Key Points
mTOR inhibition in patients with m-IC improves cTfh and T-cell dysregulation along with preservation of absolute T- and B-cell counts.
Despite favorable changes in T-cell compartment, mTOR inhibition did not universally result in improvement in B-cell maturation.
Abstract
mTOR inhibitors such as sirolimus are increasingly used in the management of multilineage immune cytopenia (m-IC) in children. Although sirolimus is effective in improving IC, it is unclear how sirolimus affects the broader immune dysregulation associated with m-IC. We profiled T- and B-cell subsets longitudinally and measured cytokines and chemokines before and after sirolimus treatment. Eleven of the 12 patients with m-IC who tolerated sirolimus were followed for a median duration of 17 months. All patients had an improvement in IC, and sirolimus therapy did not result in significant decreases in T-, B- and NK-cell numbers. However, the expansion and activation of circulating T follicular helper and the Th1 bias noted before the initiation of sirolimus were significantly decreased. Features of chronic T-cell activation and exhaustion within effector memory compartments of CD4+ and CD8+ T cells decreased with sirolimus therapy. Corresponding to these changes, plasma levels of CXCL9 and CXCL10 also decreased. Interestingly, no significant improvement in the proportion of class-switched memory B cells or frequencies of CD4+ naive T cells were noted. Longer follow-up and additional studies are needed to validate these findings and evaluate the effect of sirolimus on B-cell maturation.
Introduction
Multilineage immune cytopenias (m-IC) are increasingly recognized as a presenting manifestation of underlying immune regulatory disorders.1-5 Genetic analysis of m-IC identified several pathogenic variants in immune regulatory genes in ∼40% of these cases.6 Recently, we showed that patients with m-IC, irrespective of underlying genetic mutations, have broad immune dysregulation characterized by expansion and dysregulation of circulating T-follicular helper cells (cTfh), increased T-cell activation, and decreased frequencies of CD4+ naïve T cells and class-switched memory B (CSMB) cells.7 Tfh cells represent a specialized population of CD4+ T cells which help in B-cells class switch and make high-affinity antibodies. Dysregulation in Tfh cells affects T- and B-cell interaction leading to autoimmunity and humoral immune deficiency.8,9
Sirolimus, an mTOR inhibitor, is being increasingly used to treat patients with autoimmune lymphoproliferative syndrome (ALPS) and non-ALPS–associated m-IC, and multiple studies have shown excellent overall response rates.5,10-15 Although sirolimus improves immune cytopenias in patients with m-IC, it is still poorly understood whether sirolimus helps in ameliorating underlying broader immune dysregulation in these patients. Limited immune studies in m-IC have shown no change in absolute lymphocyte count, CD4+ T, CD8+ T and IgG levels on long term treatment with sirolimus.11 To address how sirolimus affects underlying immune dysfunction in patients with m-IC, we performed longitudinal immune evaluation in m-IC patients before and after sirolimus treatment.
Study design
Human subjects
A total of 12 patients with m-IC (aged 5-18 years, median age = 8.5 years) and 21 healthy controls (HC) (aged 1-30 years, median = 16 years) were included in this study from 2017 to 2021. All patients with m-IC were enrolled at Children’s Healthcare of Atlanta under institutional review board approved protocol. The demographics and clinical characteristics for patients with m-IC are provided in supplemental Table 1 (available on the Blood website). Clinical laboratory parameters were extracted from medical records in all 12 patients. All patients’ samples were titrated to target serum trough levels between 5 and 12 ng/mL. Of the 12 patients, 1 was excluded owing to poor adherence (sirolimus levels <3 ng/mL) leaving 11 patients with sirolimus levels of > 3 ng/mL included in the analysis. Patients with m-IC were evaluated either at disease onset or during active disease. Patients with a co-existing diagnosis of systemic lupus erythematosus, or underlying malignancy were excluded. Informed consent was obtained from all subjects as per the Declaration of Helsinki.
Genetic evaluation
All 12 patients with m-IC underwent genetic testing. Four patients were found with pathogenic or likely pathogenic gene variants, that is, LRBA (n = 1), PI3KCD (n = 1) and FAS (n = 2), whereas 2 patients were found with gene variants of uncertain significance which includes PNP, PRKCB and ADA. One patient was found with CFHR3 gene deletion (risk allele). Additional details about genetic testing and the variants are listed in supplemental Table 2.
Flow cytometry and description of different immune subsets (supplemental Table 4), cytokine/chemokine profiling and statistical analysis are included in the supplemental data.
Results and discussion
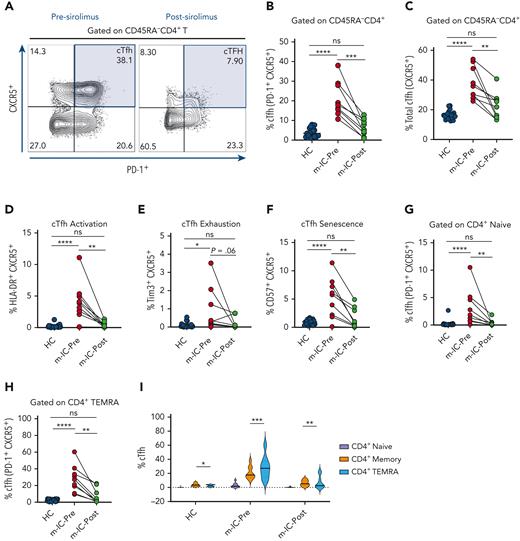
In our previous study, we found that patients with m-IC display a broad signature of T- and B-cell immune dysregulation that was markedly different from patients with chronic ITP (cITP).7 Patients with m-IC showed an expansion of cTfh population along with upregulation of activation, exhaustion and senescence markers.7 At a median follow-up of 17 months (range, 5-30; supplemental Figure 1) on sirolimus therapy, most patients achieved favorable clinical response of immune cytopenia and lymphoproliferation (supplemental Table 1). In addition, patients on sirolimus therapy showed a significant decrease in total (CXCR5+) and PD-1+ cTfh populations (Figure 1A-C). Furthermore, we noted a decrease in levels of activation, senescence and exhaustion markers on cTfh suggesting that sirolimus not only helped in restricting the abnormal expansion of cTfh but also improved the dysregulated state of this subset (Figure 1D-F). Earlier, we showed that cTfh displays early lineage commitment in CD4+ naïve T -cell populations and expands further in CD4+ memory and TEMRA cells owing to likely preferential proliferative stress in cTfh.7 We observed a significant decrease in the proportion of cTfh in both CD4+ naïve T along with CD4+ TEMRA populations on sirolimus treatment, suggesting suppression of early lineage commitment of these cells and reversal of preferential cTfh expansion (Figure 1G-I).
Attenuation of cTfh expansion and dysregulation on sirolimus treatment. (A) Representative flow plots showing frequency of cTfh cells in a patient with m-IC before (Pre) and after (Post) sirolimus treatment. (B-C) Frequency of PD-1+ cTfh (PD-1+ CXCR5+) and total cTfh (CXCR5+) are shown for HC and patients with m-IC (n = 9) before and after sirolimus therapy. (D-F) Plots showing frequencies of cTfh activation (HLA-DR+ CXCR5+), exhaustion (Tim3+ CXCR5+) and senescence (CD57+ CXCR5+) gated on total memory CD4+T (CD45RA-CD4+) cells. (G-H) cTfh population was gated on CD4+ naïve T and CD4+ TEMRA T cells and plots showing percentage of these populations pre- and post-sirolimus therapy. (I) Violin plot showing percentage of cTfh in naïve, memory and TEMRA compartments of CD4+ T -cells in HC and patients with m-IC before and after sirolimus treatment. Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test for non-normally distributed samples and ordinary 1-way ANOVA followed by Tukey’s multiple comparison test for normally distributed samples were used for statistical comparison of HC with m-IC pre- and posttreatment groups. Paired t test or Wilcoxon signed-rank test was used for paired analysis between pre- and post-m-IC groups. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001; ns, not significant.
Attenuation of cTfh expansion and dysregulation on sirolimus treatment. (A) Representative flow plots showing frequency of cTfh cells in a patient with m-IC before (Pre) and after (Post) sirolimus treatment. (B-C) Frequency of PD-1+ cTfh (PD-1+ CXCR5+) and total cTfh (CXCR5+) are shown for HC and patients with m-IC (n = 9) before and after sirolimus therapy. (D-F) Plots showing frequencies of cTfh activation (HLA-DR+ CXCR5+), exhaustion (Tim3+ CXCR5+) and senescence (CD57+ CXCR5+) gated on total memory CD4+T (CD45RA-CD4+) cells. (G-H) cTfh population was gated on CD4+ naïve T and CD4+ TEMRA T cells and plots showing percentage of these populations pre- and post-sirolimus therapy. (I) Violin plot showing percentage of cTfh in naïve, memory and TEMRA compartments of CD4+ T -cells in HC and patients with m-IC before and after sirolimus treatment. Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test for non-normally distributed samples and ordinary 1-way ANOVA followed by Tukey’s multiple comparison test for normally distributed samples were used for statistical comparison of HC with m-IC pre- and posttreatment groups. Paired t test or Wilcoxon signed-rank test was used for paired analysis between pre- and post-m-IC groups. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001; ns, not significant.
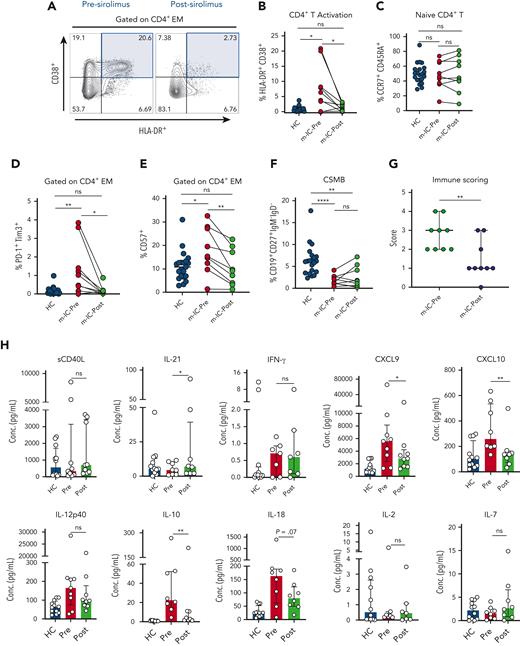
Another important feature of patients with m-IC is chronic T-cell activation.7 On sirolimus treatment, we found a significant decrease in both CD4+ and CD8+ effector memory (EM) T-cell activation than to the HC (Figure 2A-B and supplemental Figure 2A). We also observed a decrease in expression of senescence and exhaustion markers in CD4+ EM T cells (Figure 2D-E). Similarly, a decrease in exhaustion markers was seen on CD8+ EM T cells (supplemental Figure 2B). Similar findings of decrease in frequency of PD-1+CD4+ and T-cell senescence was reported earlier in APDS (activated phosphoinositide 3-kinase δ) syndrome using PI3Kδ inhibitor leniolisib which targets upstream of PI3K/Akt/mTOR pathway.16 Previous studies have shown increased mTOR activity in patients with ALPS and APDS.17,18 However, in our study cohort, despite having diverse genetic defects, we found similar response to sirolimus suggesting increased mTOR activity in patients with m-IC irrespective of underlying genetics.
Changes in T- and B-cell immune abnormalities and inflammatory milieu in patients with m-IC on sirolimus therapy. (A) FACS plots showing frequency of co-expression of activation markers HLA-DR+ CD38+ on CD4+ EM T cells in a patient with m-IC before (Pre) and after (Post) sirolimus therapy. (B-C) Dot plots showing percentage of CD4+ EM activation (HLA-DR+CD38+ gated on CD4+ EM T cells) and naïve CD4+ T in HC and m-IC groups. (D-E) Plots showing percentage of immune markers for exhaustion (PD-1+ Tim3+) and senescence (CD57+) gated on CD4+ EM T cells in HC and m-IC groups. (F) Dots plots represent CSMB cells in HC and m-IC groups. (G) Dot plot represents a combined immune score of 4 immune parameters in pre- and post-sirolimus treatment m-IC groups (n = 9). (H) Bar plots showing plasma concentrations of sCD40L, IL-21, IFN-γ, CXCL9, CXCL10, IL-12p40, IL-10, IL-18, IL-2 and IL-7 in pre- and posttreatment m-IC groups. Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test for non-normally distributed samples and ordinary 1-way ANOVA followed by Tukey’s multiple comparison test for normally distributed samples were used for statistical comparison of HC with m-IC pre- and posttreatment groups. Paired t test or Wilcoxon signed-rank test was used for paired analysis between pre- and post-m-IC groups. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001; ns, not significant.
Changes in T- and B-cell immune abnormalities and inflammatory milieu in patients with m-IC on sirolimus therapy. (A) FACS plots showing frequency of co-expression of activation markers HLA-DR+ CD38+ on CD4+ EM T cells in a patient with m-IC before (Pre) and after (Post) sirolimus therapy. (B-C) Dot plots showing percentage of CD4+ EM activation (HLA-DR+CD38+ gated on CD4+ EM T cells) and naïve CD4+ T in HC and m-IC groups. (D-E) Plots showing percentage of immune markers for exhaustion (PD-1+ Tim3+) and senescence (CD57+) gated on CD4+ EM T cells in HC and m-IC groups. (F) Dots plots represent CSMB cells in HC and m-IC groups. (G) Dot plot represents a combined immune score of 4 immune parameters in pre- and post-sirolimus treatment m-IC groups (n = 9). (H) Bar plots showing plasma concentrations of sCD40L, IL-21, IFN-γ, CXCL9, CXCL10, IL-12p40, IL-10, IL-18, IL-2 and IL-7 in pre- and posttreatment m-IC groups. Kruskal-Wallis 1-way ANOVA followed by Dunn’s multiple comparison test for non-normally distributed samples and ordinary 1-way ANOVA followed by Tukey’s multiple comparison test for normally distributed samples were used for statistical comparison of HC with m-IC pre- and posttreatment groups. Paired t test or Wilcoxon signed-rank test was used for paired analysis between pre- and post-m-IC groups. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001; ns, not significant.
We previously showed skewing of cTfh toward a Th1 phenotype in patients with m-IC.7 Longitudinal evaluation on sirolimus therapy showed a decrease in Th1 skewing in both total CD4 and cTfh compartments, with corresponding decrease in Th1/Th2 ratios, suggesting a likely decrease in Th1/IFN-γ mediated inflammation (supplemental Figure 2D-E, 2G-H and 2J-K). However, we did not find any significant change in Th17 or cTfh17 populations (supplemental figure 2F and 2I). Despite decrease in T -cell activation and Th1 polarization, we did not find any significant improvement in frequencies of CD4+ naïve T -cells (Figure 2C). Although not included in our assessment, previous observations have also shown an improvement in regulatory T (Treg)-cell numbers in m-IC patients on sirolimus therapy.15
An assessment of absolute B-cell numbers showed no difference on sirolimus therapy (supplemental figure 3D). Although CSMB cells increased in some patients on sirolimus treatment, it was not statistically significant and did not result in appreciable change in immunoglobulin profile (Figure 2F, supplemental Figure 3A-C). Another observation worth noting is that despite attenuation of T -cell activation and decrease in lymphoproliferation on sirolimus therapy, absolute counts of CD4+ T, CD8+ T and NK cells remain stable in peripheral blood of m-IC patients, alluding to its potential role in immune preservation (supplemental Figure 3E-H). A similar observation of stable T -cell numbers on sirolimus therapy was previously reported by Bride et al in 2016.11 By maintaining absolute T- and B-cell numbers and decreasing chronic activation, exhaustion and senescence, sirolimus has a unique role in improving functional immunity in patients with m-IC and may slow evolution to more significant immune deficiency states such as common variable immunodeficiency or combined immunodeficiency (CID) with worsened T- and B-cell function.
Recently we showed similar immune anomalies that exist in patients with m-IC, irrespective of presence or absence of genetic drivers.7 Therefore, we wanted to evaluate whether response to sirolimus is also similar in patients with known genetic defects versus patients with unknown or no genetic variants. Toward this goal, we segregated patients with m-IC, based on the absence or presence of pathogenic/likely pathogenic genetic variants and evaluated immune subsets in these subgroups after sirolimus therapy. We observed a similar trend in both the subgroups showing decrease in percentage of cTfh, cTfh activation, senescence and exhaustion as well as T-cell activation. However, owing to the limited number of samples in gene (+) subgroup, the samples did not meet the significance for some phenotypes (supplemental Figure 4).
Based on the unique profile of patients with m-IC, we had earlier proposed immune parameters based on the 4-point scoring system.7 We found that in our cohort of patients with m-IC, the median score decreased from 3 to 1 for those on sirolimus therapy, suggesting a global improvement in immune dysregulation (Figure 2G and supplemental figure 5). Further characterization of inflammatory milieu in these patients was performed by chemokine and cytokine profiling. Although the levels of IFN-γ were not different, more reliable markers of IFN-γ induced chemokines, that is, CXCL9 and CXCL1019,20 were significantly decreased on sirolimus therapy, suggesting decreased IFN-γ mediated inflammation and corroborating our previous observations of a decreased Th1 polarization and T-cell activation. A decreasing trend in IL-18 levels was also observed in patients on sirolimus therapy, suggesting an improvement in innate inflammation. Improvements in IL-21 levels despite a decrease in cTfh frequency could suggest a more functional cTfh compartment. However, we did not observe any change in CD40L levels which is critical for cTfh and B-cell interaction.8 A decrease in IL-10 levels was also seen in patients with m-IC. Elevated IL-10 levels in ALPS have been shown previously and correlate with disease severity.21 Cytokines important for naïve and Treg development22 such as IL-2 and IL-7 remained unchanged. IL12p40, a cytokine required for Th1 differentiation23,24 showed an overall (non-significant) decrease in median values on sirolimus therapy (Figure 2H).
Our cohort illustrates that despite different genetic drivers in m-IC, sirolimus treatment results in the improvement of immune dysregulation signatures (Supplemental Figure 6). Future studies of a larger cohort of patients will be important to validate our findings. Studies with longer follow-up are needed to determine if improvement in CD4+ naïve T-cells and CSMB populations occurs as well. Moreover, we did not test whether the improvements in immunologic profiles observed with sirolimus therapy are also observed with other immunosuppressive agents such as mycophenolate mofetil5 used to treat immune dysregulation in patients with m-IC. Overall, our study lays the foundation for future studies to explore the role of sirolimus in immune preservation and improving functional immunity in patients with m-IC.
Conclusions
In summary, our data suggest that mTOR inhibition not only improves immune cytopenia and lymphoproliferation but also significantly improves the dysregulated immune phenotype seen in patients with m-IC such as Tfh cell expansion and chronic T-cell activation, without compromising overall lymphocyte counts and immunoglobulin levels. However, longer term studies are needed to determine if the beneficial effects of sirolimus are durable and extend to other dysregulated immune subsets such as naïve CD4+ T cells and CSMB cells.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant 1K08HL141635-01A1, Atlanta Pediatric Scholars Program K12 Scholar supported by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K12HD072245 and Henagan Foundation (to S.C.). S. Prahalad is supported in part by the Marcus Foundation Inc, Atlanta.
Authorship
Contribution: D.K. collected the data, performed data analysis, created all figures and wrote the manuscript; T.H.N. and C.P. collected and analyzed the data; S. Park, C.M.B., M.B., and L.L. were involved in clinical care and wrote the manuscript; T.L., K.C., and M.I. enrolled study subjects; E.K.W. and S. Prahalad wrote the manuscript and provided critical inputs; and S.C. conceptualized the project, designed the study, supervised and oversaw the project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: S.C. serves on the advisory committee of SOBI. S. Prahalad serves on a Macrophage Activation Syndrome Adjudication Committee for Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Shanmuganathan Chandrakasan, Immune Dysregulation and Immunohematology Program, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University School of Medicine, 2015 Uppergate Dr, ECC Room 434A, Atlanta, GA 30322; e-mail: shanmuganathan.chandrakasan@emory.edu.
References
Author notes
The online version of this article contains a data supplement
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal