Abstract
Adolescent and young adult (AYA) patients with acute lymphoblastic leukemia (ALL) are recognized as a unique population with specific characteristics and needs. In adolescents age 15 to 20 years, the use of fully pediatric protocols is supported by many comparative studies of pediatric and adult cooperative groups. In young adults, growing evidence suggests that pediatric-inspired or even fully pediatric approaches may also dramatically improve outcomes, leading to long-term survival rates of almost 70%, despite diminishing indications of hematopoietic stem-cell transplantation. In the last decade, better knowledge of the ALL oncogenic landscape according to age distribution and minimal residual disease assessments has improved risk stratification. New targets have emerged, mostly in the heterogeneous B-other group, particularly in the Philadelphia-like ALL subgroup, which requires both in-depth molecular investigations and specific evaluations of targeted treatments. The remaining gap in the excellent results reported in children has many other contributing factors that should not be underestimated, including late or difficult access to care and/or trials, increased acute toxicities, and poor adherence to treatment. Specific programs should be designed to take into account those factors and finally ameliorate survival and quality of life for AYAs with ALL.
Introduction
The word adolescent derives from the Latin adolescere, which means to grow. Not surprisingly, there is thus no precise definition of adolescence or young adulthood. The word teenager encompasses the period from age 13 to 19 years. According to the World Health Organization definition, adolescents are individuals age 10 to 19 years. In 2006, the National Cancer Institute Adolescent and Young Adult (AYA) Oncology Progress Review Group considered the issue and focused on individuals diagnosed with cancer from age 15 to 39 years.1 However, currently, AYAs in the European Union are considered those up to age 29 years, whereas in the United States, the age limit is ∼40 years. Those differences are not trivial, because they affect access to different types of care structures and treatment trials or protocols and finally the data that are provided.
In many cancer subtypes, the outcomes of AYA patients age 15 to 39 years remain markedly worse than those of their younger counterparts. A recent study by EUROCARE-5 based on cancer registries of 27 European countries reported on the outcomes of 4617 AYAs with acute lymphoblastic leukemia (ALL) age 15 to 39 years compared with 15 089 children age 0 to 14 years diagnosed between the years 2000 and 2007. A dramatic decrease with age in 5-year relative survival (± standard deviation [SD]) was seen: 85.8% (± 0.4%) for patients age 0 to 14 years, 62.2% (± 1.6%) in patients age 15 to 19 years, and 52.8% (± 0.01%) in those age 20 to 39 years.2
Comparisons of the outcomes of adolescents treated in pediatric and adult trials in different countries have drawn the same conclusion: adolescents with Philadelphia (Ph)–negative ALL have better outcomes when treated according to pediatric strategies. This has led to the use noncomparative pediatric-inspired or fully pediatric strategies that have improved outcomes in young adults with Ph− ALL and challenged allogeneic hematopoietic stem-cell transplantation (HSCT) indications in this population.
This review will examine the main questions related to AYA ALL.
Is there a specific AYA ALL biology?
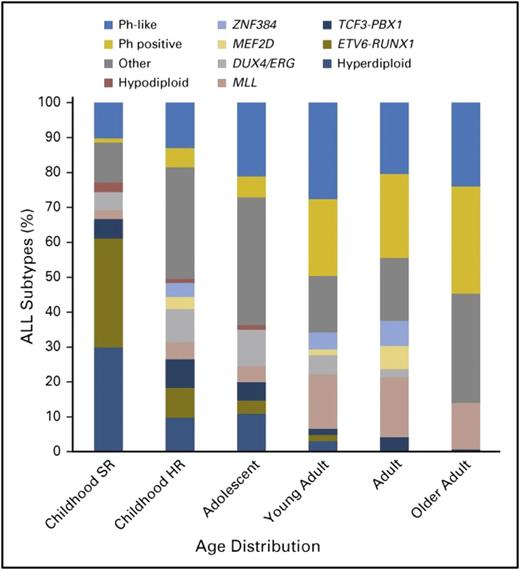
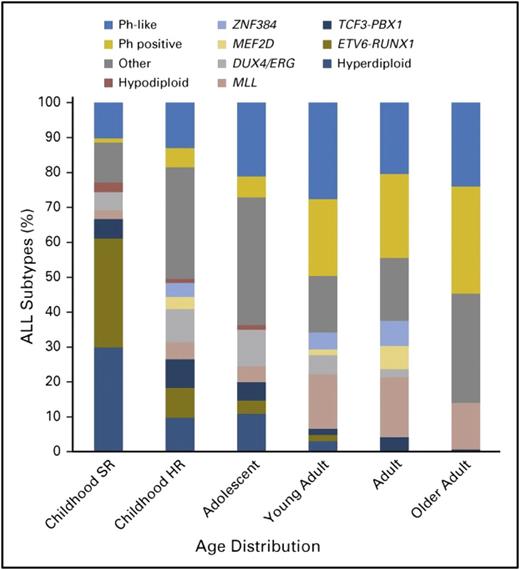
Schematically, adolescence begins at ∼10 years of age in ALL. After this fuzzy limit, an increase of high-risk factors and a decrease of good prognostic factors are seen. Incidence of hyperdiploidy in AYAs with B-lineage ALL is markedly reduced from the 30% to 40% observed in children with B-cell precursor ALL (BCP-ALL) age between 1 and 10 years to <20% in those age 10 to 15 years, <10% in those age 15 to 24 years, and <5% in those age 25 to 44 years (Figure 1).3-5 Only rare cases of ETV6-RUNX1 leukemia are observed in those age >10 years. This cryptic t(12;21) rearrangement, observed in ∼25% of cases of childhood ALL, but in <2% of cases of adult ALL, was present in 7% of adolescents age 15 to 20 years in the French Acute Lymphoblastic Leukemia Study Group 93 trial.6 The prevalence of t(9;22) Ph chromosome leading to BCR-ABL1 fusion progressively increases with age, from ∼3% in the pediatric population to 25% to 30% of BCP-ALLs in adults (Figure 1).7
Biology of BCP-ALL according to age. HR, high risk; SR, standard risk. Reprinted from Iacobucci and Mullighan7 with permission.
Biology of BCP-ALL according to age. HR, high risk; SR, standard risk. Reprinted from Iacobucci and Mullighan7 with permission.
Over the last 15 years, light has been shed on the so-called black hole of ALL biology in AYAs, and a biological continuum between childhood and adult ALL has been revealed:
A rare event in childhood ALL (∼2%), amplification of the long arm of chromosome 21 (iAMP21), is more frequent in older children and adolescents and is associated with a higher risk of relapse only partially diminished by intensified treatment.8,9 In the Nordic Society of Paediatric Haematology and Oncology (NOPHO) 2008 trial, the proportion of iAMP21 cases was 1.5% in those between age 1 and 9 years, 5.8% in those age 10 to 17 years, and 12% in those age 17 to 45 years, with outcomes still overall inferior to those of non-iAMP21 cases (5-year event-free survival [EFS; ±SD] 61% ± 12% vs 85% ± 1%).4
IGH@ rearrangements are more frequent in the AYA population and are also associated with unfavorable outcomes.10 Many partner genes may be involved, including CRLF2 in ∼25% of cases as well as CEBP family genes. The median age of patients with IGH@ translocations was 16 years, with a peak incidence of 11% among patients age 20 to 24 years. Patients with BCP-ALL and IGH@ translocation have an inferior overall survival (OS) compared with other patients.5,10
The subgroup of DUX4/ERG is characterized by translocations of the double homeobox 4 gene DUX4 into the IGH enhancer locus.11-13 DUX4 is not expressed in normal B cells, and translocation to IGH@ results in expression of a truncated DUX4 isoform in the B-cell lineage. Intragenic deletions of the ERG gene have been reported in ∼5% of childhood ALLs and are restricted to these DUX4-rearranged cases. DUX4/ERG-deregulated ALL is associated with favorable outcomes, despite the frequent presence of IKZF1 intragenic deletions (40% of patients). DUX4/ERG is specifically reported in up to 15% of AYA BCP-ALLs.11
Ph-like ALL was independently identified by 2 groups as a novel subtype of high-risk BCP-ALL, showing a gene expression profile similar to Ph+ ALL with a high frequency of alterations of the IKZF1 gene.14,15 These cases are characterized by high levels of postinduction minimal residual disease (MRD) and overall poor outcomes.16-18 Ph-like prevalence is a subject of controversy, because the set of genes used for gene expression profiling and ethnicities (higher incidence in Hispanics) differ between US and EU cohorts (Table 1). Nevertheless, the prevalence is increased in AYAs compared with children. Several subgroups have been identified, and >70 kinase fusions have been described, as reviewed by Tasian et al19 and Pui et al20 : (1) ABL-class rearrangements involving ABL1, ABL2, CSF1R, and PDGFRB; (2) EPOR and JAK2 rearrangements; (3) CRLF2 rearrangements associated with JAK mutations in ∼50% of the cases; (4) other JAK-STAT activating mutations and deletions, including those involving IL7R, FLT3, and SH2B3; and (5) rearrangement involving rare targets like NTRK3 and others.16 Case reports with EBF1-PDGFRB rearrangements have been published favoring the addition of a tyrosine kinase inhibitor (TKI) to chemotherapy in this context.21,22
The zinc finger protein 384 (ZNF384) can be part of in-frame fusion with several genes, notably EP300 or CREBBP. These fusions represent 7% to 12% of the ALLs in AYAs and older patients. They are found in very early pro-B ALLs, often CD10− with expression of myeloid markers and activation of the JAK-STAT pathway. It is estimated from Japanese, Chinese, and Canadian studies that the prognosis of this ZNF384-fusion ALL is relatively good; nevertheless, prognosis possibly depends on the fusion partner.12,23,24
Myocyte enhancer factor 2D (MEF2D) is a member of a family of transcription factors that can be fused to 1 of several partners, most commonly BCL9, in ∼4% to 7% of patients, mostly AYAs.11,12,24,25 MEF2D ALL was associated with older age, an aberrant immunophenotype (CD10− CD38+), and poor outcomes in the first published reports. The deregulation of MEF2D target genes includes histone deacetylase 9, resulting in a potential sensitivity to histone deacetylase inhibitors, such as panobinostat.26
The proportion of T-cell ALLs (T-ALLs) is higher in the AYA population than in children or older adults. Within this subgroup of ALL, the prevalence of immature T-ALLs increases with age, from 8% in children to 35% in adults.27,28 Among T-ALLs, early T-cell progenitor ALLs have been initially associated with early resistance to treatment and poor outcomes.27,29 More recently, it has been suggested by pediatric and adult groups that therapeutic intensification based on early response assessment could eliminate this unfavorable prognosis, with HSCT used as intensification in the adult series.30-32 Oncogenic processes in T-ALL involve numerous transcription factors/oncogenes (TAL1/2, LMO1/2, TLX1/3, HOXA genes, MYC/MYB) that are mostly overexpressed through translocations involving TCRB or TCRA/TCRD loci (reviewed by Girardi et al33 ). Association with age is poorly known, even if some entities including the CALM-AF10 fusion gene seem to be mostly observed in the AYA population and associated with early resistance and poor outcomes.28 Constitutive activation of the NOTCH1 signaling pathway is the most frequent alteration observed in T-ALL.34,35 Almost exclusive mutations of NOTCH1 and FBXW7, which controls NOTCH1 ubiquitin-mediated degradation, are found in approximately two thirds of patients, with no specific age distribution, and were associated with more favorable outcomes.36-38 This favorable prognosis may be mitigated by the presence of RAS or PTEN alterations that define a subgroup of patients with higher risk of relapse.38-40
Treat adolescents as children
Adolescents, considered high-risk patients per se by pediatricians, have been for a long time considered good-risk patients when seen by adult hematologists.41 In this population, pediatric protocols provide the best outcomes. After the first fully reported French study,6 numerous studies have confirmed this observation (Table 2).42-46 Disparities in chemotherapy or in dose-intensity were pointed out as the major explanation for these observations.47 Higher cumulative doses of asparaginase, vincristine, and steroids and delayed intensifications were used in pediatric protocols, whereas higher doses of cytarabine and increased rates of HSCT were characteristic of adult trials.6,45
Results from more recent pediatric trials have demonstrated a steady improvement in older adolescents age 15 up to 18 to 24 years (Table 3).48-51 Impressive outcome data were reported by the St. Jude Children’s Research Hospital investigators using an MRD-directed treatment in a cohort of 45 patients age 15 to 18 years treated in Total Therapy XV, with a 5-year EFS and OS of 86.4% and 87.9%, respectively.51 Nevertheless, these excellent results were obtained with significant toxicities, including 11.7%, 32.9%, 23.8%, and 27.7% 3-year cumulative risks of grade 4 to 5 severe infection, grade 3 to 4 osteonecrosis, grade 2 to 4 thrombosis, and grade 3 to 4 hyperglycemia, respectively.51
Overall, most of these trials more or less abolished the gap in survival between older adolescents and younger patients, despite increased short-term and long-term toxicities.
Success and limitations of pediatric approach in young adults age 20 to 40 years
In the young adult population, 2 main strategies have been used according to cooperative groups (Table 4):
The first has been to develop so-called pediatric-inspired strategies to include not only AYA patients but also older adults up to age 50 to 60 years. The Dana-Farber Cancer Institute reported on the treatment of 74 patients age 18 to 50 years (median age, 28 years) with Ph− ALL. In this pediatric-inspired protocol using a 30-week consolidation course with pharmacokinetically dose-adjusted L-asparaginase, the 4-year disease-free survival and OS rates were, respectively, 71% and 70%.52 The Princess Margaret Hospital reported on the outcomes of 85 patients with Ph− ALL treated in a modified Dana-Farber Cancer Institute protocol.53 For the whole cohort, 5-year OS was 63% and reached 83% in patients age <35 years.
In Europe, the German Multicenter Trials for Adult ALL and the French Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) adopted a Berlin-Frankfurt-Münster (BFM)–like induction regimen for a large population of adults up to 59 years of age, keeping wide indications for allogeneic HSCT in CR1. In the German Multicenter Trials for Adult ALL 07/03 study, 5-year OS was 65% for patients age 15 to 35 years and reached 73% in adolescents age 15 to 17 years.54 In the French GRAALL 2005 studies, patients age 18 to 24 years and those age 25 to 34 years had comparable outcomes, with 5-year OS of 60% and 58%, respectively.55
The second has been to adopt fully pediatric trials in AYAs up to 40 years of age. The Spanish Programa Español de Tratamientos en Hematología was the first group to report on the outcomes of 81 patients age 15 to 30 years with standard-risk ALL (white blood cells [WBCs] ≤30 × 109/L and absence of t(9; 22), t(1;19), or 11q23 abnormality) included in the pediatric ALL-96 study.56 The 6-year EFS and OS rates were 61% and 69%, respectively, with no differences between adolescents and young adults. The hematological toxicity in consolidation and reinforcement cycles was higher in young adults than in adolescents. The Japanese Acute Leukemia Study Group included 139 patients age 15 to 24 years in the pediatric ALL202-U study.57 The 5-year disease-free survival and OS rates were 67% and 73%, respectively. Severe adverse events were observed at a frequency that was similar to or lower than that in children treated with the same protocol. A US intergroup reported on early results of the C10403 trial, with 2-year EFS and OS rates of 66% and 78%, respectively, for 214 patients age 16 to 39 years.58 In contrast, the MD Anderson Cancer Center compared 106 patients age 13 to 39 years treated according to an augmented BFM regimen with 102 patients receiving hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyperCVAD) and found no difference in outcome.59
The recently published prospective NOPHO 2008 trial included and stratified 1509 patients age 1 to 45 years into 3 risk groups using only high WBC (≥100 × 109/L), central nervous system (CNS) involvement, T or B phenotype, cytogenetics, and MRD evaluation (D15, D29, D79), but not age.4 At 5 years, EFS rates (±SD) were 89% ± 1%, 80% ± 3%, and 74% ± 4% for patients age 1 to 9 years (n = 1022), 10 to 17 years (n = 266), and 18 to 45 years (n = 221), respectively. At 5 years, EFS rates (±SD) were 75% ± 4% and 68% ± 5% when young adults were split into age groups of 18 to 25 years (n = 110) and 26 to 45 years (n = 111) respectively. The death rates during induction and CR1 were 1% and 5% for the 18- to 25-year age group and 2% and 7% for the 26- to 45-year age group, respectively. These results are among the best published in this age range. Interestingly, the anticipated increased toxicity was found to be significant in only 3 of 19 toxicity items (ie, pancreatitis, thrombosis, and osteonecrosis), with no difference between the 10- to 17-year age group and the 18- to 45-year age group.4
In practice, these recent studies point toward the feasibility of using a common protocol for children and AYAs until age 40 to 45 years, relying on a close collaboration between pediatric and adult hematologists (Table 4). Because these studies have emerged only as a rather recent trend, and because few were population based, it is not surprising that population-based epidemiological data like those reported by EUROCARE-5 or Surveillance, Epidemiology, and End Results differ. Indeed, 5-year overall relative survival of ∼60% is reported, differing significantly from the more recent integrated studies; the multiple potential reasons for this include differences in health care systems, access to clinical trials, and adoption of common pediatric and AYAs protocols.
To transplant or not to transplant?
Consensus on indications for HSCT in AYAs has long been poor, based on a multiplicity of factors primarily related to disease characteristics. These indications were mainly defined before the era of protocol intensification for AYAs. In this context, a large study in adult patients concluded there was a benefit of HSCT in standard-risk patients, defined by age <35 years, low WBCs (<30 or 50 × 109/L in BCP-ALL or T-ALL, respectively), and absence of Philadelphia chromosome.60
Over the last 15 years, most pediatric protocols have progressively restricted indications of HSCT to patients exhibiting suboptimal early responses to treatments, essentially now based on MRD at a second time point obtained after consolidation therapy.61 In more recent adult protocols with a more intensive pediatric-inspired backbone, the role of MRD evaluation as a predictor of HSCT benefit has been also strongly suggested.62,63 Indications of HSCT in the NOPHO 2008 trial were also relying on D29 high MRD (≥5%) and/or D79 high MRD (≥0.1%) leading to transplantation in 15.8% of the patients age 18 to 45 years vs 5.5% of those in the 1- to 17-year age range.4
Early toxicity with HSCT in AYAs remains a significant problem, with treatment-related mortality in CR1 ranging from 10% to 30% according to studies.4,60,64-66
As a tentative conclusion, because of its associated short- and long-term toxicities, progress in chemotherapy management, and the advent of new drugs (as described in “New drugs/new therapeutic approaches”), HSCT should be reserved for AYA patients with ALL exhibiting early resistance to chemotherapy assessed by predefined evaluations of MRD.
Conflicting strategies in Ph+ ALL
In both pediatric and adult patients, the prognosis for Ph+ ALL has been markedly improved using TKIs in combination with chemotherapy. However, adult and pediatric cooperative groups have engaged in different strategies for the management of these patients, mostly driven by the older age of Ph+ patients in adult cohorts. Where most young adult (age <55-60 years) protocols have taken advantage of the favorable safety profile and efficacy of TKIs to decrease the dose-intensity of the chemotherapy backbone, pediatricians have combined TKIs with chemotherapy regimens designed for high-risk patients with the aim of reducing the use of HSCT. Moreover, specific TKI safety concerns in children have resulted in a more cautious assessment of the latest generation of TKIs in this population of patients.67
The Children’s Oncology Group AALL0031 study showed no advantage of HSCT in children continuously exposed to high-dose imatinib in combination with chemotherapy.68 Subsequently, the European Study group for Phildelphia-positive ALL amended its protocol in 2010 to continuously expose patients to imatinib and reduce HSCT eligibility criteria, based on early response and MRD. The rate of patients receiving transplants could be reduced from 80% to 38% without changes in 5-year EFS or OS rates (57.0% and 71.8%, respectively).69 With the objective of reducing HSCT, the international CA180-372 reported on the combination of EsPhALL chemotherapy backbone with the second-generation TKI dasatinib with an MRD-based eligibility for HSCT. The rate of patients undergoing HSCT was 14%, with promising 3-year EFS and OS rates (66.0% and 92.3%, respectively).70
In contrast, an almost fully chemotherapy-free induction was considered by the GIMEMA group using imatinib or dasatinib combined with prednisone and CNS prophylaxis, leading to a 96% to 100% CR rate.71,72 In the LAL-1205 study based on dasatinib, the reported 5-year survival was 48.8% at 5 years. The French GRAAPH 2005 study addressed the question of induction dose-intensity and reported a better CR rate in the reduced-intensity arm (98% vs 91%; P = .006) as a consequence of decreased toxicity, with no difference in terms of major molecular response rate (66% vs 64%).73 All patients were eligible for HSCT in CR1, and no difference in terms of OS was observed between the 2 arms (5-year OS, 45.6%). Besides these attempts to decrease the chemotherapy burden in adults, the MD Anderson Cancer Center retained a full hyperCVAD induction in young adults to assess the benefit of different TKIs.74,75 Markedly, the combination of the third-generation TKI ponatinib with hyperCVAD resulted in a 100% CR rate, 83% complete molecular response, and promising 3-year OS of 84%, with no impact of HSCT so far.75
Considering that hypofertility is a major long-term effect in AYA with cancer, the best strategy for this population of patients with Ph-positive ALL should be further evaluated. Incorporation of MRD-based strategies, more potent last-generation TKIs, and recently approved immunotherapies in adult trials will probably bridge the gap between pediatric and adult approaches.
New drugs/new therapeutic approaches
Despite the progressive use of pediatric-inspired or fully pediatric protocols, issues remain in terms of both efficacy and toxicity, leading to the evaluation of newer treatments in this population.
Immunotherapy has attracted much attention recently:
Blinatumomab, a first-in-class bispecific T-cell engager that binds both CD19 and CD3, has shown very promising results for patients with relapsed/refractory (R/R) BCP-ALL. In the recently published phase 3 TOWER study, the difference in remission rate was also significant in the AYA subgroup (age 18-35 years): 43% in the blinatumomab group vs 25% in the chemotherapy group.76 A recent study of blinatumomab for patients with high MRD in CR1 or CR2 demonstrated a 91% rate complete negativation of MRD in 32 AYAs age 18 to 34 years after 1 cycle.77 Efficacy in children and adolescents with R/R BCP-ALL has also been demonstrated: 32% CR rate in 40 children and adolescents age 7 to 17 years.78 Trials are to begin for bridging patients with high MRD at end of consolidation to HSCT at a lower toxicity cost and with a lower pre-HSCT MRD. The possibility of decreasing chemotherapy intensity and increasing efficacy particularly in the AYA field is appealing but will need carefully designed collaborative trials to be assessed.
Inotuzumab ozogamicin (IO) is a monoclonal anti-CD22 bound to calicheamicin. A phase 3 study recently demonstrated that patients with R/R BCP-ALL who received IO had higher rates of remission than patients treated with standard intensive therapy.79 Among patients age <55 years, the CR rate was 80% in the IO group as compared with 32% in the chemotherapy group. Liver-related adverse events were more common in the IO group, especially veno-occlusive disease (11% vs 1%), as well grade ≥3 thrombocytopenia and neutropenic fever.80
The very exciting field of chimeric antigen receptor T cells is a new avenue for patients with BCP-ALL. Striking responses have been observed in children, AYAs, and adults with R/R BCP-ALL, with CR rates as high as 90% with chimeric antigen receptor T cells targeting the B-cell specific antigen CD19.81,82 This has led to the recent approval of tisagenlecleucel by the US Food and Drug Administration. The complexities of setting up these programs, their price, the manufacturing waiting time, the loss of persistence, clonal evolution, and acute and long-term toxicities (particularly cytokine release syndrome, macrophage activation syndrome, neurotoxicity, tumor lysis syndrome, and B-cell aplasia) are not to be overlooked. Nevertheless, all these hurdles and toxicities are to be balanced with those of HSCT.
Targeted therapies in Ph-like ALL lead to questions similar to those addressed in the management of Ph+ leukemia (eg, to transplant or not, dose-intensity de-escalation). Nonrandomized current pediatric and adolescent programs are evaluating the feasibility of defining Ph-like ALL in real time and applying, in addition to high-risk chemotherapy, dasatinib or JAK inhibitor ruxolitinib (ClinicalTrials.gov #NCT02883049, #NCT02723994, and #NCT02420717). Because of the heterogeneity of the ABL class fusions and signal transduction abnormalities and their low frequency in children, the need for additional specific studies within AYA cohorts can be anticipated as the frequency of these abnormalities increases with age.
Support as adults, as children, or as AYAs?
The use of intensified regimens and the following improvements in survival in AYAs with ALL raise the need for monitoring and preventing acute and late effects in this population of patients. Increasing toxicities with age were reported in almost all cohorts of patients treated with fully pediatric or pediatric-like approaches, including in younger age ranges. Most of the studies have shown that the induction death rate, death in remission, delays in chemotherapy administration, and occurrence of severe adverse events are higher in the AYA population compared with children, thus affecting survival as well as quality of life. Of note, specific to ALL treatment are the toxicities related to asparaginase and steroids. Recommendations concerning the use of asparaginase in AYAs have been discussed in previous reviews.83,84
Increase in thrombosis according to age is 1 of the main issues with asparaginase.4 Of note in NOPHO 2008, CNS bleeding was also significantly more frequent in patients age 18 to 45 years.4 Specific programs for prevention of CNS thrombosis are not easy to set up. The results of the Thrombotect study within the BFM 2000 study comparing low molecular weight heparin, antithrombin, and no prevention are thus eagerly awaited.
Pancreatitis incidence obviously depends on the intensity of asparaginase use in a defined protocol. This complication is more common in older children and adolescents, with global rates varying from 2.3% to 7% in large cohorts. Its odds ratio was comparable for patients age 10 to 17 and 17 to 45 years vs those age 1 to 9 years in NOPHO 2008 (2.2 and 2.4, respectively; P = not significant).4
Other asparaginase acute toxicities include hypersensitivity (clinical allergy and silent inactivation) and liver toxicities, including hyperbilirubinemia and hypertriglyceridemia. Of note, incidence of hypersensitivity to pegaspargase was lower in adults compared with children included in the NOPHO 2008 and the Dana-Farber Cancer Institute studies,4,52 and incidence and severity of hypertriglyceridemia were related to asparaginase activity levels.85
The risk of developing avascular osteonecrosis is at its maximum in teenagers and decreases in young adults. As an example, avascular osteonecrosis incidence in the NOPHO 2008 study was 1.5% for patients age 1 to 9 years, 13.4% for those age 10 to 17 years, and 8.5% for those age >18 years.4 This trend is found across protocols. The only proven intervention for prevention has been the successful split of dexamethasone during delayed intensification proposed by the Children’s Oncology Group.86
The pediatric Ponte di Legno Toxicity Working Group recently published definitions of 14 severe acute effects for childhood ALL treatment.87 Because most of these complications are also encountered in the AYA population, this initiative should be an opportunity for adult oncologists to work further with pediatricians to capture these adverse effects and propose adapted recommendations in AYA patients.
Hypofertility/infertility is a particularly relevant late effect in the AYA population. Cyclophosphamide and doxorubicin have the greatest impact on fertility through testicular and ovarian damage, as well as on occurrence of early menopause. Cyclophosphamide is administered at a significantly higher cumulative dose in adult protocols such as hyperCVAD (7.2 g/m2) as compared with pediatric protocols relying on a BFM backbone (3 g/m2), supporting pediatric protocols in AYA patients with ALL.88 Sperm cryopreservation should be offered to male patients before chemotherapy initiation. No clear method for preserving ovarian function is available, because randomized controlled trials evaluating the possible role of gonadotropin-releasing hormone agonists have not been conclusive. Despite discussions about a potential deleterious effect of removing ovarian tissue and the risk of disease reintroduction, cryopreservation should be proposed before HSCT. The lack of precise data on long-term fertility after the use of intensified protocols should support the offering of specific fertility monitoring after treatment to all young men and women.
Specific to ALL is the importance of so-called maintenance or continuation therapy, a long-lasting therapy (mean, 18 months) key to prevention of undue relapses. Many studies have shown the higher risk of nonadherence in the adolescent population, stressing the need for specific adherence programs including monitoring measures.89 Finally, because of the impact of a severe disease and more specifically to the use of high-dose steroids, some frequent psychological disorders may worsen during treatment of leukemia (eg, eating sleeping disorders, psychotic disorders).90 Multidisciplinary teams must be aware of these complex situations to ensure that they are anticipated and treated appropriately.
Conclusion
Progress in the field of AYA ALL has been dramatic, through the identification of new genetic entities and the adoption of fully pediatric or pediatric-inspired protocols. Risk stratification based on recent biological findings and sequential MRD evaluations should now be implemented, as should new therapeutic options including immunotherapy and targeted therapies, preferably within the setting of integrated pediatric and AYA protocols. All these questions should be addressed through specific trials, to which it has been acknowledged AYAs have not been satisfactorily recruited.91,92 Increasing recruitment to trials in the first-line and relapse settings and/or to early-phase trials is an important component of the strategy to improve the outcome of patients in this age range.
Highly involved and specialized teams are necessary, because treatment strategies, although crucial, are only part of the complex care of AYAs. Many challenges remain to address their specific needs: increasing access to clinical trials and new drugs; reinforcing treatment protocol adherence; proposing appropriate psychosocial support; ensuring proper fertility preservation; maintaining access to health care, insurance, education, employment and career paths; and improving survivorship care. Further and stronger collaboration between adult and pediatric hematologists, with involvement of multidisciplinary teams, is of utmost importance to improve the survival and quality of life of AYAs.
Authorship
Contribution: A.B. and N.B. planned the review methodology, analyzed the literature together, and wrote the manuscript.
Conflict-of-interest disclosure: A.B. has been involved in advisory boards for Amgen, Celgene, Erytech, Jazz, Novartis, Roche, Sanofi, and Servier and received research funding from Shire; N.B. has been involved in advisory boards for Amgen, Novartis, Sanofi, and Servier and received research funding from Amgen and Bristol-Myers Squibb.
Correspondence: André Baruchel, Hématologie et Immunologie Pédiatrique, Assistance Publique–Hôpitaux de Paris, Hôpital Robert-Debré, 48, boulevard Sérurier, 75019 Paris, France; e-mail: andre.baruchel@aphp.fr.