In this issue of Blood, Kumar et al1 investigate the impact of sirolimus on immune health in a cohort of children treated with sirolimus for multilineage immune cytopenias (m-ICs). Sirolimus, also known as rapamycin, is an antifungal compound first identified in a soil sample obtained from Easter Island (Rapa Nui) as part of a 1960s drug discovery program.2 Shortly after its isolation, sirolimus was found to be a potent immunosuppressive agent, forming a complex with FK-binding protein-12 that blocks activation of the protein kinase, mammalian target of rapamycin (mTOR), arresting the cell cycle and inducing autophagy.2 In 1999, sirolimus was approved by the US Food and Drug Administration to prevent solid organ transplant rejection, and over the past 20 years, it has been found to be a well-tolerated drug with an excellent safety profile.2
In the early 2000s, sirolimus was studied as a treatment for autoimmune lymphoproliferative syndrome (ALPS), a disorder driven by defects in Fas-mediated apoptosis that causes inappropriate proliferation of CD4− and CD8− T lymphocytes, termed “double-negative” T cells (DNTs), resulting in symptoms including lymphadenopathy, splenomegaly, and m-ICs.3,4 Multiple studies have since confirmed that sirolimus is a highly effective therapy for ALPS, inducing apoptosis of DNTs while promoting the developing of regulatory T cells, leading to the resolution of m-ICs without causing significant immune compromise when used as monotherapy.5,6 Interestingly, sirolimus has subsequently been shown to be effective in the treatment of m-ICs in patients without ALPS.7 In many of these patients, sirolimus does not significantly change absolute lymphocyte counts or immunoglobulin levels, and its mechanism of activity remains unclear. However, prior studies of the impact of sirolimus on immune health are not robust.8
There is increasing recognition that m-ICs in children are commonly a manifestation of disorders of immune dysregulation, which may or may not be monogenic, and are often amenable to therapy with immune modulatory medications. To determine how sirolimus affects immune dysfunction in pediatric m-IC, Kumar et al performed robust longitudinal quantitative and qualitative immune profiling of 12 patients with m-ICs before and after initiation of sirolimus, with a median follow-up time of 17 months, compared with 21 healthy pediatric controls. Although 4 patients were found to have pathogenic gene variants in LRBA, PI3KCD, and FAS, an underlying genetic etiology was not identified in 8 patients. All patients responded to sirolimus, with 2 experiencing partial remission of their cytopenias and 10 achieving complete remission. The majority also experienced improvement in lymphoproliferation (lymphadenopathy and/or splenomegaly).
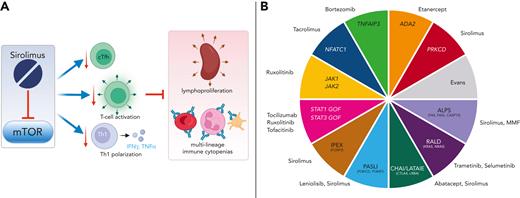
Sirolimus treatment was consistently associated with normalization of T-cell distribution and characteristics, without changes in absolute numbers of CD4+ and CD8+ T cells or immunoglobulin levels (see figure panel A). All patients had a significant reduction in circulating T follicular helper cells (cTfh), a cell type often elevated in the setting of autoimmunity and immune dysregulation.9 Sirolimus also led to a decrease in markers of cell activation, senescence, and exhaustion on CD4+ and CD8+ effector memory T cells, along with a reduction of Th1 polarization. Of note, the authors did not report whether they observed changes in DNT counts following initiation of mTOR inhibition. Sirolimus use was associated with a decrease in CXCL9 and CXCL10 levels, consistent with flow cytometry results demonstrating decreased Th1 polarization. Strikingly, similar trends were observed in m-IC patients regardless of etiology or whether they were found to have an underlying pathogenic genetic variant.
(A) Sirolimus, an mTOR inhibitor, is effective in children with m-IC syndromes, improving and/or eliminating abnormal lymphocyte populations that drive immune dysregulation, including cTfh and decreasing markers of abnormal T-cell activation, senescence, and exhaustion on CD4+ and CD8+ effector memory T cells, along with a reduction of Th1 polarization. Ultimately, sirolimus improved disease manifestations without leading to immune deficiency. Panel B depicts targeted therapies currently available to treat children and adults with different monogenic causes of m-IC. Similar studies evaluating the mechanism of action and short- and long-term impacts on immune health are needed. ADA2, adenosine deaminase deficiency 2; ALPS, autoimmune lymphoproliferative syndrome; CHAI, CTLA4 haploinsufficiency with autoimmune infiltration; Evans, syndrome characterized by m-ICs without known cause; GOF, gain of function; IFN, interferon; IPEX, immune dysregulation polyendocrinopathy, enteropathy, X-linked; LATAIE, LRBA deficiency with autoantibodies, regulatory T (Treg) cell defects, autoimmune infiltration, and enteropathy; MMF, mycophenolate mofetil; PASLI, p110d-activation with senescent T cells, lymphadenopathy, and immunodeficiency; PRKCD, protein kinase C delta deficiency; RALD, ras-associated leukoproliferative disease; TNF, tumor necrosis factor; TNFAIP3, tumor necrosis factor alpha infused protein 3.
(A) Sirolimus, an mTOR inhibitor, is effective in children with m-IC syndromes, improving and/or eliminating abnormal lymphocyte populations that drive immune dysregulation, including cTfh and decreasing markers of abnormal T-cell activation, senescence, and exhaustion on CD4+ and CD8+ effector memory T cells, along with a reduction of Th1 polarization. Ultimately, sirolimus improved disease manifestations without leading to immune deficiency. Panel B depicts targeted therapies currently available to treat children and adults with different monogenic causes of m-IC. Similar studies evaluating the mechanism of action and short- and long-term impacts on immune health are needed. ADA2, adenosine deaminase deficiency 2; ALPS, autoimmune lymphoproliferative syndrome; CHAI, CTLA4 haploinsufficiency with autoimmune infiltration; Evans, syndrome characterized by m-ICs without known cause; GOF, gain of function; IFN, interferon; IPEX, immune dysregulation polyendocrinopathy, enteropathy, X-linked; LATAIE, LRBA deficiency with autoantibodies, regulatory T (Treg) cell defects, autoimmune infiltration, and enteropathy; MMF, mycophenolate mofetil; PASLI, p110d-activation with senescent T cells, lymphadenopathy, and immunodeficiency; PRKCD, protein kinase C delta deficiency; RALD, ras-associated leukoproliferative disease; TNF, tumor necrosis factor; TNFAIP3, tumor necrosis factor alpha infused protein 3.
Unlike single-lineage autoimmune cytopenias, m-ICs in children are often chronic, regardless of whether they are found to be secondary to a monogenic disorder or are classified as idiopathic (eg, Evans syndrome). Although some of these children, especially those with certain monogenic disorders, may benefit from hematopoietic stem cell transplant (HSCT), HSCT is often avoided because graft rejection is high in patients with aberrant immune activation, and HSCT is fraught with significant morbidity and mortality.10 Accordingly, during formative stages of growth and development, many of these children require long-term immunomodulation/suppression, and it is critical that we understand the short- and long-term complications of these therapies, particularly their effect on immune health. The authors’ data suggesting that mTOR inhibition ameliorates underlying immune dysregulation in patients with m-ICs irrespective of underlying genetic etiology without suppressing the immune response are highly encouraging and support long-term use of monotherapeutic sirolimus in pediatric m-ICs.
Although the total number of patients studied was small, the consistency of results is compelling, especially in light of the genetic heterogeneity in the cohort. Nevertheless, larger studies focused on individual cohorts of patients with longer follow-up are needed to confirm these findings. For example, sirolimus and abatacept have been anecdotally used successfully for patients with LRBA- and CTLA4-mutant disease, and multicenter studies are needed to assess the short- and long-term impacts of immunomodulatory medications including sirolimus in each of these diseases.7 We have entered an exciting time in the management of treatment of children with disorders of immune dysregulation, as multiple targeted precision therapies are now available (see figure panel B). For example, patients with inherited mutations in Jak/Stat pathway genes may benefit from ruxolitinib, patients with gain of function mutations in STAT3 may benefit from tocilizumab, and patients with Ras-associated leukoproliferative disorder may benefit from MAPK inhibitors.7 It is critical that we continue to study the impact of these medicines on immune health in these populations to determine whether novel targeted approaches are acting through expected mechanisms and to confirm whether they are safe for long-term use.
Conflict-of-interest disclosure: D.T.T. serves on advisory boards for Sobi, Janssen, and BEAM Therapeutics and receives research funding form NeoImmune Tech, BEAM Therapeutics, Servier, and Jazz. K.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal