Abstract
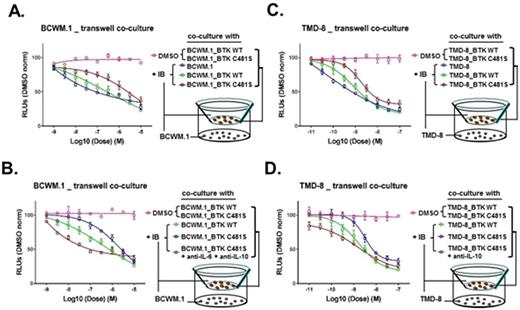
Activating mutations in MYD88 promote NF-kB survival signaling in WM and ABC DLBCL cells through BTK and HCK, both targets of ibrutinib (Yang et al, Blood 2013; 2016). These findings likely explain the high rates of response observed in MYD88 mutated WM and ABC DLBCL patients (Treon et al, NEJM 2015; Wilson et al, Nature Med 2015). Resistance to ibrutinib is increasingly being recognized with prolonged therapy in patients with various B-cell malignancies. Mutations at the BTKCys481 site are observed in half of WM patients with disease progression following initial response (Xu et al, Blood 2017), and are also associated with clinical resistance in patients with CLL and MCL. However, in many WM and CLL cases, BTKCys481 mutations are subclonal and their relevance to clinical disease progression remains unclear. Moreover, the signaling mechanisms that promote ibrutinib resistance remain to be clarified. We therefore performed lentiviral transduction studies in MYD88 mutated WM (BCWM.1, MWCL-1) and ABC DLBCL (TMD-8, HBL-1) cells with vector alone, wild-type BTK (BTKWT), and the BTK variant most frequently observed in ibrutinib resistant WM and CLL cases (BTKCys481Ser). BTKCys481Ser but not BTKWT or vector only transduced WM and ABC DLBCL cells uniformly demonstrated persistent BTK and PLCγ2 activation in the presence of ibrutinib (100, 500 nM), along with a 1-3 log fold increase in EC50 to ibrutinib. Western blot analysis showed persistent BTK signaling in BTKCys481Ser expressing cells that was accompanied by ERK1/2 hyperactivation. Use of highly selective and potent ERK1/2 inhibitors (ulixertinib, GDC-0994) induced apoptosis of BTKCys481Ser expressing WM and ABC DLBCL cells, and showed synergistic tumor cell killing with ibrutinib by CellTiter-Glo® Luminescent Cell Viability Assays using Calcasyn, as well as enhanced apoptosis by Annexin V staining. Using a multiplex cytokine assay and confirmed by TaqMan® quantitative RT-PCR assay for cytokine mRNA levels, we identified that ERK1/2 activation in ibrutinib treated BTKCys481Ser WM and ABC DLBCL cells was accompanied by persistent release of pro-inflammatory and growth enhancing cytokines including CXCL13, IL-2R, IL-6, IL-10, IL-12p40, and TNF-a that was blocked by ulixertinib. In contrast, ibrutinib blocked their release in BTKWT WM and ABC DLBCL cells. We next sought to clarify if cytokine release by ibrutinib treated BTKCys481Ser WM and ABC DLBCL could impact survival of bystander tumor cells. We performed experiments using a Transwell system in which BTKCys481Ser or BTKWT transduced WM or ABC DLBCL cells were co-cultured with their native counterpart cells in the presence or absence of ibrutinib (Figures 1A, B). These studies showed that native WM or ABC DLBCL cells were rescued in the presence of ibrutinib when co-cultured with their BTKCys481Ser but not BTKWT transduced counterparts. Finally, use of IL-6 and IL-10 blocking antibodies abolished the protective effect on native tumor cells conferred by co-culture with BTKCys481Ser expressing cells in the presence of ibrutinib (Figures 1C, D).
Our findings show that BTKCys481Ser mutation drives ibrutinib resistance through hyperactivation of ERK1/2, and that paracrine mediated ERK1/2 dependent pro-survival signaling by BTKCys481Ser expressing cells can confer a protective effect on bystander Waldenstrom's Macroglobulinemia and ABC DLBCL cells in the presence of ibrutinib.
Castillo: Janssen: Consultancy, Research Funding; Abbvie: Research Funding; Pharmacyclics: Consultancy, Research Funding; Millennium: Research Funding. Treon: Pharmacyclics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract