Key Points
In MM patients who became sIFE-negative, 100% (serum M-protein only at diagnosis)/98.6% (serum/urine M-protein) were also uIFE-negative.
Patients meeting all criteria for CR but without uIFE assessment have outcomes comparable to those in CR and superior to those with VGPR.
Abstract
Response criteria for multiple myeloma (MM) require monoclonal protein (M-protein)–negative status on both serum immunofixation electrophoresis (sIFE) and urine (uIFE) immunofixation electrophoresis for classification of complete response (CR). However, uIFE is not always performed for sIFE-negative patients. We analyzed M-protein evaluations from 384 MM patients (excluding those with light-chain-only disease) treated in the GEM2012MENOS65 (NCT01916252) trial to determine the uIFE-positive rate in patients who became sIFE-negative posttreatment and evaluate rates of minimal residual disease (MRD)–negative status and progression-free survival (PFS) among patients achieving CR, CR but without uIFE available (uncertain CR; uCR), or very good partial response (VGPR). Among 107 patients with M-protein exclusively in serum at diagnosis who became sIFE-negative posttreatment and who had uIFE available, the uIFE-positive rate was 0%. Among 161 patients with M-protein in both serum and urine at diagnosis who became sIFE-negative posttreatment, 3 (1.8%) were uIFE positive. Among patients achieving CR vs uCR, there were no significant differences in postconsolidation MRD-negative (<10−6; 76% vs 75%; P = .9) and 2-year PFS (85% vs 88%; P = .4) rates; rates were significantly lower among patients achieving VGPR. Our results suggest that uIFE is not necessary for defining CR in MM patients other than those with light-chain-only disease.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2731.
Disclosures
Associate Editor Hervé Avet-Loiseau served as speaker or a member of a speakers bureau for AbbVie Inc., Amgen Inc., Celgene Corporation, Janssen-Cilag, Sanofi, and Takeda and received grants for clinical research from Amgen Inc., Celgene Corporation, Sanofi, and Takeda. Author Juan-José Lahuerta received honoraria for lectures and advisory boards from Amgen Inc.; Celgene Corporation, Janssen-Cilag, and Takeda. Author Bruno Paiva served as advisor or consultant for Celgene Corporation and Janssen-Cilag; received grants for clinical research from Celgene Corporation; received honoraria from Celgene Corporation and Janssen-Cilag; and received travel, accommodation, and expenses from Celgene Corporation and Janssen-Cilag. Author Joaquín Martínez-López served as advisor or consultant for Bristol-Myers Squibb Company, Celgene Corporation, Janssen-Cilag, and Novartis Pharmaceuticals Corporation; served as a speaker or a member of a speakers bureau for Bristol-Myers Squibb Company, Celgene Corporation, Janssen-Cilag, and Novartis Pharmaceuticals Corporation; and received grants for clinical research from Bristol-Myers Squibb Company, Celgene Corporation, Janssen-Cilag, and Novartis Pharmaceuticals Corporation. Author Noemi Puig served as advisor or consultant for Janssen-Cilag; received honoraria from Amgen Inc., Janssen-Cilag, and Takeda; and received travel, accommodations, and expenses from Janssen-Cilag. Author Albert Oriol served as advisor or consultant for Amgen Inc. and Janssen-Cilag. Author Luis Palomera served as advisor or consultant for Celgene Corporation and Janssen-Cilag and received honoraria Celgene Corporation and Janssen-Cilag. Author María-Victoria Mateos served as a speaker or a member of a speakers bureau for Celgene Corporation and Janssen-Cilag and received honoraria from Celgene Corporation and Janssen-Cilag. Author Laura Rosiñol received honoraria from Celgene Corporation and Janssen-Cilag. Author Jesús F. San Miguel Served as advisor or consultant for Amgen Inc., Bristol-Myers Squibb Company, Celgene Corporation, Janssen-Cilag, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Roche, Sanofi, Takeda, and Squibb. Author Joan Blade received honoraria for lectures and advisory boards from Amgen Inc., Celgene Corporation, Janssen-Cilag, and Takeda. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC and the remaining authors declare no relevant financial relationships.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the value of urine immunofixation electrophoresis (uIFE)–negative status to define complete response (CR) among patients enrolled in the PETHEMA GEM2012MENOS65 randomized phase-3 clinical trial
Compare progression-free survival from the postinduction, post–autologous stem cell transplantation, and postconsolidation landmarks according to response achieved at each landmark among patients enrolled in the PETHEMA GEM2012MENOS65 randomized phase-3 clinical trial
Identify clinical implications of the value of uIFE-negative status to define CR among patients enrolled in the PETHEMA GEM2012MENOS65 randomized phase 3 clinical trial
Release date: June 20, 2019; Expiration date: June 20, 2020
Introduction
In multiple myeloma (MM), reproducible disease response and progression criteria are critical to ensure consistent reporting from clinical trials. Since 19981 and per the latest International Myeloma Working Group (IMWG) response criteria,2,3 a complete response (CR) requires the confirmed absence of monoclonal protein (M-protein) on serum immunofixation electrophoresis (sIFE) and urine immunofixation electrophoresis (uIFE), resolution of any soft tissue plasmacytomas, and <5% plasma cells (PCs) in bone marrow (BM) aspirates. However, especially for sIFE-negative patients, the uIFE evaluation is not always performed.
With the rationale that absence of uIFE confirmation of CR could bias comparisons between trials, the Independent Response Adjudication Committee (IRAC) of the Study to Determine Efficacy and Safety of Lenalidomide Plus Low-dose Dexamethasone Versus Melphalan, Prednisone, Thalidomide in Patients With Previously Untreated Multiple Myeloma (FIRST) trial recommended that patients meeting all criteria for CR except for uIFE availability should be classified as having a very good partial response (VGPR).4 However, whether the availability of uIFE results affects clinical outcomes in patients meeting all other criteria for CR has not been investigated to date. If patients achieving CR have similar prognosis regardless of documented uIFE-negative status, it would be clinically erroneous to classify those lacking uIFE information as achieving only VGPR, because this would underestimate the true CR rate in the trial, thereby increasing bias and magnifying the problem that the original recommendation was intended to correct. Here, we analyze the value of uIFE-negative status in the definition of CR among patients enrolled in the Programa para el Tratamiento de Hemopatías Malignas (PETHEMA) GEM2012MENOS65 (NCT01916252; Bortezomib [Velcade®], Lenalidomide [Revlimid®] and IV Busulfan [Busilvex®] in Patients Under 65 Years Old) randomized phase 3 clinical trial.
Study design
This ad hoc analysis incorporated data from patients age 65 years or younger with newly diagnosed symptomatic MM enrolled in GEM2012MENOS65). Of 458 patients, 8 discontinued early and were not evaluable. All patients provided written informed consent according to local ethical committee requirements and the Declaration of Helsinki. Patients received six 28-day cycles of bortezomib-lenalidomide-dexamethasone as induction, were randomly assigned (1:1) to receive high-dose melphalan or busulfan-plus-melphalan conditioning followed by autologous stem cell transplantation (ASCT), and then received 2 cycles of bortezomib-lenalidomide-dexamethasone as consolidation. BM aspirates were performed to quantitate PCs and monitor minimal residual disease (MRD) at the time of achieving sIFE negativity, as well as postinduction (after cycle 6), post-ASCT, and postconsolidation, regardless of M-protein status. After completing treatment on GEM2012MENOS65, evaluable patients were enrolled in a subsequent trial to receive lenalidomide-based maintenance therapies.
Responses were defined according to IMWG criteria.3 For this analysis, we pooled CR and stringent CR data because of the similar outcomes of these two populations.5-7 We investigated separately the patients described in the FIRST trial IRAC recommendation4 (ie, sIFE-negative patients with <5% BM PCs but uIFE unavailable; for this analysis, we defined them as having uncertain CR [uCR]).
Progression-free survival (PFS) was measured from the end of induction, the time of ASCT, and the end of consolidation to the date of progression or death. PFS distribution was estimated using Kaplan-Meier methodology, with patients censored at their last visit if they were alive and progression free. Between-group differences in MRD-negative rate and PFS were evaluated by using the χ2 test and the log-rank test, respectively.
MRD was assessed using next-generation flow cytometry, based on a standardized lyse-wash-and-stain sample preparation protocol and an optimized 2-tube 8-color antibody panel for accurate identification of phenotypically aberrant PCs (tube 1: CD138-BV421, CD27-BV510, CD38-FITC, CD56-PE, CD45-PerCPCy5.5, CD19-PECy7, CD117-APC, CD81-APCH7; tube 2: CD138-BV421, CD27-BV510, CD38-FITC, CD56-PE, CD45-PerCPCy5.5, CD19-PECy7, cyKAPPA-APC, cyLAMBDA-APCH7).8,9 This allowed detectionbof MRD with specific confirmation of light-chain monoclonality on phenotypically aberrant PCs identified either by antigen underexpression (CD19, CD27, CD38, CD45, CD81) or overexpression (CD56, CD117, CD138). The median limit of MRD detection was 3 × 10−6.
Results and discussion
Overall, among 450 evaluable patients in GEM2012MENOS65, 3779 M-protein evaluations were performed. Sixty-six patients had light-chain-only disease at diagnosis and were excluded. Of the initial 384 patients, 173 had M-protein detected only in serum at diagnosis, and 211 had detectable M-protein in both serum and urine.
Of 173 patients with M-protein exclusively in serum at diagnosis, 107 reached sIFE-negative response status at some point during treatment and had <5% BM PCs and uIFE available. For the 107 patients who had achieved this response status, 235 serum and urine M-protein evaluations were performed simultaneously; the uIFE-positive rate was 0%. Of 211 patients with M-protein detectable in both serum and urine at diagnosis, 161 reached sIFE-negative response status at some point during treatment and had <5% BM PCs and uIFE available. For the 161 patients who had achieved this response status, 337 serum and urine M-protein evaluations were performed simultaneously. uIFE was positive in only 3 patients (1.8%); moreover, in these 3 patients, the uIFE fluctuated from positive (n = 7) to negative (n = 20) in one-quarter of the sequential evaluations performed.
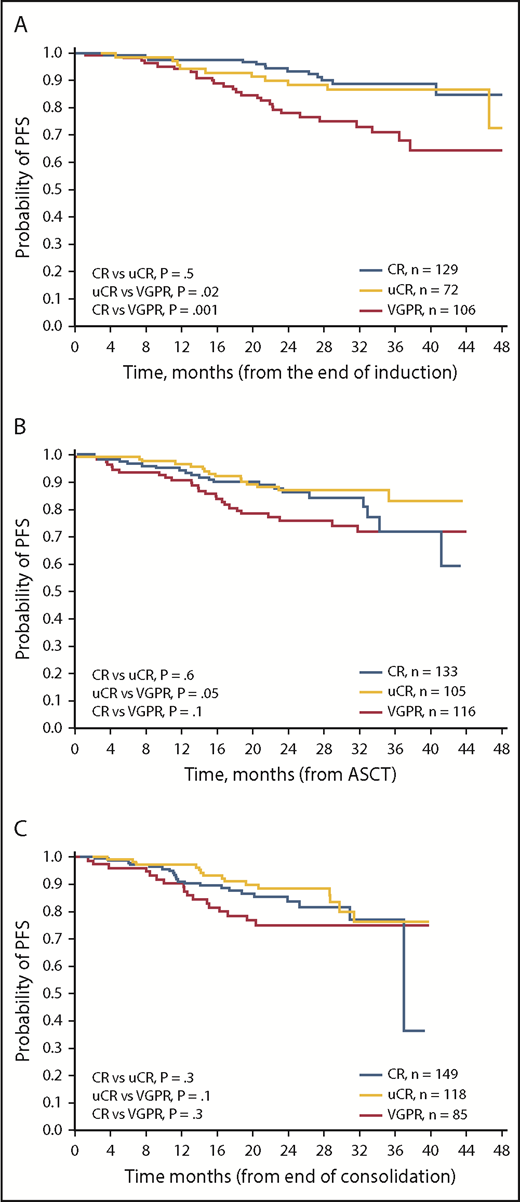
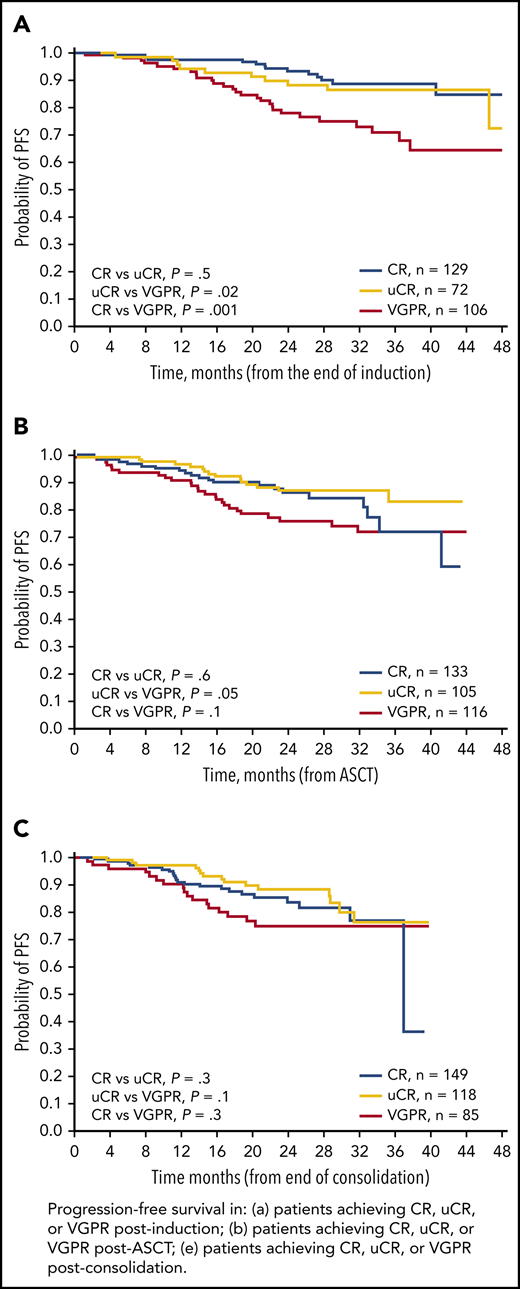
We also analyzed PFS from the postinduction, post-ASCT, and postconsolidation landmarks according to response achieved at each landmark (Figure 1). The 2-year PFS rates were not significantly different between patients achieving CR or uCR at any of the landmarks (Table 1). By contrast, the postinduction 2-year PFS rate in patients achieving VGPR (78%) was significantly lower compared with patients achieving uCR (88%; P = .001) or CR (93%; P = .02). Numerical differences in 2-year PFS rates between groups seemed similar at the post-ASCT and postconsolidation landmarks; however, these differences were not statistically significant, likely because of insufficient follow-up times.10
Kaplan-Meier analyses of PFS. PFS in patients achieving CR, uCR, or VGPR (A) postinduction, (B) post-ASCT, and (C) postconsolidation.
Kaplan-Meier analyses of PFS. PFS in patients achieving CR, uCR, or VGPR (A) postinduction, (B) post-ASCT, and (C) postconsolidation.
MRD status is a robust surrogate of long-term survival and the quality of conventional responses.11,12 We compared MRD-negative rates in patients achieving CR, uCR, or VGPR postinduction, post-ASCT, and postconsolidation (Table 1). Interestingly, there were no significant differences in postconsolidation MRD-negative rates between patients achieving CR and uCR (76% vs 75%; P = .9), whereas the difference in MRD-negative rates between patients achieving uCR or VGPR (28%) was highly significant (P < .0001). Similar findings were shown for postinduction and post-ASCT evaluations (Table 1).
Although the definition of CR in the response criteria for MM and its prognostic importance have been extensively validated,13-16 the requirement for negative uIFE in sIFE-negative patients has never been analyzed. Our results indicate that in MM patients with M-protein exclusively in serum at diagnosis, uIFE is not necessary for establishing CR. Moreover, in patients with M-protein in serum and urine at diagnosis, sIFE-negative response is accompanied by a uIFE-negative finding in 98.2% of patients. In addition, patients who meet the criteria for CR but without uIFE available display MRD-negative and 2-year PFS rates similar to those in true CR, whereas MRD-negative and 2-year PFS rates are inferior in patients achieving VGPR. Thus, these results suggest that uIFE is not necessary for defining CR in sIFE-negative patients, which eliminates the need for 24-hour urine collection in these patients. Consequently, patients fulfilling the criteria for CR but with uIFE unavailable should be classified as achieving CR and not VGPR. This statement, does not apply for patients with light-chain-only disease, although the French group has reported that serum free light chains, not urine specimens, should be used to evaluate response in pure light-chain MM.17,18
Finally, our conclusions should be limited to the scope of the CR assessment, because the investigations of monoclonal proteins in urine are still needed in clinical surveillance for MM19 and others gammopathies20,21 at diagnosis, in controlling tumor reduction, and in detecting progression. Although our results need to be validated in independent studies,3 they support revisiting the IMWG criteria for CR.
For original data, please contact jjlahuerta@telefonica.net.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the patients and their families, all participating members of the Grupo Español de Mieloma (GEM)/PETHEMA study group, Arturo Touchard, Esther Blade, and Roberto Maldonado for data management and processing support, Alfonso Santiago and Carmen López Carrero for administrative support, and Stephen Hill for editorial assistance with the English, which was funded by the Foundation PETHEMA and which complied with Good Publication Practice 3 ethical guidelines.
This work was supported by grants from the Fondo de Investigación Sanitaria (PI1201761 and PI1502062), Red Temática de Investigación Cooperative en Cancer, and Centro de Investigación Biomédica en Cancer of the Instituto de Salud Carlos III (Ministry of Science, Innovation and Universities).
Authorship
Contribution: J.-J.L., A.J.-U., J.M.-L., and J. Blade conceived the analysis; J.-J.L., A.J.-U., J.M.-L., B.P., M.-V.M., J. Blade., J.F.S.M., and L.R. designed the analysis protocol; B.P., M.-T.C., and N.P. analyzed the flow cytometry data; L.L.-A. performed data curation; J.-J.L., A.J.-U., J.M.-L., B.P., J.F.S.M., and J. Blade analyzed and interpreted data; A.J.-U., J.G.-M., and L.L.-A. performed statistical analysis; and A.J.-U., J.-J.L, B.P., M.-V.M., J.F.S.M. and J. Blade wrote the manuscript; and all authors provided study material or patients and reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.-J.L. received honoraria for lectures from and participated in advisory boards for Janssen-Cilag, Celgene, Takeda, and Amgen. B.P. received honoraria from, served in a consulting or advisory role for, and received travel, accommodation, and expenses from Janssen and Celgene, and received research funding for his institution from Celgene. J.M.-L. served in a consulting or advisory role for, served on a Speakers’ Bureau for, and received research funding for his institution from Novartis, Janssen-Cilag, Celgene, and Bristol-Myers Squibb. N.P. received honoraria from Janssen-Cilag, Takeda, and Amgen, and served in a consulting or advisory role and received travel, accommodations, and expenses from Janssen-Cilag. A.O. served in a consulting or advisory role for Amgen and Janssen-Cilag. L.P. received honoraria from and served in a consulting or advisory role for Janssen-Cilag and Celgene. M.-V.M. received honoraria from and served on a Speakers’ Bureau for Janssen-Cilag and Celgene. L.R. received honoraria from Janssen-Cilag and Celgene. J.F.S.M. served as a consultant for Bristol-Myers Squibb, Janssen-Cilag, Celgene, Merck, Takeda, Novartis, Amgen, Sanofi, and Roche. J. Blade received honoraria for lectures and advisory boards from Janssen-Cilag, Celgene, Amgen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Juan-José Lahuerta, Hospital Universitario 12 de Octubre, Av de Córdoba s/n, 28045 Madrid, Spain; e-mail: jjlahuerta@telefonica.net.
REFERENCES
Author notes
J.-J.L. and A.J.-U. contributed equally to this study.