Abstract
Adolescents and young adults (AYAs) comprise the largest age group affected by Hodgkin lymphoma (HL). Despite excellent overall survival of AYA patients with HL due to advances in treatment regimens, therapy-associated late effects continue to be a concern in HL survivors, especially for younger patients who have decades of life remaining. Since the first clinical trial for HL with chemotherapy in 1964, subsequent protocols have attempted to reduce chemotherapy-induced toxicities and yet maintain high overall survival rates. Today, new analytic methods applied to data from survivorship cohorts, such as the recently described cumulative burden of disease metric, can be used to inform changes for future protocols. Although pediatric and adult trial consortia have followed this process, the AYA population, an age cohort split between pediatric and adult health care services, faces many barriers to care and is the least likely to be enrolled in clinical trials. AYA patients with HL theoretically have a choice to be treated in pediatric or adult protocols when presented with these options. Recent efforts by the National Clinical Trials Network, the Children’s Oncology Group, and others have been made to ensure that the burden of choice for the AYA population is not greater than the burden of disease.
Introduction
Hodgkin lymphoma (HL) is one of the most common cancers among adolescents and young adults (AYAs).1 Since the 1970s, the 5-year relative survival rate of HL among AYA patients, an age range defined by the National Cancer Institute as between 15 and 39 years, has exceeded 80% and has risen to over 90% today.1 Despite excellent outcomes, past treatment exposures, such as high-dose radiation with expansive dose fields, anthracyclines, and other chemotherapy agents cause an increased risk of early mortality and higher rates of long-term cardiac, pulmonary, gonadal, and endocrine toxicity and secondary neoplasms.2 As a consequence, the combined effect of lifelong morbidities and high survival rates has created a unique shift toward prioritization of late effect–oriented objectives for clinical studies of pediatric HL.3 Pediatric clinical trials for HL now have survivorship objectives, as well as a reduction in therapy intensity, as their main priorities. For example, the Children’s Oncology Group (COG) trial AHOD0431 was designed to assess intensive therapy–free survival rather than event-free survival (EFS),4 and the recent adult trial RATHL randomized the omission of bleomycin for patients with a negative positron emission tomography (PET) scan after 2 cycles of therapy to successfully lower pulmonary toxicity.5 Consequently, data generated from childhood and adolescent cancer survivorship cohorts over the past 4 decades in North America and Europe have heavily influenced modern HL treatment regimens for all age groups.6
Despite advances in HL treatment and high survival rates,7 the AYA patient population faces many well-known barriers to care,8 as mortality and survival trends of AYA patients continue to lag behind those of pediatric patients with HL. This difference may be attributed to many factors, but, in general, pediatric and AYA patients have similar histologic subtypes, with most patients presenting with nodular sclerosis, whereas older adults have an increased incidence of mixed cellularity and lymphocyte depletion.9 However, because dedicated research efforts are lacking, no single treatment approach is considered a standard of care for AYA patients.7,10,11 Consequently, identifying optimal treatment opportunities is perhaps the most pressing challenge for AYA patients with HL, as very few individuals aged 15 to 39 years are enrolled in clinical trials. Instead, they are often treated as either pediatric or adult patients, per published treatment protocols, according to their geographical location at the time of initial presentation.
The AYA population: no home for the bulk of patients
Unlike most pediatric and adult cancers, HL primarily occurs in the AYA population. In general, HL has a bimodal distribution: the incidence increases between the ages of 10 to 14 years, crests between the ages of 20 and 24 years, and then slowly decreases thereafter with a nadir in the late 40s. A second peak begins in the late 40s and increases to incidence rates similar to those of AYAs after the age of 65 years.1
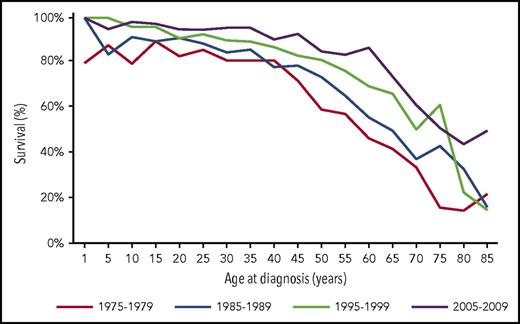
After collapsing Surveillance, Epidemiology, and End Results (SEER) data for patients with HL into 3 cohorts, pediatric (<15 years), AYA (15-39 years), and adults (≥40 years), the 5-year relative survival rates of each group between the years of 2003 and 2007 were 97.4%, 95.8%, and 77.1%, respectively (Figure 1).1 Interestingly, when these trends are analyzed by decade, survival across all age groups has increased over time but has always remained above 80% for pediatric and AYA patients before declining after 40 years of age. The relative survival rate is used when assessing HL to compare the survival difference between those with and without the disease, as the desired information is the proportion of patients who survived cancer after accounting for background mortality in the general population. These data from SEER are likely an accurate representation of outcomes, as it includes AYA patients treated in clinical protocols and those who were not.
Survival curves for HL from the SEER database throughout decades of treatment.1
Survival curves for HL from the SEER database throughout decades of treatment.1
Although the goal of maintaining overall survival (OS) rates while lowering long-term therapy–related late effects has driven recent pediatric and adolescent clinical research, the proportionally large population of AYA patients with HL has generally been overlooked because this population is frequently segregated into either pediatric or adult studies. As protocols strive to deliver the best possible treatments and build on prior successes, one major struggle that remains is the lack of enrollment of the AYA population into clinical trials, with estimates of enrollment between 10% and 15% for patients aged 15 to 19 years and <2% for patients aged 20 to 30 years.7,8,11 This low rate of enrollment corresponds with a marked lag in the reduction of cancer mortality rates among the AYA population, with an increase in 5-year survival rates >1.5% per year for both children and adults but <0.5% per year for patients aged 15 to 24 years and no improvement for patients aged 25 to 34 years.8
Survival trends for AYA patients treated in either adult or pediatric protocols
Although pediatric and adult protocols can differ, data demonstrating how outcomes are affected by treatment regimens in subgroup analyses of AYA patients are limited. Historically, adolescents treated in adult protocols have experienced inferior outcomes. Older data from Britain demonstrated poor long-term outcomes for adolescents aged 15 to 17 years when they were treated according to adult protocols between 1970 and 1997. Further review of these data suggests that this difference may be due to the secondary procedures and treatments provided during this time period that are no longer considered appropriate and contributed to deaths. Examples of this include staging laparotomy, splenectomy, and high-dose, extended-field radiation alone for low-stage disease that led to treatment failures and caused cardiac toxicity and secondary neoplasms.12
In more recent reports, Foltz et al demonstrated equivalent outcomes for patients aged 16 to 21 years as for those aged 22 to 45 years when treated in the same adult protocol with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) therapy from 1981 to 2004 in British Columbia, Canada.13 The 10-year progression-free survival rate for adolescents vs young adults was 77% and 80%, respectively, and 10-year OS was 96% for both age groups with limited-stage disease and 88% and 86%, respectively, for those with advanced-stage disease.13 This suggests that outcomes are similar within the AYA age group when treated in the same protocol as adults, but this analysis was limited by its retrospective nature and lack of comparison with a pediatric cohort. In 2009, the German Hodgkin Study Group reported a similar retrospective analysis comparing the outcomes of AYA patients aged 15 to 20 years with those of patients aged 21 to 45 years.14 Their results showed comparable rates of freedom from treatment failure (80.2% and 79.7%, respectively) and 6-year OS (93.6% and 90.9%, respectively) for both groups. This and other similar outcomes between the 2 age groups suggest that it is appropriate to treat AYA patients the same as adults, but long-term data describing treatment-associated late effects, such as infertility, risk for secondary neoplasms, and early mortality are lacking. Another limitation is the lack of comparison of these outcomes with those of pediatric or adult patients treated during this time frame14 to determine whether the AYA outcomes were superior or inferior.
An analysis of AYA patients treated in a pediatric protocol vs an adult protocol between the years of 1999 and 2006 was reported by Henderson et al in 201715 and their findings contradicted those of previous studies. Specifically, AYA patients aged 17 to 21 years (21 years was the upper age limit for this pediatric protocol) treated in the COG pediatric protocol AHOD0031 exhibited a 5-year failure-free survival (FFS) rate of 85%, as compared with a 68% 5-year FFS rate of this same age group in the adult ECOG-ACRIN trial E2496. Furthermore, this age group experienced inferior outcomes when compared with patients aged 22 to 44 years, with 5-year FFS rates of 68% and 76%, respectively.15 Although the inclusion criteria differed for the 2 trials and a greater number of AYA patients with stage III or IV disease and B symptoms were enrolled in the ECOG-ACRIN trial, when the survival analysis controlled for stage, B symptoms, and bulk disease, AYA patients on the AHOD0031 still had superior outcomes than did those on the E2496 trial.15 Differences in staging and response evaluation criteria are always present among trials, hindering true direct comparisons, but these prospectively collected data are important and revealed that AYA patients experience superior outcomes when they are treated according to a pediatric protocol.15 Continued prospective research is needed to better delineate the outcomes of AYA patients treated in pediatric vs adult protocols.
The shifting paradigm of HL therapeutic goals toward late effects
With excellent 5- and 10-year OS rates above 90% among AYA patients with HL, optimization of treatment must focus on long-term reductions in morbidity and mortality that remain unapparent during 10-year follow-up time frames. In pediatric and adolescent patients treated between the 1960s and 1990s, HL survivors were found to carry an increased risk of early mortality from second cancers, cardiac disease, and infection when followed for up to 28 years after diagnosis.16 To address this, a major shift to maintain survival while minimizing treatment intensity and late effects has evolved over time.3 This shift has led to a variation in the standard of care between adult and pediatric groups, resulting in a historical divergence that is particularly relevant for AYAs with HL.
Successful HL therapy evolved from total-body irradiation at the turn of the century to a standard-of-care regimen with ABVD in the United States and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and procarbazine (BEACOPP) among European groups (Table 1). Although ABVD is efficacious in the pediatric population,13 efforts to decrease the cumulative dose of each chemotherapy agent, most notably anthracyclines, to reduce late effects (eg, cardiac, pulmonary, and gonadal toxicity) and secondary neoplasms led to a variety of clinical trials of different chemotherapy backbones derived from these first regimens. To test new drug combinations, several large collaborative groups have formed over the last 40 years to perform risk-adapted clinical trials and monitor survivors for therapy-associated late effects. These trials have driven the development of many subsequent protocols (Table 1). However, only a portion of AYA patients are eligible for any given trial, as most protocols limit enrollment to patients aged less than or older than 18 years, splintering the AYA group. The following are 3 examples of successful pediatric consortia.
Treatment of patients with high-risk HL
| Trial . | Group . | Age, y . | Treatment . | Anthracycline, mg/m2 . | Radiation, Gy . |
|---|---|---|---|---|---|
| High-risk clinical trials | |||||
| AHOD133121 | COG | <19 | Bv-AVEPC vs ABVE-PC | 250 | 21 + 9 boost if incomplete metabolic response at end of therapy |
| 250 | |||||
| HLHR1324 | SJ–Stanford–Dana-Farber Consortium | <19 | AEPA/CAPDac | 160 | 25.5 ISRT to individual sites with an inadequate response at ERA |
| HOD9939 | SJ–Stanford–Dana-Farber Consortium | ≤21 | Stanford V | 150 | 25.5 IFRT (15 if in CR at ERA) |
| C219 | EuroNet-PHL | <18 or <25 in France, Italy, and UK | OEPA/COPDAC vs OEPA/DECOPDAC | 160 | 19.8 + 10 boost to LRA PET+ lesions 30 only to PET+ lesions at LRA |
| 260 | |||||
| NYMC 56840 | New York Medical Center | <30 | BV-AVD-R (+2 cycles ifosfamide/vinorelbine) for slow early responders | 300 | RT only foreseen for patients that do not achieve a CR at the EOT |
| HD2141 | German Hodgkin Study Group | ≥18 | Escalated BEACOPP × 4 or 6 vs BrECADD × 4 or 6 | 140 or 210 | 30 Gy to residual PET+ lesions at EOT |
| 140 or 210 | 30 Gy to residual PET+ lesions at EOT | ||||
| Checkmate 205 | Bristol-Myers Squibb | ≥18 | N + N-AVD | 300 | No RT |
| ECHELON-125 | Seattle Genetics | ≥18 | A-AVD × 6 vs ABVD × 6 | 300 | No RT (on this phase of trial) |
| 300 | |||||
| EORTC-2001242 | EORTC | ≥16 | ABVD × 8 vs Escalated BEACOPP × 4 then BEACOPP × 4 | 400 | No RT |
| 240 | |||||
| CA209-44743 | Memorial Sloan Kettering | ≥18 | ABVD × 6 + nivolumab × 8 doses | 300 | No RT |
| Standards of care–NCCN guidelines | |||||
| n/a | n/a | ≥18 | ABVD × 6 | 300 | 30-36 36-45 for sites of partial response |
| n/a | n/a | ≥18 | ABVD × 2 + 4 escalated BEACOPP | 240 | To initially bulky sites or PET + sites |
| n/a | n/a | ≥18 | Escalated BEACOPP × 6 | 210 | 30-36 36-45 for sites of partial response |
| n/a | n/a | ≥18 | Stanford V | 150 | 30-36 to initial sites >5 cm |
| Trial . | Group . | Age, y . | Treatment . | Anthracycline, mg/m2 . | Radiation, Gy . |
|---|---|---|---|---|---|
| High-risk clinical trials | |||||
| AHOD133121 | COG | <19 | Bv-AVEPC vs ABVE-PC | 250 | 21 + 9 boost if incomplete metabolic response at end of therapy |
| 250 | |||||
| HLHR1324 | SJ–Stanford–Dana-Farber Consortium | <19 | AEPA/CAPDac | 160 | 25.5 ISRT to individual sites with an inadequate response at ERA |
| HOD9939 | SJ–Stanford–Dana-Farber Consortium | ≤21 | Stanford V | 150 | 25.5 IFRT (15 if in CR at ERA) |
| C219 | EuroNet-PHL | <18 or <25 in France, Italy, and UK | OEPA/COPDAC vs OEPA/DECOPDAC | 160 | 19.8 + 10 boost to LRA PET+ lesions 30 only to PET+ lesions at LRA |
| 260 | |||||
| NYMC 56840 | New York Medical Center | <30 | BV-AVD-R (+2 cycles ifosfamide/vinorelbine) for slow early responders | 300 | RT only foreseen for patients that do not achieve a CR at the EOT |
| HD2141 | German Hodgkin Study Group | ≥18 | Escalated BEACOPP × 4 or 6 vs BrECADD × 4 or 6 | 140 or 210 | 30 Gy to residual PET+ lesions at EOT |
| 140 or 210 | 30 Gy to residual PET+ lesions at EOT | ||||
| Checkmate 205 | Bristol-Myers Squibb | ≥18 | N + N-AVD | 300 | No RT |
| ECHELON-125 | Seattle Genetics | ≥18 | A-AVD × 6 vs ABVD × 6 | 300 | No RT (on this phase of trial) |
| 300 | |||||
| EORTC-2001242 | EORTC | ≥16 | ABVD × 8 vs Escalated BEACOPP × 4 then BEACOPP × 4 | 400 | No RT |
| 240 | |||||
| CA209-44743 | Memorial Sloan Kettering | ≥18 | ABVD × 6 + nivolumab × 8 doses | 300 | No RT |
| Standards of care–NCCN guidelines | |||||
| n/a | n/a | ≥18 | ABVD × 6 | 300 | 30-36 36-45 for sites of partial response |
| n/a | n/a | ≥18 | ABVD × 2 + 4 escalated BEACOPP | 240 | To initially bulky sites or PET + sites |
| n/a | n/a | ≥18 | Escalated BEACOPP × 6 | 210 | 30-36 36-45 for sites of partial response |
| n/a | n/a | ≥18 | Stanford V | 150 | 30-36 to initial sites >5 cm |
A-AVD, brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; ABVD, Adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine; ABVE-PC, Adriamycin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; AEPA/CAPDac; brentuximab vedotin, etoposide, prednisone and doxorubicin/ cyclophosphamide, brentuximab vedotin, prednisone and dacarbazine; BrECADD, brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine, dexamethasone; BV-AVD-R, brentuximab vedotin, doxorubicin, vinblastine, dacarbazine, rituximab; Bv-AVEPC, brentuximab vedotin, Adriamycin, vincristine, etoposide, prednisone, cyclophosphamide; COG, Children’s Oncology Group; CR, complete response; EORTC, European Organization for Research and Treatment of Cancer; EOT, end of therapy; ERA, early response assessment; Escalated BEACOPP, bleomycin, etoposide, Adriamycin, cyclophosphamide, Oncovin (vincristine), procarbazine, prednisone; EuroNet-PHL, European Network Group on Pediatric Hodgkin Lymphoma; HOD, studies by the St. Jude–Stanford–Dana-Farber Pediatric Hodgkin Consortium; IFRT, involved field radiation therapy; ISRT, involved site radiation therapy; LRA, late response assessment; N + N-AVD, nivolumab + nivolumab, doxorubicin, vinblastine, dacarbazine; n/a, not applicable; NCCN, National Comprehensive Cancer Network; OEPA/COPDAC, vincristine, etoposide, prednisone and doxorubicin/ cyclophosphamide, vincristine, prednisone and dacarbazine; OEPA/DECOPDAC, vincristine, etoposide, prednisone and doxorubicin/doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone and dacarbazine; PET, positron emission tomography; RT, radiation therapy; SJ, St. Jude Children’s Research Hospital; Stanford V, doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone; UK, United Kingdom.
An early European collaboration began between Germany and Austria17 and has consistently reported survival rates above 90%, which led to a focus in the reduction of late effects after 1982 with the DAL-HD 82 trial.18 This was exemplified by the replacement of procarbazine with etoposide in the vincristine, procarbazine, prednisone, and doxorubicin (OPPA) regimen to reduce gonadal toxicity. The current iteration of this trial (C2) led by the European Network on Pediatric Hodgkin Lymphoma (EuroNet-PHL), a group consisting of >20 European nations, Israel, and Australia, is now testing the efficacy of vincristine, etoposide, prednisone, and doxorubicin (OEPA) in combination with cyclophosphamide, vincristine, prednisone, and dacarbazine (COPDac) vs doxorubicin, etoposide, cyclophosphamide, vincristine, prednisone, and dacarbazine (DECOPDac).19
The COG, formed after the merge of the Children’s Cancer Group and the Pediatric Oncology Group, has tested a variety of therapies beginning with Mustargen (mechlorethamine), Oncovin, procarbazine, prednisone (MOPP)/ABVD. In 1997, the COG moved to an evolving Adriamycin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) backbone. This regimen replaced dacarbazine in ABVD with etoposide, similar to that done by the EuroNet-PHL group, and added cyclophosphamide and prednisone to increase efficacy. The regimen also changed from vinblastine in ABVD to vincristine in an attempt to limit myelosuppression, and added filgrastim to allow for a dose-dense regimen with less delays for count recovery. Additionally, the ABVE-PC regimen limited the cumulative dose for each drug, as compared with ABVD, in an attempt to lower long-term cardiopulmonary toxicities.20 The current trial for patients with high-risk HL AHOD1331 is testing a brentuximab vedotin (ie, a novel anti-CD30 antibody-drug conjugate), Adriamycin, vincristine, etoposide, prednisone, and cyclophosphamide (BV-AVEPC) regimen vs an ABVE-PC regimen.21
The St. Jude–Stanford–Dana-Farber Consortium, initiated in 1990, has also tested several treatment backbones, most notably the Stanford V regimen (ie, doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone) and a vinblastine, doxorubicin, methotrexate, and prednisone (VAMP22,23 ) regimen. It is currently using a brentuximab vedotin, etoposide, prednisone, doxorubicin/cyclophosphamide, brentuximab vedotin, prednisone, and dacarbazine (AEPA/CAPDac) combination in the high-risk trial HLHR13, which was built on the EuroNet-PHL OEPA/COPDac backbone by substituting brentuximab vedotin (Adcetris) for vincristine in patients with high-risk disease.24
Similar to pediatric HL trials, many cooperative group trials are open for adult patients 18 years and older to test new combinations of therapies and novel agents. For patients at least 18 years of age, numerous treatment options are recommended from the current standard of care published in the National Comprehensive Cancer Network guidelines, including brentuximab vedotin in the recently completed ECHELON-125 trial or the PD-1 inhibitor nivolumab in a trial conducted at Memorial Sloan Kettering and in the Checkmate 205 trial26 (Table 1).
For both pediatric and adult patients, radiation therapy has historically been used at doses of 35 to 40 Gy. Today, radiation is delivered to only small defined areas and with different doses for adult and pediatric patients, with many adult protocols still using ∼36 Gy and pediatric protocols using 15 to 25.5 Gy.27,28 As efforts are made to decrease toxicity of therapy, an interim PET-adapted response has been incorporated into most adult and pediatric protocols to allow for the complete elimination of radiation therapy for patients with an “adequate early response,” as defined by the protocol. However, because radiation therapy is efficacious for HL, between 30% and 100% of patients still receive radiation as part of their curative regimen in many protocols. There is no known standard-of-care dose of radiation for AYA patients, and currently the dose they receive varies according to the decision to be treated as a pediatric or adult patient (see Table 1).
Assessing the disease burden of surviving HL
With the knowledge that AYA patient survival is excellent, a major task for clinicians lies in how to design protocols that minimize therapy-related late effects and take into account an estimated life expectancy exceeding 80 years. HL trials aiming to increase survival are not statistically feasible because of their historically high survival rates, which limits most protocols to a noninferiority statistical design. HL clinical trials are also expensive, take a long time to accrue an adequate number of patients, and often require multicenter collaborations. Therefore, clinical investigators must build on recent successes29 and continue to iterate studies that reduce treatment intensity. To this end, future clinical trial designs must either rely on HL-specific data from trials that are now considered obsolete or innovate the application of survivorship data when selecting objectives.
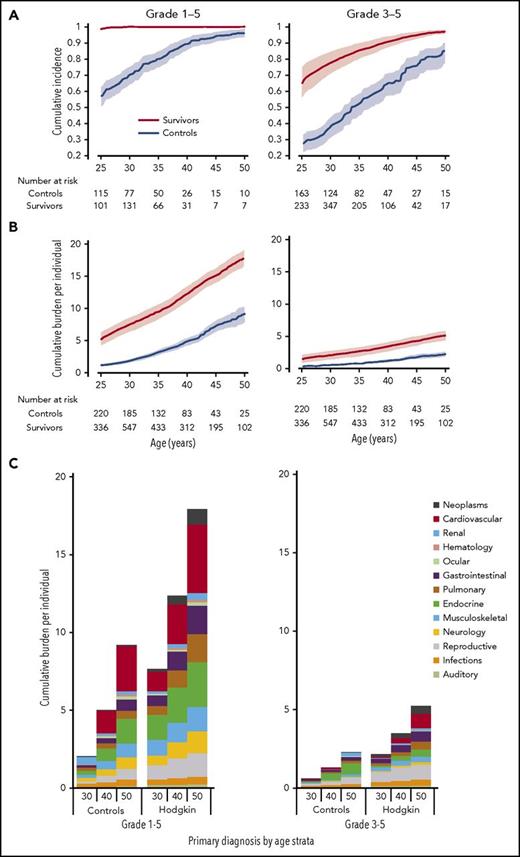
To address how to preclinically apply survivorship data for the purposes of understanding therapy-related tradeoffs, a comprehensive descriptive account of the lifelong disease burden associated with HL therapy has recently been described.30 Given the large number of independently associated long-term late effects with HL, standard statistical approaches have not provided a complete appraisal of the disease burden survivors experience because measures such as incidence only take into account the time to first adverse event. Figure 2A depicts the cumulative incidence of 168 chronic health conditions among HL survivors and matched community controls in the St. Jude Lifetime Cohort Study (SJLIFE).
The burden of disease among survivors of childhood and adolescent HL. (A) The cumulative incidence of 168 chronic health conditions: grade 1-5 and grade 3-5, respectively, among SJLIFE HL survivors and community controls. (B) The cumulative burden of 168 chronic health conditions: grade 1-5 and grade 3-5. (C) The distribution of cumulative burden among both groups by organ system at 30, 40, and 50 years.
The burden of disease among survivors of childhood and adolescent HL. (A) The cumulative incidence of 168 chronic health conditions: grade 1-5 and grade 3-5, respectively, among SJLIFE HL survivors and community controls. (B) The cumulative burden of 168 chronic health conditions: grade 1-5 and grade 3-5. (C) The distribution of cumulative burden among both groups by organ system at 30, 40, and 50 years.
The cumulative burden metric,30-32 as presented in Figure 2B-C, offers a new approach to quantify disease burden within large observational cohorts. Survivors of HL often develop multiple and/or recurrent morbidities and experience higher rates of competing risks, such as premature mortality. Unlike other measures of disease burden, cumulative burden quantifies multimorbidity by counting the average number of conditions present at specific time points, yet taking into account the effect of early mortality on the estimated figures. Because of the large number of conditions measured and the high prevalence of grade 1 conditions among survivors, cumulative incidence curves for grade 1-5 chronic health conditions approach 100% by 25 years of age and are not clinically useful. Furthermore, the cumulative incidence of grade 1-5 and grade 3-5 chronic health conditions among community controls also approaches 100% by 50 years of age. Previously, this observed convergence in the cumulative incidence of individual or groups of chronic health conditions has led to hypotheses that cancer survivors develop an early aging phenotype that mimics the disease burden observed in older adult populations.33 However, when recurrent and multiple morbidities are considered with the new cumulative burden approach, the curves do not converge, and the distribution of disease differs between survivors and community controls.
These multiple early-onset severe morbidities most likely contribute to the reduced health-related quality of life (HRQOL) that has been observed among HL survivors34,35 who report worse emotional and social functioning and increased fatigue and sleep disturbances than do matched controls. Early analyses of the association between the cumulative burden of chronic health conditions and HRQOL suggest that a greater disease burden is associated with reduced HRQOL.36 Continued investigations of which chronic health conditions and HRQOL domains drive these associations and how they are adjusted by variations in treatment are ongoing to define what reductions in toxicity are a priority in future HL protocols.
Translating observed survivorship outcomes to guide upfront therapeutic decision-making
Although advocating for more AYA patients to be treated in clinical trials is the first and most important step for improving their outcomes, identifying the specific study objectives to reduce long-term treatment toxicities is another equally complex challenge for clinical investigators. The decision of which strategy to choose, and in which to invest the time, resources, and patient numbers, is not self-evident, and waiting decades for recently treated HL survivors to age is not a viable option either. To address this issue, analyses of observational data from survivorship cohorts has emerged as a major preclinical innovation. The guiding principle for this approach is the concept that long-term toxicities are not related to the primary neoplasm but rather to treatment type and dose. Using this framework, investigators can use the historical heterogeneity among treatment regimens across all cancers to inform future protocol designs.
Several methods have been used to leverage decades worth of survivorship data into diagnosis-specific actionable guidance. The most frequently applied approaches of regression-based and/or standard-risk prediction models allow investigators to study the tradeoffs between dose modifications and compare quantifiable preclinical outcomes. As previously described, recent work using the cumulative burden metric has provided a blueprint for future opportunities to study complex disease burden interactions. Using marked point regression methods, we assessed the tradeoffs between anthracycline and radiation dose effects on 21 distinct cardiac conditions (excluding strokes).31 In this analysis, an increasing anthracycline dose was associated with more cardiovascular conditions overall (grade 1-5). Radiation dose exhibited no increase in cardiac events, but the severity of the cardiac conditions was augmented, as patients treated with at least 35 Gy were significantly more likely to develop severe, life-threatening, or fatal cardiac conditions. In contrast with standard-risk prediction models, the cumulative burden approach allows clinical investigators to understand the broad implications of differing treatment strategies across chronic health conditions simultaneously.
Burden of choice
For AYA patients with HL, our goal is to maintain excellent cure rates with lower treatment intensities that will reduce long-term complications for survivors who have decades of life left to live. To do this, measured excess disease burden and observed decrements in HRQOL among HL survivors, together with novel treatment strategies and therapies, can be used to drive protocol changes that further optimize treatment regimens and reduce therapy-associated late effects. Barriers to optimization of treatment of AYA patients have been longstanding and are extensive, not the least of which include access to sites with clinical trials, insurance coverage, treatment adherence, and pregnancy.37,38 The COG was the first group in North America to have a formal committee dedicated to AYA patients with cancer. To further address this issue, in 2014, the National Cancer Institute formed the National Clinical Trials Network (NCTN), bringing together both adult and pediatric groups such as The Alliance for Clinical Trials in Oncology, COG, SWOG, and others to form a coordinated research network. The NCTN has a specific emphasis on enhancing care for AYA patients through the expansion of clinical trial access via cross-group enrollment, the formation of trials with broader age eligibility, and improved availability of trials in communities with a high prevalence of AYA patients. In addition, the NCTN aims to enhance “supportive care, access to care, health care transitions, mobile health technologies, and health care delivery systems,” all of which are known barriers for AYA patients.37 Today, the COG continues to work together with The Alliance Advocacy Group to form a clinical trial open specifically to AYA patients with HL7 that will have substantial positive repercussions for this population.
Increased enrollment of AYA patients in protocols will be expected when they are afforded the opportunity to choose between many different regimens. When presented with these alternatives, AYA patients with HL will be empowered to choose one that may be better suited to reduce their long-term risk of a morbidity that is important to them or one that better suits their lifestyle. It is inexcusable for AYA patients to be provided only one choice of therapy driven by the location of their first presentation. Nevertheless, additional data are needed to facilitate the comparison of AYA outcomes in current trials because a choice between therapies is not particularly beneficial when the knowledge to support informed decision-making is absent.
Conclusions
AYA patients comprise the largest cohort of patients with HL, and dedicated efforts to develop a standard-of-care treatment regimen and increase enrollment on clinical trials are needed for this group. As new regimens are proposed, the next clinical trials are designed, and novel agents applicable to HL, such as brentuximab vedotin and PD-1 inhibitors, are considered for inclusion into treatment regimens, late effects of therapy must be at the center of risk-benefit analyses. The cumulative burden approach provides a new, more comprehensive perspective of total disease burden and patterns of illness within a population of interest that can inform decisions for future trials. Further efforts are needed to ensure that the burden of choice for AYA patients is not greater than the burden of their disease.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute (Cancer Support Core grant CA-21765), St. Baldrick’s Foundation, and American Lebanese Syrian Associated Charities.
Authorship
Contribution: J.E.F. prepared the manuscript, with contributions from M.L.M. and N.B.; and all authors were involved in the editing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jamie E. Flerlage, Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: jamie.flerlage@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal