 32D cells into sublethally irradiated C3H/HeJ mice and monitored their survival. Although ectopic expression of PRL2 had no effect on the survival of C3H/HeJ mice transplanted with FLT3-ITD–expressing 32D cells, expression of PRL2-CSDA significantly extended the survival of C3H/HeJ mice (Figure 4L).
Genetic and pharmacological inhibition of PRL2 decrease leukemia burden and extend the survival of mice transplanted with human leukemia cell lines
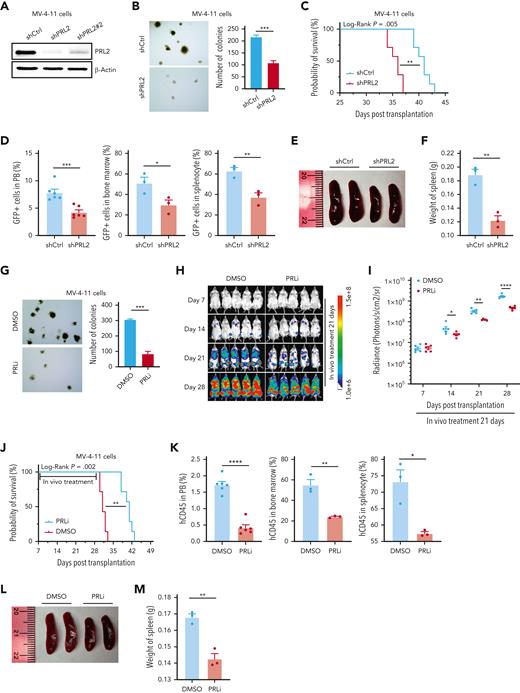
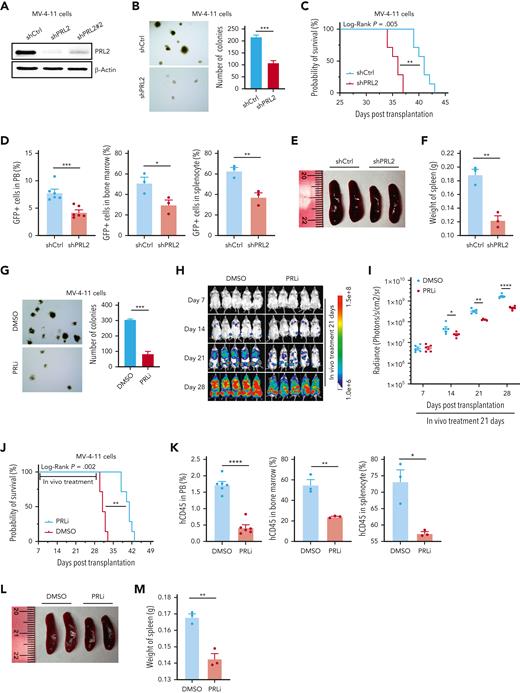
MV-4-11, MOLM-13, and K562 are human AML cell lines.34 To examine the impact of PRL2 deficiency on human leukemia cell proliferation, we have developed 2 short hairpin RNAs (shRNAs) targeting different regions of human PRL2.27,30 Both shRNAs can efficiently decrease PRL2 proteins in MV-4-11 cells (Figure 5A). We focused our studies using one of the PRL2 shRNAs and found that knockdown of PRL2 decreased the colony formation of MV-4-11, MOLM-13, and K562 cells (Figure 5B and supplemental Figure 6A-C). To determine the impact of PRL2 deficiency on leukemia development in vivo, we transplanted 3 × 106 MV-4-11 or MOLM-13 cells expressing control or PRL2 shRNA into sublethally irradiated NSG mice and monitored their survival. We found that loss of PRL2 significantly extended the survival of recipient mice transplanted with MV-4-11 or MOLM-13 cells (Figure 5C and supplemental Figure 6D). In addition, we found that the genetic inhibition of PRL2 significantly decreases the engraftment of MV-4-11 cells in PB, BM, and spleen of recipient mice (Figure 5D). Furthermore, knockdown of PRL2 significantly decreased splenomegaly seen in recipient mice transplanted with MV-4-11 cells (Figure 5E-F).
Genetic and pharmacological inhibition of PRL2 decrease leukemia burden and extend the survival of mice transplanted with human leukemia cell lines. (A) Western blot analysis for PRL2 in MV-4-11 cells transduced with lentiviruses expressing a shCtrl or PRL2 shRNAs (shPRL2 and shPRL2#2). (B) Knocking down of PRL2 significantly decreased the colony formation of MV-4-11 cells (n = 3). Representative images of the colonies are shown. (C) Kaplan-Meier survival curve of sublethally irradiated NSG mice transplanted with 3 × 106 MV-4-11 cells expressing shCtrl or shPRL2 (n = 7 mice group). (D) Flow cytometry quantification of GFP+ cells in PB, BM, and spleen of NSG mice transplanted with MV-4-11 cells expressing shCtrl or shPRL2 (n = 3 mice per group). (E-F) The size and weight of spleen from NSG mice transplanted with MV-4-11 cells expressing shCtrl or shPRL2 (n = 3 mice per group). (G) PRLi treatment significantly decreased the colony formation ability in MV-4-11 (n = 3). Representative images of the colonies are displayed. (H) 3 × 106 MV-4-11 cells expressing luciferase were injected into sublethally irradiated NSG mice. One week after the transplantation, NSG mice were treated with DMSO or PRLi (25 mg/kg, ip) daily for 3 weeks. The leukemia burdens in NSG mice were monitored by in vivo imaging system once a week for 3 weeks (n = 5 mice per group). (I) Quantitative results from bioimaging (n = 5 mice per group). (J) Kaplan-Meier survival curve of NSG mice treated with DMSO or PRLi (n = 7 mice per group). (K) Flow cytometry analysis of human CD45+ cells in PB, BM, and spleen of NSG mice transplanted with MV-4-11 cells after 3 weeks of DMSO or PRLi treatment (n = 3 mice per group). (L) PRLi treatment reduced splenomegaly seen in NSG mice transplanted with MV-4-11 cells. (M) The spleen weights of NSG mice transplanted with MV-4-11 cells following 3 weeks of DMSO or PRLi treatment (n = 3 mice per group). Mean values (±SEM) are shown (∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001). DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; PRLi, PRL inhibitor; shCtrl, control shRNA, NSG, NOD-scid IL2Rgammanull.
Genetic and pharmacological inhibition of PRL2 decrease leukemia burden and extend the survival of mice transplanted with human leukemia cell lines. (A) Western blot analysis for PRL2 in MV-4-11 cells transduced with lentiviruses expressing a shCtrl or PRL2 shRNAs (shPRL2 and shPRL2#2). (B) Knocking down of PRL2 significantly decreased the colony formation of MV-4-11 cells (n = 3). Representative images of the colonies are shown. (C) Kaplan-Meier survival curve of sublethally irradiated NSG mice transplanted with 3 × 106 MV-4-11 cells expressing shCtrl or shPRL2 (n = 7 mice group). (D) Flow cytometry quantification of GFP+ cells in PB, BM, and spleen of NSG mice transplanted with MV-4-11 cells expressing shCtrl or shPRL2 (n = 3 mice per group). (E-F) The size and weight of spleen from NSG mice transplanted with MV-4-11 cells expressing shCtrl or shPRL2 (n = 3 mice per group). (G) PRLi treatment significantly decreased the colony formation ability in MV-4-11 (n = 3). Representative images of the colonies are displayed. (H) 3 × 106 MV-4-11 cells expressing luciferase were injected into sublethally irradiated NSG mice. One week after the transplantation, NSG mice were treated with DMSO or PRLi (25 mg/kg, ip) daily for 3 weeks. The leukemia burdens in NSG mice were monitored by in vivo imaging system once a week for 3 weeks (n = 5 mice per group). (I) Quantitative results from bioimaging (n = 5 mice per group). (J) Kaplan-Meier survival curve of NSG mice treated with DMSO or PRLi (n = 7 mice per group). (K) Flow cytometry analysis of human CD45+ cells in PB, BM, and spleen of NSG mice transplanted with MV-4-11 cells after 3 weeks of DMSO or PRLi treatment (n = 3 mice per group). (L) PRLi treatment reduced splenomegaly seen in NSG mice transplanted with MV-4-11 cells. (M) The spleen weights of NSG mice transplanted with MV-4-11 cells following 3 weeks of DMSO or PRLi treatment (n = 3 mice per group). Mean values (±SEM) are shown (∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001). DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; PRLi, PRL inhibitor; shCtrl, control shRNA, NSG, NOD-scid IL2Rgammanull.
To further substantiate the PRL2 knockdown results, we also used compound 43,35 a small molecule PRL inhibitor (PRLi) that blocks PRL trimerization, which is essential for PRL function.35-37 Consistent with previous findings,35 PRLi treatment reduces the colony formation of MV-4-11, MOLM-13, and K562 cells (Figure 5G and supplemental Figure 6E-F). To determine the efficacy of PRLi on human leukemia cells in vivo, we transplanted luciferase-labeled MV-4-11 cells into sublethally irradiated NSG via tail vein injection. One week after the transplantation, we treated NSG mice with vehicle (10% DMSO) or PRLi (25 mg/kg, I.P.) daily for 3 weeks. Leukemia burden in NSG mice was monitored via bioluminescence imaging weekly. Serial imaging of luminescence showed that PRLi treatment dramatically decreased leukemia burden compared with the control group (Figure 5H). The radiance of the NSG mice was significantly reduced after exposure to PRLi (Figure 5I). Furthermore, PRLi substantially extended the survival of NSG mice transplanted with human leukemia cells (Figure 5J). PRLi also considerably decreased the engraftment of human leukemia cells in PB, BM, and spleen of NSG mice (Figure 5K). PRLi treatment significantly reduced the size and weight of spleen of NSG mice (Figure 5L-M). Finally, we found that PRLi is specific for PRL2 as it does not affect the colony formation of MV-4-11, MOLM-13, and K562 cells expressing a shRNA targeting PRL2 (supplemental Figure 6G). Furthermore, PRLi inhibits the proliferation of MV-4-11 and MOLM-13 cells expressing PRL2, but not MV4-11 and MOLM-13 cells expressing PRL2-CSDA (supplemental Figure 6H).
Pharmacological inhibition of PRL2 reduces leukemia burden and extends the survival of mice transplanted with primary human AML cells
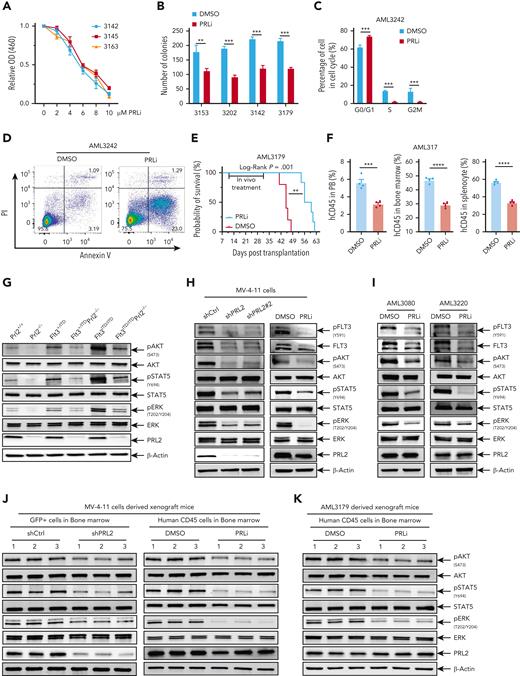
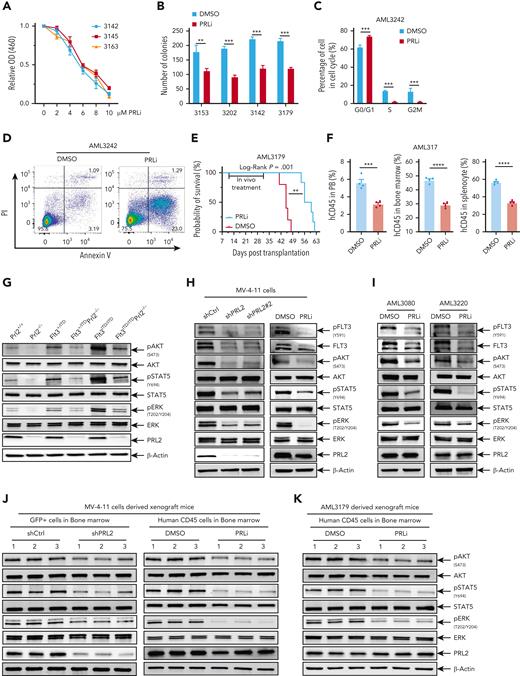
PRLi decreases the proliferation of primary human AML cells in vitro in a dosage-dependent manner (Figure 6A). In addition, PRLi treatment decreases the colony formation of primary human AML cells with or without FLT3 mutations (Figure 6B). PRLi treatment also arrested primary AML cells with FLT3-ITD mutation at the G0/G1 phase of the cell cycle and decreased the percentage of cells in S or G2M phase (Figure 6C and supplemental Figure 6I). Furthermore, PRLi treatment significantly increased the apoptosis of primary human AML cells with FLT3-ITD mutation (Figure 6D and supplemental Figure 6J).
Pharmacological inhibition of PRL2 reduces leukemia burden and extends the survival of mice transplanted with primary human AML cells. (A) PRLi treatment decreased the viability of primary human AML cells with FLT3-ITD mutation in a dosage-dependent manner. (B) PRLi treatment reduced the colony forming ability of primary human AML cells with or without FLT3-ITD mutation. Samples 3153 and 3202 are from AML patients with WT FLT3, whereas samples 3142 and 3179 are from AML patients with FLT3-ITD. (C) Cell cycle analysis of primary AML cells with FLT3-ITD mutation (AML3242) at 24 hours following DMSO or PRLi (10 μM) treatment. (D) Apoptosis analysis of primary AML cells with FLT3-ITD (AML3242) at 24 hours following DMSO or PRLi (10 μM) treatment. (E) Kaplan-Meier survival curve of NSG mice transplanted with 4 × 106 human CD45+ leukemia cells (AML3179) following 3 weeks of DMSO or PRLi treatment (n = 6 mice per group). (F) Flow cytometry analysis of human CD45+ cells in PB, BM, and spleen of NSG mice transplanted with 4 × 106 human CD45+ leukemia cells (AML3179) after 3 weeks of DMSO or PRLi treatment (n = 4 mice per group). (G) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− BM mononuclear cells. (H) Representative western blot analysis of FLT3, AKT, STAT5, and ERK phosphorylation in MV-4-11 cells expressing shCtrl, shPRL2, or shPRL2#2 (left) and following 24 hours of DMSO or 5 μM PRLi treatment (right). (I) Representative western blot analysis of FLT3, AKT, STAT5, and ERK phosphorylation in primary AML cells with FLT3-ITD mutation (AML3080 and AML3220) following 24 hours of DMSO or PRLi (10 μM) treatment. (J) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in human CD45+ cells isolated from the BM of NSG mice at 4 weeks after transplantation with MV-4-11 cells expressing shCtrl or shPRL2 (left panel, n = 3 mice per group); human CD45+ cells in the BM of NSG mice transplanted with MV-4-11 cells following 3 weeks of DMSO or PRLi treatment (right panel, n = 3 mice per group). (K) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in human CD45+ cells isolated from the BM of NSG mice transplanted with patient-derived xenograft cells (AML3179) following 3 weeks of DMSO or PRLi treatment (n = 3 mice per group).
Pharmacological inhibition of PRL2 reduces leukemia burden and extends the survival of mice transplanted with primary human AML cells. (A) PRLi treatment decreased the viability of primary human AML cells with FLT3-ITD mutation in a dosage-dependent manner. (B) PRLi treatment reduced the colony forming ability of primary human AML cells with or without FLT3-ITD mutation. Samples 3153 and 3202 are from AML patients with WT FLT3, whereas samples 3142 and 3179 are from AML patients with FLT3-ITD. (C) Cell cycle analysis of primary AML cells with FLT3-ITD mutation (AML3242) at 24 hours following DMSO or PRLi (10 μM) treatment. (D) Apoptosis analysis of primary AML cells with FLT3-ITD (AML3242) at 24 hours following DMSO or PRLi (10 μM) treatment. (E) Kaplan-Meier survival curve of NSG mice transplanted with 4 × 106 human CD45+ leukemia cells (AML3179) following 3 weeks of DMSO or PRLi treatment (n = 6 mice per group). (F) Flow cytometry analysis of human CD45+ cells in PB, BM, and spleen of NSG mice transplanted with 4 × 106 human CD45+ leukemia cells (AML3179) after 3 weeks of DMSO or PRLi treatment (n = 4 mice per group). (G) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in Prl2+/+, Prl2−/−, Flt3+/ITD, Flt3+/ITDPrl2−/−, Flt3ITD/ITD, and Flt3ITD/ITDPrl2−/− BM mononuclear cells. (H) Representative western blot analysis of FLT3, AKT, STAT5, and ERK phosphorylation in MV-4-11 cells expressing shCtrl, shPRL2, or shPRL2#2 (left) and following 24 hours of DMSO or 5 μM PRLi treatment (right). (I) Representative western blot analysis of FLT3, AKT, STAT5, and ERK phosphorylation in primary AML cells with FLT3-ITD mutation (AML3080 and AML3220) following 24 hours of DMSO or PRLi (10 μM) treatment. (J) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in human CD45+ cells isolated from the BM of NSG mice at 4 weeks after transplantation with MV-4-11 cells expressing shCtrl or shPRL2 (left panel, n = 3 mice per group); human CD45+ cells in the BM of NSG mice transplanted with MV-4-11 cells following 3 weeks of DMSO or PRLi treatment (right panel, n = 3 mice per group). (K) Representative western blot analysis of AKT, STAT5, and ERK phosphorylation in human CD45+ cells isolated from the BM of NSG mice transplanted with patient-derived xenograft cells (AML3179) following 3 weeks of DMSO or PRLi treatment (n = 3 mice per group).
To determine the efficacy of PRLi on primary human leukemia cells in vivo, we generated 2 patient-derived xenograft (PDX) models of FLT3-ITD–positive AML in NSGS mice. We confirmed engraftment of human CD45+ (hCD45+) AML cells in NSGS mice 12 to 16 weeks after primary transplantation (data not shown), and generated secondary recipients for drug administration. After confirmation of human leukemia cell engraftment in PB of NSG mice (>1% human CD45+ cells), NSG mice were treated with vehicle (10% DMSO) or PRLi (25 mg/kg, I.P.) daily for 3 weeks. PRLi substantially extended the survival of NSG mice transplanted with human CD45+ leukemia cells (Figure 6E and supplemental Figure 6K). PRLi also considerably decreased the engraftment of human CD45+ leukemia cells in PB, BM, and spleen of NSG mice at the end point of treatment (Figure 6F and supplemental Figure 6L).
PRL2 is a positive mediator of oncogenic FLT3 signaling in murine hematopoietic cells and human leukemia cells
To determine the impact of PRL2 on FLT3 signaling, we examined STAT5, AKT, and ERK phosphorylation and found that loss of Prl2 decreased STAT5, AKT, and ERK phosphorylation in both Flt3+/ITD and Flt3ITD/ITD BM cells (Figure 6G). These observations suggest that PRL2 is a positive mediator of FLT3-ITD signaling in hematopoietic cells. To determine the impact of PRL2 deficiency on FLT3 signaling in leukemia cells, we found that knockdown of PRL2 significantly decreased pFLT3, FLT3 expression, AKT, ERK, and STAT5 phosphorylation in MV-4-11 cells (Figure 6H, left panel). In addition, we showed that PRLi treatment decreased pFLT3, FLT3 expression, AKT, ERK, STAT5, STAT3, and MEK phosphorylation in MV-4-11 cells (Figure 6H right panel and supplemental Figure 7A). Moreover, we observed decreased pFLT3, FLT3 expression, phosphorylation of AKT, STAT5, STAT3, STAT1, and MEK in K562 cells following PRLi treatment (supplemental Figure 7B), but there was no change in the levels of BCR-ABL, BCR, and c-ABL (supplemental Figure 7C). We also found that PRLi treatment reduces FLT3 expression and decreases the phosphorylation of AKT, ERK, and STAT5 in U937 cells expressing WT FLT3 or FLT3-ITD (supplemental Figure 7D). PRLi treatment signficantly decreased the phopshorylation of AKT, STAT5, and ERK in primary AML cells with FLT3-ITD mutation (Figure 6I). We observed decreased phosphorylation of AKT, STAT5, and ERK in MV4-11 cells expressing shPRL2 isolated from NSG mice at 4 weeks following transplantation (Figure 6J left and supplemental Figure 7E left). Notably, we observed decreased phosphorylation of AKT, STAT5, and ERK in MV4-11 and primary human AML cells isolated from NSG mice following 3 weeks of PRLi treatment (Figure 6J-K and supplemental Figure 7E). Although PTEN (Phosphatase and tensin homolog) is a negative regulator of the AKT signaling pathway,28 PRLi treatment did not affect PTEN expression in MV4-11 cells (supplemental Figure 7F). Finally, we showed that PRLi is synergic with FLT3 inhibitor AC220 or gilteritinib in inhibiting the proliferation of MV-4-11 cells (supplemental Figure 7G).
PRL2 associates with and dephosphorylates CBL at tyrosine 371 in leukemia cells
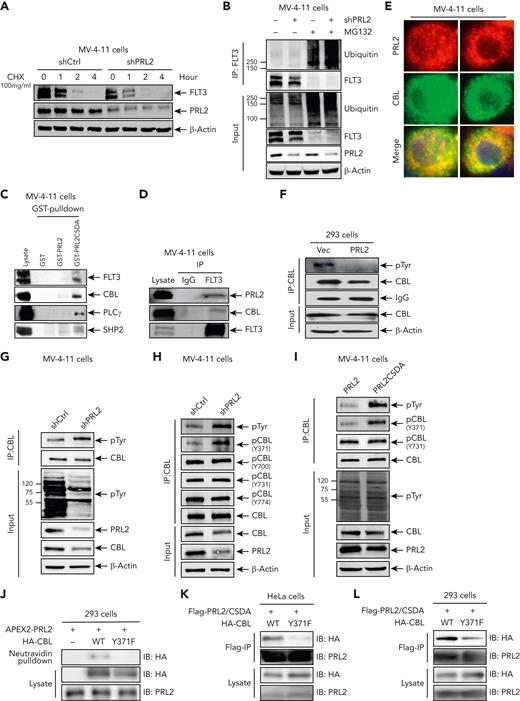
To investigate the mechanism by which PRL2 promotes FLT3 signaling, we determined the effect of PRL2 inhibition on FLT3 stability. We discovered that both knockdown of PRL2 and PRLi treatment can lead to a reduction in FLT3 protein level as a result of a decrease in FLT3 half-life in MV-4-11 cells (Figure 7A and supplemental Figure 8A). In line with this observation, we found that both knockdown of PRL2 and PRLi treatment increased FLT3 ubiquitination in MV-4-11 cells (Figure 7B and supplemental Figure 8B).
PRL2 associates with and dephosphorylates CBL at tyrosine 371 in leukemia cells. (A) Knockdown of PRL2 decreased FLT3 half-life in MV-4-11 cells. (B) Knockdown of PRL2 enhanced FLT3 ubiquitination in MV-4-11 cells. (C) Total cellular proteins from MV-4-11 cells were isolated, incubated with GST, GST-PRL2, or GST- PRL2-CSDA and immunoblotted with antibody against FLT3, CBL, SHP2, and PLC-γ. (D) Co-immunoprecipitation assays showed that PRL2 interacts with FLT3 and CBL in MV-4-11 cells. (E) Immunofluorescence analysis showed that PRL2 colocalizes with CBL in MV-4-11 cells. (F) Representative western blot analysis showed that ectopic PRL2 expression decreases tyrosine phosphorylation of CBL in 293 cells. (G) Representative western blot analysis showed that knocking down of PRL2 increases the tyrosine phosphorylation of CBL in MV-4-11 cells. (H) Representative western blot analysis showed that knocking down of PRL2 increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells. (I) Representative western blot analysis showed that ectopic expression of PRL2-CSDA increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells. (J) APEX2-PRL2 proximity labeling was performed in HA-CBL or HA-CBLY371F transiently expressed 293 cells stably expressing APEX2-PRL2. After labeling, biotinylated proteins are enriched with neutravidin beads and examined with anti-HA and anti-PRL2 antibodies by western blot analysis. (K-L) PRL2-CSDA substrate trapping assays was performed in HA-CBL or HA- CBLY371F transiently expressed HeLa (K) or 293 (L) cells stably expressing Flag-PRL2-CSDA. After anti-FLAG pulldown, bound proteins were boiled in 50 μL Laemmli sample buffer and examined with anti-HA, anti-PRL2 antibodies by western blot analysis. CBL, Casitas B-lineage lymphoma.
PRL2 associates with and dephosphorylates CBL at tyrosine 371 in leukemia cells. (A) Knockdown of PRL2 decreased FLT3 half-life in MV-4-11 cells. (B) Knockdown of PRL2 enhanced FLT3 ubiquitination in MV-4-11 cells. (C) Total cellular proteins from MV-4-11 cells were isolated, incubated with GST, GST-PRL2, or GST- PRL2-CSDA and immunoblotted with antibody against FLT3, CBL, SHP2, and PLC-γ. (D) Co-immunoprecipitation assays showed that PRL2 interacts with FLT3 and CBL in MV-4-11 cells. (E) Immunofluorescence analysis showed that PRL2 colocalizes with CBL in MV-4-11 cells. (F) Representative western blot analysis showed that ectopic PRL2 expression decreases tyrosine phosphorylation of CBL in 293 cells. (G) Representative western blot analysis showed that knocking down of PRL2 increases the tyrosine phosphorylation of CBL in MV-4-11 cells. (H) Representative western blot analysis showed that knocking down of PRL2 increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells. (I) Representative western blot analysis showed that ectopic expression of PRL2-CSDA increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells. (J) APEX2-PRL2 proximity labeling was performed in HA-CBL or HA-CBLY371F transiently expressed 293 cells stably expressing APEX2-PRL2. After labeling, biotinylated proteins are enriched with neutravidin beads and examined with anti-HA and anti-PRL2 antibodies by western blot analysis. (K-L) PRL2-CSDA substrate trapping assays was performed in HA-CBL or HA- CBLY371F transiently expressed HeLa (K) or 293 (L) cells stably expressing Flag-PRL2-CSDA. After anti-FLAG pulldown, bound proteins were boiled in 50 μL Laemmli sample buffer and examined with anti-HA, anti-PRL2 antibodies by western blot analysis. CBL, Casitas B-lineage lymphoma.
To understand how PRL2 promotes FLT3 stabilization, we carried out substrate trapping experiments to identify potential PRL2 substrates in leukemia cells. To that end, we used the GST-tagged PRL2-CSDA mutant, which is competent for substrate binding but unable to catalyze substrate turnover. 33,38 Indeed, we found that PRL2-CSDA shows enhanced association with CBL, FLT3, PLCγ, and SHP2 compared with WT PRL2 in MV-4-11 cells (Figure 7C). We confirmed that PRL2 associates with FLT3 and CBL in MV-4-11 cells using co-immunoprecipitation (Co-IP) assays (Figure 7D). We also found that PRL2 and CBL colocalize in MV-4-11 (Figure 7E) and U2OS cells (supplemental Figure 8C). Given that CBL is an E3 ubiquitin ligase which is responsible for ubiquitination and degradation of FLT3 in hematopoietic cells,15 these findings suggest that CBL may be a PRL2 substrate.
CBL becomes phosphorylated on several tyrosine residues following cytokine stimulation (supplemental Figure 8D). To determine whether CBL can serve as a substrate for PRL2, we expressed PRL2 in HEK293 cells and found that ectopic PRL2 expression decreased CBL tyrosine phosphorylation in HEK293 cells (Figure 7F). In contrast, knockdown of PRL2 increases CBL tyrosine phosphorylation in MV-4-11 cells (Figure 7G). CBL becomes activated upon tyrosine 371 phosphorylation, which enables it to target receptor protein tyrosine kinases for ubiquitin-mediated degradation.15,22,39-41 Indeed, we found that knockdown of PRL2 increases CBL phosphorylation at tyrosine 371, whereas the levels of CBL phosphorylation at tyrosine 700, 731, and 774 were not affected by PRL2 inhibition in MV-4-11 cells (Figure 7H). We detected that ectopic expression of the catalytically inactive PRL2-CSDA mutant increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells (Figure 7I). Furthermore, we found that PRLi treatment increases CBL phosphorylation at tyrosine 371 in MV-4-11 cells (supplemental Figure 8E).
To further examine the enzyme-substrate interaction between PRL2 and CBL at the molecular level, we used APEX2 proximity labeling, which is a widely used method for rapid covalent labeling of neighboring proteins within a 10 to 20 nm radius of a protein of interest in living cells.42-44 To that end, APEX2-PRL2 fusion protein was used to perform proximity labeling to identify its interacting proteins. To our satisfaction, we identified CBL as a PRL2 neighboring protein, but not the nonphosphorylatable CBLY371F mutant, in live cells (Figure 7J). Consistently, the PRL2-CSDA substrate trapping mutant showed enhanced association with CBL compared with the CBLY371F mutant in both HeLa and 293 cells (Figure 7K-L). Notably, CBL expression is correlated with PRL2 expression in human patients with leukemia (supplemental Figure 8F). Collectively, the data presented above demonstrate that CBL is a substrate of PRL2 and that PRL2 associates with and dephosphorylates CBL at tyrosine 371 in leukemia cells. It follows that dephosphorylation of CBL at tyrosine 371 by PRL2 blocks CBL-mediated FLT3 ubiquitination and degradation, leading to heightened FLT3 signaling in leukemia cells.
Discussion
Members of the PTP family dephosphorylate target proteins and counter the activities of protein tyrosine kinases to control the strength and duration of tyrosine phosphorylation–mediated cellular signaling.45,46 FLT3 is a major oncogenic receptor tyrosine kinase aberrantly activated in leukemia.6,7 PRL2 is known to be overexpressed in some subtypes of AML.27 Here, we demonstrate that PRL2 enhances oncogenic FLT3 signaling and promotes leukemia cell proliferation and survival. We further establish that PRL2 dephosphorylates CBL at tyrosine 371 and inhibits its E3 ligase activity toward FLT3, leading to decreased ubiquitination and degradation of FLT3, thereby activating its downstream signaling pathways in leukemia cells. Finally, we also show that genetic and pharmacological inhibition of PRL2 significantly reduce the burden of FLT3-ITD–driven leukemia and extend the survival of leukemic mice. Together, our work validates PRL2 as a novel druggable target for AML.
We previously found that loss of PRL2 does not change HSC number in the BM but decreases adult HSPC proliferation.28 We now show that receptor tyrosine kinase, PI3K/AKT, and ERK signaling gene signatures are significantly downregulated in Prl2 null fetal liver HSPCs. In addition, loss of Prl2 significantly decreased AKT, STAT5, and ERK phosphorylation in fetal liver cells. Given that fetal liver HSPCs are characterized by a massive expansion of HSCs, whereas BM HSCs are much more quiescent, the PRL2 effect could be associated with cell proliferation instead of "stem" ability in fetal livers.
Members of the CBL family of E3 ubiquitin ligases share a highly conserved N-terminal tyrosine kinase–binding domain, a short linker helical region (LHR), and a RING finger (RF) domain.15 The LHR and RF domains dictate the E3 activity of CBL family members by serving as a structural platform for optimal binding of a ubiquitin-conjugating enzyme, E2.15 CBL’s ubiquitination activity is stimulated by phosphorylation of a Tyr residue in a LHR.39-41 Structural and biochemical studies show that phosphorylation of Tyr 371 activates CBL by inducing LHR conformational changes that eliminate autoinhibition and enable direct participation of LHR phosphotyrosine in the activation of E2-ubiquitin complex for catalysis.41,47 This activation is required for receptor tyrosine kinase ubiquitination. We found that PRL2 associates with and dephosphorylates CBL in human leukemia cells and that inhibition of PRL2 activity increases CBL Tyr 371 phosphorylation in human leukemia cells. Our results suggest that CBL/pTyr371 is a novel PRL2 substrate in leukemia cells.
Most CBL mutations in myeloid malignancies are found in the RF domain and the linker region of CBL.19-21 Some CBL mutants, such as CBLY371H and CBL-70Z, do not have E3 ubiquitin ligase activity but compete against WT CBL and CBL-B, leading to prolonged activation of receptor tyrosine kinases after cytokine stimulation.39,40 Inactivating CBL mutations–mediated hematopoietic transformation in AML depends on FLT3 signaling.48 Indeed, loss of CBL E3 ubiquitin ligase activity enhances the development of myeloid leukemia in FLT3-ITD mutant mice.49 Furthermore, myeloid leukemia development in CBL RF mutant mice is dependent on FLT3 signaling.50 Our finding that PRL2 dephosphorylates CBL at Tyr 371 thereby compromising CBL’s ability to ubiquitinate FLT3 is consistent with a tumor suppressor role for CBL in hematological malignancies. We previously showed that PRL2 is important for SCF/KIT signaling in HSPCs.28 Thus, decreased AKT, ERK, and STAT5 phosphorylation seen in Prl2 null fetal HSPCs could be because of diminished FLT3 and KIT signaling. Given that CBL is the E3 ligase for both FLT3 and KIT,15 it is possible that PRL2 could also promote KIT signaling in HSPCs through dephosphorylation of CBL at tyrosine 371.
Despite substantial efforts devoted to the development of FLT3 inhibitors, the effectiveness of these agents in AML has been limited.6-8,51 Even though FLT3 inhibitors show relative success at prolonging survival rates compared with the standard therapies, the short duration of response and therapeutic resistance are still a clinical challenge in AML treatment.42,51,52 The strategies to overcome resistance mutations and provide durable remissions, such as a combination of inhibitors or use of more potent FLT3 inhibitors, have been evaluated.9 Here, we show that PRL2 functions upstream of FLT3 and promotes oncogenic FLT3 signaling in leukemia cells by inhibiting CBL–mediated FLT3 ubiquitination and degradation. We further demonstrate that PRL2 deletion or inhibition decreases leukemia burden and extends the survival of mice transplanted with human leukemia cells. Consequently, PRL2 inhibitors may offer an alternative strategy for AML treatment. To therapeutically target the PRL family members in cancer, we sought to exploit a unique regulatory property of the PRLs, namely their propensity for trimer formation, which is required for PRL-mediated cell growth and migration. 35-37,53 Using structure-based virtual screening, we identified compound 43 (PRLi), which disrupts PRL trimerization and blocks PRL-induced cell proliferation and migration.35 PRLi displays a respectable pharmacokinetic profile and exhibits no obvious toxicity to major tissues and organs in mice.35 Notably, PRLi did not affect the viability of human cord blood mononuclear cells and CD34+ cells.27 PRLi treatment significantly reduced tumor volume in NSG mice transplanted with human melanoma cells.35 Furthermore, we found that both human AML and acute lymphoblastic leukemia cells are sensitive to PRLi treatment in vitro.27,30 We now show that in vivo PRLi treatment significantly reduces leukemia burden and extends the survival of NSG mice transplanted with primary human leukemia cells with FLT3-ITD mutations. Our ex vivo studies showed that FLT3 WT and FLT3 mutated primary AML samples are equally sensitive to PRL2 inhibition, suggesting that there is an underlying mechanism that is different among AML samples based on their mutations. PRL2 is highly expressed in some subtypes of AML27 and AML patients with high PRL2 expression have reduced overall survival compared with those with low PRL2 expression. It is possible that PRL2 uses distinct mechanisms to promote cell proliferation and enhance oncogenic signaling in different cellular contexts. We thus demonstrate that PRL2 is a novel druggable target in human AML.
Acknowledgments
The authors thank Flow Cytometry Facility, Molecular and Translational Imaging Core Facility, Pathology Core Facility, Quantitative Data Sciences Core Facility, and Center for Comparative Medicine at the Northwestern University and Robert H. Lurie Comprehensive Cancer Center. The authors also acknowledge the Flow Cytometry Core and In vivo Therapeutic Core Laboratories at the Indiana University, which were sponsored, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases Cooperative Center of Excellence in Hematology (CCEH) grant U54 DK106846.
Y.L. was supported by National Institutes of Health (R01 HL150624, R56 DK119524, R56 AG052501), Department of Defense (DoDW81XWH-18-1-0265, DoD W81XWH-19-1-0575), the Leukemia & Lymphoma Society Translational Research Program award 6581-20 and the St. Baldrick’s Foundation Scholar Award. Y.B. and Z.-Y.Z. were supported by National Institutes of Health (R01 CA069202) and the Robert C. and Charlotte Anderson Chair Endowment. S.B. was supported by a National Institutes of Health F31 Award (F31HL160120). H.C. was supported by Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0969).
Authorship
Contribution: H.C., Y.B., M.K., Z.-Y.Z., and Y.L. were responsible for the conception and/or design of the research; H.C., Y.B., M.K., S.X., W.C., S.B., S.C., J.M., F.N.M., S.V., J.P.R., J.W., Y.J., H.L., P.J., Z.-Y.Z., and Y.L. were involved in acquisition, analysis, or interpretation of data; J.M.C., H.S.B., L.S.L., J.K.A., E.A.E., W.T., H.B., D.T.H., and L.C.P. provided reagents and constructive advice to the study; H.C., Y.B., Z.-Y.Z., and Y.L. wrote the manuscript; and all authors read, commented on, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhong-Yin Zhang, Department of Medicinal Chemistry and Molecular Pharmacology, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, West Lafayette, IN; e-mail: zhang-zy@purdue.edu; and Yan Liu, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL; e-mail: yan.liu@northwestern.edu.
References
Author notes
RNA-seq data are available at GEO under accession number GSE208136.
Data are available on request from the corresponding authors Yan Liu (yan.liu@northwestern.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
H.C. and Y.B. contributed equally to the study.