Abstract
Adolescents and young adults (AYAs) form a unique group of patients with newly diagnosed acute myeloid leukemia (AML). They differ in terms of disease biology, psychosocial challenges, survival, and in other important respects from children as well as from middle-aged and older adults. AYAs may be treated using pediatric protocols developed in trials composed primarily of younger patients, or using adult protocols developed in trials composed primarily of older patients. After reviewing the distinguishing characteristics of AYAs with AML, we compare and contrast the chemotherapy approaches and argue that neither the pediatric nor adult approaches may be ideally suited for AYAs and the development of AYA-specific approaches merits further consideration. We finish by putting forth ideas for future research to optimize chemotherapy treatment of AYAs with AML.
Introduction
In this review of treatment of acute myeloid leukemia (AML) in adolescents and young adults (AYAs), we will focus on treatment of newly diagnosed primary AML. Although AML that is secondary to myelodysplastic syndrome, previous cancer treatment, and marrow failure disorders are all important entities, as is the treatment of relapsed AML, AYA-specific research in these areas is sparse. For the same reason, we will not cover acute promyelocytic leukemia.
Is there a need to consider the specific treatment needs of AYAs in AML?
The field of AYA oncology, defined by the US National Cancer Institute (NCI) as encompassing patients from 15 to 39 years of age (www.cancer.gov/types/aya), was founded on the recognition that AYA patients differ in important respects from younger and older patients, psychosocially and biologically (both host and disease), and that these differences impact response to treatment.
Currently, a task central to the field is assessing the appropriateness of existing therapies for AYAs. In AML, this assessment is challenging because AML, unlike many cancers, occurs throughout life. And under the cooperative group structure found in most countries (the British National Cancer Research Institute trials, which encompass children, AYAs, and older adults, is a notable exception), the age range is split into pediatric and adult, sometimes dividing the AYA age group at ∼18 to 21 years of age. Moreover, as we will discuss, typical pediatric and adult approaches to AML therapy differ in important respects.
Distinguishing characteristics of AYAs
Before considering treatment, we will first discuss why it is important in AML to consider the needs of AYA patients apart from younger and older ones, highlighting the important ways in which AYAs differ.
Age-related differences in AML biology
It has long been appreciated that the biology of AML changes with age, with the early recognition of age-related variation in common cytogenetic abnormalities helping to establish this understanding.1 The recent Central European study of 5564 patients with de novo AML spanning infancy through adulthood by Creutzig et al comprehensively captures the influence of age on cytogenetic abnormalities in AML (Table 1),2 demonstrating that favorable cytogenetics have a low frequency in infants, increase in frequency in children and young adults, and then decrease in frequency in middle-aged and older adults. Normal karyotype increases in prevalence from 13.7% in infants to ∼25% in children, 44% in AYAs, and 50% in adults. Most unfavorable cytogenetic abnormalities are rare across all age groups, though complex cytogenetics are relatively more frequent in infants, decrease in frequency in AYAs, and then increase in frequency beyond AYAs. Overall, the AYA subgroup is characterized by the high prevalence of normal cytogenetics; the relatively high prevalence of t(8;21), inv(16)/t(16;16), and 11q23 abnormalities; and the low prevalence of complex cytogenetics, monosomy 7, and chromosome 5 abnormalities.
Prevalence of age-specific chromosome abnormalities in AML
| Chromosome aberration . | Infants, 0-2 y (%) . | Children, 2-11 y (%) . | Children, 12-17 y (%) . | AYAs, 18-39 y (%) . | Adults, 40-59 y (%) . | Risk category . |
|---|---|---|---|---|---|---|
| t(15;17) | 2 | 5.9 | 11.7 | 12.4 | 8.1 | Favorable |
| t(8;21) | <1 | 17.2 | 13.3 | 7.8 | 4.3 | Favorable |
| Inv(16) | 6.3 | 10 | 8.8 | 5.5 | 4.4 | Favorable |
| Normal karyotype | 14 | 20 | 27 | 44 | 52 | Intermediate |
| t(9;11) | 19.6 | 8.3 | 4.5 | 2.6 | 1.2 | Intermediate |
| 11q23/MLL | 25.2 | 10 | 7.4 | 5.1 | 2.0 | Unfavorable for adults, variable for children |
| Complex karyotype | 14 | 7.0 | 4.3 | 2.3 | 7.8 | Unfavorable for adults |
| Monosomy 7 | <1 | 1.9 | 1.1 | 0.9 | 1.2 | Unfavorable |
| Chromosome 5 abnormalities | <1 | 1.3 | 1.4 | <1 | <1 | Unfavorable |
| 17p abnormalities | 0 | 0 | 0 | 0.2 | 0.3 | Unfavorable for adults |
| 12p abnormalities | 1.5 | 0.4 | 1.1 | 0.5 | 0.2 | Unfavorable for adults |
| t(6;9) | 0 | 0 | 0.5 | 1.4 | 0.3 | Unfavorable for adults |
| Inv(3) | 0 | 0.2 | 0.2 | 1.2 | 0.7 | Unfavorable for adults |
| Chromosome aberration . | Infants, 0-2 y (%) . | Children, 2-11 y (%) . | Children, 12-17 y (%) . | AYAs, 18-39 y (%) . | Adults, 40-59 y (%) . | Risk category . |
|---|---|---|---|---|---|---|
| t(15;17) | 2 | 5.9 | 11.7 | 12.4 | 8.1 | Favorable |
| t(8;21) | <1 | 17.2 | 13.3 | 7.8 | 4.3 | Favorable |
| Inv(16) | 6.3 | 10 | 8.8 | 5.5 | 4.4 | Favorable |
| Normal karyotype | 14 | 20 | 27 | 44 | 52 | Intermediate |
| t(9;11) | 19.6 | 8.3 | 4.5 | 2.6 | 1.2 | Intermediate |
| 11q23/MLL | 25.2 | 10 | 7.4 | 5.1 | 2.0 | Unfavorable for adults, variable for children |
| Complex karyotype | 14 | 7.0 | 4.3 | 2.3 | 7.8 | Unfavorable for adults |
| Monosomy 7 | <1 | 1.9 | 1.1 | 0.9 | 1.2 | Unfavorable |
| Chromosome 5 abnormalities | <1 | 1.3 | 1.4 | <1 | <1 | Unfavorable |
| 17p abnormalities | 0 | 0 | 0 | 0.2 | 0.3 | Unfavorable for adults |
| 12p abnormalities | 1.5 | 0.4 | 1.1 | 0.5 | 0.2 | Unfavorable for adults |
| t(6;9) | 0 | 0 | 0.5 | 1.4 | 0.3 | Unfavorable for adults |
| Inv(3) | 0 | 0.2 | 0.2 | 1.2 | 0.7 | Unfavorable for adults |
Adapted from Creutzig et al2 with permission.
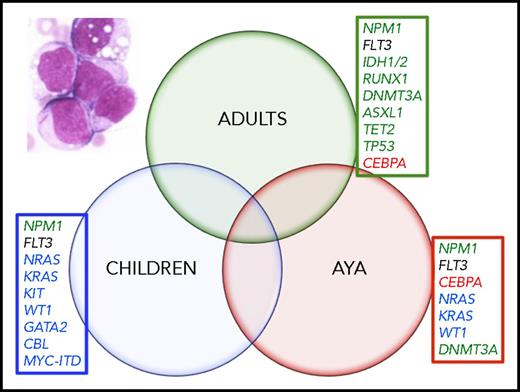
It is also now becoming apparent that age also influences the presence of AML-related molecular abnormalities. The adult AML genome has been characterized in several large studies and important recurrent genetic mutations have been identified.3-5 The recently published results of the Children’s Oncology Group (COG)–National Cancer Institute (NCI) Therapeutically Applicable Research to Generate Effective Treatments (TARGET) AML Initiative elegantly highlights the effect of age on the type and relative frequency of recurrently mutated genes. Several comparisons between infants (<3 years), children (3-14 years), AYAs (15-39 years), and adults (40+ years) show strong associations between age and the prevalence of common somatic gene mutations. For example, KRAS mutations were most prevalent in infants (23%), decreasing with age to 11% in children, 7% in AYAs, and 6% in adults. NPM1 mutations, on the other hand, increased in prevalence from 3% in infants to 10% in children, 18% in AYAs, and 34% in adults. Unlike these abnormalities, which waxed or waned across the age spectrum, mutations in genes associated with aging and clonal hematopoiesis (like DNMT3A, IDH1/2, RUNX1, and TP53) occurred almost exclusively in middle-aged and older adults.3 These findings have important implications for the design of trials of targeted agents. The challenge will be to use the data to develop novel therapies for patients with AML, including the AYA cohort based on the disease drivers (Figure 1). For example, based on the data from the TARGET study, only the tyrosine kinase inhibitors for FLT3 internal tandem duplication (ITD)-positive AML will be useful for patients across all age groups. In contrast, the IDH1 and IDH2 inhibitors will be most beneficial for older adult patients, and are unlikely to be incorporated into pediatric or AYA AML. The recognition of the age-related differences in AML biology will provide the best opportunity to improve the clinical outcomes that have been static for decades.
Somatic mutations have variable frequencies across age groups. Mutations most commonly found in adults are noted in green. Mutations most commonly found in AYAs are noted in red print. Mutations most commonly found in children are in blue print. FLT3-ITD (black) mutations occurred in nearly equal frequency across the age groups. Inset, blood film of acute myelogenous leukemia; multiple myeloblasts, one contains a prominent Auer rod. (Wright-Giemsa stain; original magnification ×1000).
Somatic mutations have variable frequencies across age groups. Mutations most commonly found in adults are noted in green. Mutations most commonly found in AYAs are noted in red print. Mutations most commonly found in children are in blue print. FLT3-ITD (black) mutations occurred in nearly equal frequency across the age groups. Inset, blood film of acute myelogenous leukemia; multiple myeloblasts, one contains a prominent Auer rod. (Wright-Giemsa stain; original magnification ×1000).
Predisposition to treatment-related mortality with pediatric AML therapy
Pediatric cooperative groups that encompass the younger segment of the AYA age range have studied the impact of AYA age on risk for treatment-related mortality (TRM) and have shown that AYA patients are much more likely to suffer TRM than younger patients. Tomizawa et al, for example, examined the impact of AYA age across 3 cooperative group trials from Japan. In multivariate analysis, they demonstrated that age ≥15 years is associated with a nearly threefold increase in the risk for TRM (hazard ratio [HR] = 2.789; 95% confidence interval [CI], 1.437-5.412; P = .002).6
Studies from the COG have also highlighted the high risk for TRM among AYA patients. In an analysis encompassing 4 COG trials (Children’s Cancer Study Group [CCG] 2891, CCG 2941, CCG 2961, and AAML 03P1) and 1840 patients, Canner et al compared outcomes in patients 16 to 20 years of age to those in younger patients.7 As in the Japanese study, TRM was higher in AYA patients (HR = 2.3; 95% CI, 1.59-3.33; P < .001). Secondary analyses suggested that excess TRM stemmed primarily from chemotherapy-associated TRM rather than the effects of blood and marrow transplantation. They also demonstrated that the excess in TRM was due primarily to infection.7
In a second cross-study analysis from the COG, covering a more recent time period, August et al combined data from the single-arm pilot AAML 03P1 trial (also included in the analysis by Canner et al7 ) and the much larger randomized AAML 0531 trial.8 Importantly, these trials involved a shift from the intensive timing approach used in the earlier CCG trials to an approach similar to that used by the British National Cancer Research Institute (NCRI). The primary objective of these trials was to evaluate the addition of gemtuzumab ozogamicin (GO) to induction and consolidation chemotherapy. In 03P1, all patients were assigned to GO; in 0531, patients were randomly assigned to GO or standard induction and consolidation chemotherapy. GO was administered at 3 mg/m2 per dose IV with the first induction cycle (cytarabine, daunorubicin, and etoposide [ADE]) and the second intensification cycle (mitoxantrone and cytarabine). Although the incidence of TRM was lower for both children and AYAs in this study compared with that by Canner et al, TRM remained excessive in AYA patients: from study entry it was 13.3% in AYA patients and 7.3% in younger patients (P = .005). Importantly, TRM was markedly higher in AYA patients receiving GO, but not in their younger counterparts. Most of the excess stemmed from the use of GO with mitoxantrone, cytarabine intensification. Importantly, although GO was associated with improved event-free survival (EFS) in children, GO did not impact EFS in AYAs.8
There, unfortunately, has been little attempt in recent adult trials to rigorously compare TRM in AYAs and older patients. A preliminary comparison from the Southwest Oncology Group (SWOG) 1203 trial suggests, however, that there could be important differences. In this trial, 28-day mortality was 1% in those younger than 40 years of age, but 4% in those 40 to 60 years of age (Megan Othus, Southwest Oncology Group, written communication). There is a critical need for further research in this area.
Middling survival
The validity of the AYA classification in AML is evidenced by the relative survival rates in AYAs, younger and older patients. Data from national cancer registries and clinical trials suggest that the overall survival of AYA patients is superior to that of middle-aged and older adults, but inferior to that of children. Two recent registry studies have compared outcomes of AYAs to younger patients in AML. Nasir et al completed an analysis in AML using the US NCI’s Surveillance Epidemiology and End Results 18 (SEER-18) registry.9 Using a more narrow definition of AYAs, 19 to 30 years, they compared outcomes in AYAs and younger patients for the years between 1973 and 2012, dividing this time span into 3 intervals: 1973 to 1991, 1992 to 2000, and 2001 to 2012. Although, over time, survival improved for AYA patients like it did for younger patients, within each interval mortality was higher among AYA patients. In a multivariate analysis, AYA age was associated with mortality (HR = 1.34; 95% CI = 1.26-1.44; P < .01).9 In another study using SEER data, this one defining AYAs as 15 to 39 years of age, Kahn et al also demonstrated that survival in AYA patients is inferior to survival in children.10 Another objective of their study was to examine the impact of ethnicity on outcomes in AYA patients with varying hematologic malignancies. They showed that in AYAs with AML, African Americans, but not Hispanic Americans, fare especially poorly relative to European Americans.10 By comparison, disparity between African Americans and European Americans with acute lymphoblastic leukemia (ALL) was small. The authors suggest that an important reason for the marked disparity in AML is limited access to allogeneic blood and marrow donors among African Americans.
Pulte et al have used registry data from the United States and Germany to define the outcomes of AYA patients relative to those of middle-aged and older adults. Using 10-year age groupings (15-24 years, 25-34 years, 35-44 years, 45-54 years, 55-64 years, 65-74 years, and 75+ years), their research suggests that patients up through 34 years, and possibly through 44 years, have comparable survival, which is clearly superior to that of older age groups.11,12 Pemmaraju et al analyzed the outcomes of nearly 4000 patients with AML treated at the University of Texas MD Anderson Cancer Center and compared AYA patients to older patients, defining AYAs as 16 to 29 years of age.13 In a multivariate analysis, AYA age was significantly associated with the achievement of remission and inversely associated with relapse. There was also a trend toward better overall survival (HR = 0.74; P = .085).13
Most adult cooperative group trials cover the entire AYA age range, providing a unique opportunity to compare outcomes in AYAs and older patients. There have been few systematic attempts within adult cooperative groups to do so, however. One notable exception is the Korean Cooperative Study Group A, which has done so in 2 randomized controlled trials. The first trial compared standard-dose daunorubicin (45 mg/m2 per day for 3 days) to high-dose daunorubicin (90 mg/m2 per day for 3 days) in induction. In this trial, which extended from 15 to 60 years, patients ≤40 years had both superior EFS (HR for age >40 years = 1.404; P = .011) and overall survival (HR for age >40 years of age = 1.475; P = .007). There was no interaction between age and treatment.14 In a more recent randomized controlled trial, comparing high-dose daunorubicin to idarubicin in induction chemotherapy, AYA age was associated with superior overall survival, but not EFS. Again, there was no interaction between age and treatment.15
The UK’s Medical Research Council (MRC)/NCRI, which encompasses patients from birth through adulthood in its trials, is in a unique position to assess outcomes in AYA patients relative to younger and older patients. An analysis of data from the MRC 10 and 12 trials comparing overall survival of 2- to 15-year olds, 16- to 24-year olds, and 25- to 39-year olds demonstrated no differences between the younger and older AYA groups. Survival in AYA patients, however, was clearly inferior to that in younger patients. Analyses from the MRC/NCRI comparing AYA patients to 40- to 60-year olds would be a welcome addition to the literature.16
Psychosocial stressors
There is little doubt that psychosocial challenges are abundant among AYAs.17 Some of these arise from disruption of normal life-stage processes, such as acquiring personal independence, forming peer and romantic relationships, initiating sexual exploration, completing education, establishing careers, and raising families.17,18 Additionally, several psychological conditions develop in this age range, including panic, generalized anxiety, posttraumatic stress, mood, and psychotic disorders.19 Due to the dearth of studies describing psychosocial issues in AML generally, insights about AYAs must come from studies of more diverse AYA cancer populations. In a recent review, the prevalence of clinical distress among AYAs with cancer ranged from 5.4% to 56.5%.20 A longitudinal study of AYAs during the first year after cancer diagnosis found that 28% exhibited psychological distress exceeding population norms at diagnosis and 1 year later.21 AYAs report a wide variety of unmet needs during cancer treatment related to information (57%), counseling (41%), and practical support (39%).22 Historically, high-intensity chemotherapy regimens effective in AML routinely result in prolonged inpatient admissions characterized by long periods of boredom awaiting marrow recovery punctuated by life-threatening emergencies, such as sepsis. This may exacerbate chronic anxiety related to uncertainty affecting AYAs with cancer. In focus groups, important psychosocial themes for AYAs include physical/bodily changes, barriers to academic/vocational goals, and social isolation.23 Interestingly, whereas treatment nonadherence is a substantial problem among AYAs prescribed oral mercaptopurine for ALL24 and tyrosine kinase inhibitors for chronic myeloid leukemia (CML),25 currently this may be less so for AML using predominantly IV inpatient chemotherapy regimens. However, given the recent introduction of effective oral therapy, such as FLT3 inhibitors, treatment nonadherence could well emerge as a major outcome determinant for AML, as it is in CML.26,27 In short, AYAs with AML are at high risk for psychosocial distress and should routinely receive assessment and intervention directed toward health-related information concerning diagnosis, prognosis, and treatment; financial stressors; emotional health; role of family and friends; spiritual concerns; and individualized support via counseling, peer support groups, and/or social media networking.17,18 In regard to the latter, the diversity of social media platforms and their applicability to the internet-savvy AYA population has recently been reviewed.28
Low clinical trial participation
In explaining improved cancer survival statistics over the past several decades, the success of cancer clinical trials (CCTs) is thought to be a major factor.29 As in the past, continued progress in improving outcomes through CCTs is dependent upon enrollment of sufficient numbers of participants. In this respect, AYAs with AML are in jeopardy. Low participation of AYAs in CCTs is a well-documented problem. Whereas 40% to 60% of children enroll onto CCTs, only 10% to 20% of AYAs aged 15 to 21 years do so.30,31 Among AYAs aged 21 to 39 years, enrollment proportion is <10%.31 This nonparticipation has serious consequences. Low enrollment of AYAs into CCTs has been directly correlated with lower cancer survival improvement compared with children.32 Additionally, low CCT enrollment of AYAs limits their access to promising new agents; accession of valuable biospecimens; and representation in cancer control, supportive care, and epidemiology studies. Low accrual may even threaten successful completion of the CCT itself. All of these considerations are especially relevant in AML, where AYAs may be disproportionately underrepresented due to poor CCT participation that compounds their relatively low incidence of AML, especially among younger patients. Potential reasons for low AYA enrollment into CCTs are diverse, including reduced access to cancer centers and providers that offer CCTs.33 However, at least for younger AYAs, a recent study showed that nonavailability of appropriate CCTs did not account for their low participation, suggesting instead the importance of factors related to the patient/family or providers.34 Regardless of the specific barriers, it is critical to acknowledge the challenge and impact of low CCT enrollment in this age group. Continued progress in understanding and treating AML in AYAs is reliant upon effective efforts to maximize enrollment of this population into CCTs through developing appropriate studies, leveraging NCI National Clinical Trials Network (NCTN) intergroup collaborations, expanding study availability through mechanisms such as the NCI Community Oncology Research Program (NCORP), and exploring targeted AYA recruitment efforts at the site level.33
Defining the optimal chemotherapy approach for AYAs
The focus of trials for newly diagnosed AML has increasingly shifted to the evaluation of targeted agents added to standard chemotherapy regimens. The 2 most important yields of this research are the US Food and Drug Administration’s recent approvals of GO for adults and children with CD33+ AML and midostaurin for adults with FLT3-mutated AML.
Pediatric vs adult chemotherapy
The standard chemotherapy regimens or “backbones” that adult and pediatric groups are embedding investigational agents into differ in critical respects. Because both approaches are used for AYAs, it is important to consider these differences. The most commonly used adult chemotherapy regimen has long been “7 + 3” (cytarabine plus daunorubicin) induction followed by high-dose cytarabine consolidation. Over the past decade, there has been a shift to the use of higher-dose daunorubicin (90 mg/m2 × 3 doses) based on the results of randomized controlled trials.14,35 Efforts to investigate alternatives to this approach continue. These include attempts to enhance induction by replacing daunomycin with idarubicin,15 by increasing the dose of cytarabine,36 by doing both,37 or by incorporating cladribine or fludarabine.38 There have also been efforts to develop effective, multiagent consolidation regimens.39
In pediatrics, unlike adults, there is nothing that begins to approach being a standard. Most pediatric protocols are very intensive, involving greater anthracycline exposure and the use of multiagent consolidation. To help illustrate the differences in pediatric and adult chemotherapy approaches, we have outlined the salient features of the standard-arm protocols used in the recent adult US Intergroup SWOG-led phase 3 trial, S1203, open to patients from 15 to 60 years of age,37 and in the COG AAML 0531 trial, open to patients from 0 to 30 years of age,40 in Table 2.
Comparison of cumulative chemotherapy doses in standard therapy arms of COG AAML 0531 and SWOG 1203 trials
| . | Daunorubicin, mg/m2 . | Cytarabine, mg/m2* . | Etoposide, mg/m2 . | E colil-asparaginase, IU/m2 . |
|---|---|---|---|---|
| COG 0531 | 444 | 45 600 | 1750 | 12 000 |
| SWOG 1203 | 270† | 72 700 | 0 | 0 |
| . | Daunorubicin, mg/m2 . | Cytarabine, mg/m2* . | Etoposide, mg/m2 . | E colil-asparaginase, IU/m2 . |
|---|---|---|---|---|
| COG 0531 | 444 | 45 600 | 1750 | 12 000 |
| SWOG 1203 | 270† | 72 700 | 0 | 0 |
E coli, Escherichia coli; IU, international unit.
Includes induction dose(s) of cytarabine.
A 405 mg/m2 total dose if second course of induction needed due to residual blasts on day +14 bone marrow assessment.
To compare adult and pediatric approaches, Woods et al41 performed a cross-study analysis in AYAs aged 16 to 21 years treated for newly diagnosed AML between the years 1986 and 2008 on COG, Cancer and Leukemia Group B (CALGB), and SWOG trials. There were notable differences in outcome. The incidence of relapse was much lower and the incidence of TRM much higher in COG trials. Both EFS and overall survival were higher in the COG trials. There were, however, potentially important confounding variables including differences in age and cytogenetics. A multivariate analysis was performed for EFS, adjusting for these potential confounders that showed nonsignificantly worse outcome with the adult studies (HR = 1.32; 95% CI = 0.99-1.77; P = .062). This study has important limitations, including its being a retrospective analysis of studies spanning more than 2 decades and its only covering the lower end of the AYA age range. Also, most of the pediatric data were drawn from trials of intensive timing induction therapy, an approach no longer used by COG. And none of the adult data were drawn from studies using higher-dose daunorubicin, now considered the standard by many. Nevertheless, the results of this study do raise the possibility that AYA patients benefit from more intensive pediatric approaches to therapy.
When weighing the potential benefits of pediatric therapy, however, it is important to factor in the potential added risk for late cardiomyopathy stemming from the more intensive anthracycline exposure.42 Importantly, it remains unclear whether routine use of the cardioprotectant dexrazoxane could reduce this excess cardiac risk.43
Is an AYA-specific strategy needed?
It is quite possible that neither adult nor pediatric chemotherapy regimens are ideally suited for AYA patients. Although intensive pediatric therapy may benefit AYAs, its use is associated with excessive TRM. We believe that it may be feasible to create effective yet safer chemotherapy backbones by “mixing and matching” elements of adult and pediatric regimens. For instance, in the aforementioned SWOG 1203 trial, the use of 7 + 3 daunorubicin and cytarabine induction with high-dose cytarabine consolidation was associated with low risk for relapse and excellent survival in patients with favorable-risk cytogenetics. It is very possible, then, that an approach to AYA treatment that used adult therapy for those with favorable-risk disease, while utilizing a pediatric regimen for those with higher-risk disease, could be advantageous. We believe that such AYA-specific approaches warrant assessment in clinical trials.
Transplantation
While an in-depth review of the role of hematopoietic cell transplantation in the treatment of AML in AYA is beyond the scope of this paper, we believe it important to consider this option in the management of these patients. It is critical that pediatricians are mindful that generally speaking, transplant outcomes in AYA patients are inferior to those in children. Likewise, it is important for internists to be cognizant that outcomes in AYA patients are superior to those in middle age adults.44 As with the chemotherapy, then, we believe it is important to tailor the use of transplantation to best meet the needs of AYA patients.
Future steps
We hypothesize that AYA patients would benefit from AYA-specific therapy. This hypothesis, we believe, should be assessed in future clinical trials. We also think that further research is needed to form the foundation for such trials. To forge this foundation, we propose the following 3 measures:
Conduct cross-study analyses to compare the efficacy of contemporary adult and pediatric approaches in AYA patients.
Conduct within-adult-cooperative-group cross-study analyses, comparing outcomes in AYAs to middle-aged adults.
Incorporate prospective AYA analyses into future pediatric and adult cooperative group trials. Work to increase enrollment of AYA patients on pediatric and adult trials, so that AYAs make up more than a relatively small minority of patients in both settings and provide ample statistical power for such analyses. Consideration could be given to using targeted AYA enrollment goals and recruitment strategies.
Authorship
Contribution: K.O., D.R.F., and J.T.H. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John T. Horan, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University, Atlanta, GA 30322; e-mail: john.horan@choa.org.
REFERENCES
Author notes
K.O. and D.R.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal