Key Points
Survival of ATL cells depends on continuous Tax expression.
Arsenic/interferon combination induces SUMO/PML/RNF4-mediated Tax degradation.
Abstract
The human T-cell lymphotropic virus type I (HTLV-1) Tax transactivator initiates transformation in adult T-cell leukemia/lymphoma (ATL), a highly aggressive chemotherapy-resistant malignancy. The arsenic/interferon combination, which triggers degradation of the Tax oncoprotein, selectively induces apoptosis of ATL cell lines and has significant clinical activity in Tax-driven murine ATL or human patients. However, the role of Tax loss in ATL response is disputed, and the molecular mechanisms driving degradation remain elusive. Here we demonstrate that ATL-derived or HTLV-1-transformed cells are dependent on continuous Tax expression, suggesting that Tax degradation underlies clinical responses to the arsenic/interferon combination. The latter enforces promyelocytic leukemia protein (PML) nuclear body (NB) formation and partner protein recruitment. In arsenic/interferon-treated HTLV-1 transformed or ATL cells, Tax is recruited onto NBs and undergoes PML-dependent hyper-sumoylation by small ubiquitin-like modifier (SUMO)2/3 but not SUMO1, ubiquitination by RNF4, and proteasome-dependent degradation. Thus, the arsenic/interferon combination clears ATL through degradation of its Tax driver, and this regimen could have broader therapeutic value by promoting degradation of other pathogenic sumoylated proteins.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy that involves transformation of CD4+ T cells on infection by human T-cell lymphotropic virus type I (HTLV-1), the prototypic oncoretrovirus.1 ATL typically develops after a long latency period with a progressive clonal expansion of activated T cells and bears a poor prognosis due to intrinsic chemotherapy resistance.2,3 The viral oncoprotein Tax plays a central role in T-cell transformation through deregulation of multiple cellular pathways, including activation of the nuclear factor-κ B, cyclic AMP response element binding protein, and serum response factor pathways to promote cell proliferation. Tax also silences tumor suppressors and induces genetic instability.1 However, Tax expression is often undetectable at the leukemic stage, and some ATL clones bear mutations in Tax predicted to abrogate its expression.4 This has suggested that, although Tax may have an initiating role in ATL, it could allow the accumulation of subsequent genetic changes that are the actual drivers of transformation.
We previously demonstrated, in human ATL-derived cell lines or murine ATL derived from Tax transgenics, the remarkable efficacy of a combined arsenic trioxide (arsenic, AS) and interferon-α (IFN) treatment.5,6 Moreover, in chronic ATL patients, the triple combination of AS, IFN, and the nucleotide analog zidovudine triggers complete and durable clinical remissions.7 At the cellular level, AS synergizes with IFN to induce cell cycle arrest and apoptosis ex vivo.6,8 At the molecular level, AS/IFN combination leads to Tax degradation through the proteasome, suggesting that the very selective apoptosis of HTLV-1-transformed cells could reflect the targeting of the Tax oncoprotein.6,9 Nevertheless, the role of Tax as an ATL driver, the actual contribution of its degradation to treatment response, and the biochemical mechanism of Tax loss remain disputed or unknown.
Arsenic-initiated Tax degradation is reminiscent of arsenic-triggered PML/RARA catabolism in acute promyelocytic leukemia (APL), one of the cornerstones of oncogene-targeted therapy.10 We and others have shown that PML nuclear bodies (NBs) play a central role in promyelocytic leukemia/retinoic acid receptor alpha (PML/RARA) degradation by AS, through PML/RARA oxidation, followed by its hyper-sumoylation, recruitment of the SUMO-targeted ubiquitin ligase RNF4, eventually leading to its proteasomal degradation.11-15 Interestingly, IFNs initiate a drastic transcriptional induction of the PML gene,16 whereas AS enforces PML NB nucleation through direct binding and oxidation.12,15,17 PML NBs facilitate the sumoylation of partner proteins through their recruitment together with UBC9.18 For at least some of these partners, this is followed by RNF4-mediated polyubiquitination and degradation. Finally, IFN enhances SUMO expression, further facilitating global sumoylation.19 Thus, the AS/IFN combination promotes hyper-sumoylation, ubiquitination, and degradation of a class of NB-associated proteins. Tax may also directly bind RNF4, raising the possibility that PML nuclear bodies may contribute to Tax catabolism and ATL therapy.20,21
Here, we establish that continued Tax expression is required for the survival of multiple ATL-derived or HTLV-1-transformed cell lines. On arsenic/interferon exposure, Tax is degraded through stepwise poly-sumoylation and SUMO-dependent ubiquitination that require both PML and RNF4. Thus, Tax is a key target in ATL specifically targeted by AS/IFN therapy.
Materials and methods
Cells, plasmids, siRNA, and short hairpin RNA
HeLa cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 2 mM glutamine, and antibiotics. Plasmid transfections were performed by Lipofectamine or Lipofectamine 2000 (Gibco, Invitrogen). small interfering RNA (siRNA) transfections were carried out using HiPerFect (QIAGEN) according to the manufacturer’s recommendations. ATL-derived (HuT-102, MT1 [gift from K. Ishitsuka] and HPB-ATL2 [gift from M. Matsuoka]) or HTLV-1-transformed (MT2 and C91PL) cells, as well as HTLV-I-negative CEM and Jurkat malignant T-cell lines, were maintained in RPMI medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, antibiotics, and 5% glucose (Sigma-Aldrich). Cells were transduced with murine stem cell virus green fluorescent protein (GFP)-lentiviral vectors encoding scrambled (SCR/CTRL) short hairpin RNA (shRNA) or shRNA against PML (sequence available on request) or different shRNA constructs against Tax (sh1-Tax: CTCAGCTCTACAGTTCCTTAT, sh2-Tax:CAGGCCTTATTTGGACATTTA, sh3-Tax: ACGGCCTCATACAGTACTCTT). Lentiviruses were produced by transient transfections of HEK-293T cells. Infection of target cells was performed by spinoculation. PSG5M-Tax lysine mutants Tax K4-8R and Tax K1-3R, in which 5 or 3 lysines, respectively, were substituted for arginines, have been described previously.20 pCDNA3-FLAG-RNF4 is a gift from J. Palvimo (University of Kuopio, Kuopio, Finland). The following siRNAs were used: SUMO1 (ggacaggauagcagugaga–dTdT), SUMO2 (agggaugaaucuguaacuuaa–dTdT), SUMO3 (gaggcauacaccacuuagu–dTdT), RNF4(2) (aaccaacauctgauauguaaa–dTdT), RNF4(3) (ucccaucugcauggacggaua–dTdT), RNF4(4) (cccuguuuccuaagaacgaaa–dTdT), or control siRNA (siCTRL) directed against p75/LEDGF (aacagcuacauuauacaguaa–dTdT). RNF4(1) was a commercial pool of 4 siRNAs (Dharmacon; reference L-006557-00) validated and provided by R. Hay (University of Dundee). Tax transfection was performed 24 hours after siRNA transfection.
Treatments
Arsenic was obtained from Sigma-Aldrich, and recombinant human IFN-α (Roferon) was obtained from Roche. The proteasome inhibitor MG132 was purchased from Calbiochem. Arsenic was used at 1 μM for ATL cells and 2 μM for HeLa cells. IFN was used at 1000 IU/mL, and MG132 was used at 1 μM.
Immunofluorescence, in situ proximity ligation assays (Duolink), and confocal microscopy
ATL-derived or HTLV-1-transformed cells were fixed onto glass slides by cytospin (5 minutes, 1000g) and then fixed with methanol at 20°C. Protein-protein interactions were visualized using the Duolink in situ proximity ligation assay (PLA) system (Olink Bioscience). Assays were performed using anti-Tax and anti-SUMO1, anti-SUMO2/3, anti-PML, or anti-RNF4 primary antibodies, following the manufacturer's instructions. Images were acquired by confocal microscopy using either a Zeiss LSM 510 META confocal laser microscope or a Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany) with a Plan Apochromat 63/1.4 numeric aperture oil-immersion objective using Zen 2009 (Carl Zeiss). High-resolution images were obtained with a deconvolution program (Autodeblur; Image Quant), using blind iterative algorithms.
Antibodies
Tax was detected using the monoclonal antibody 168-A51 (National Institutes of Health AIDS Research and Reference Reagent Program). A homemade chicken polyclonal antibody directed against PML was used at a dilution of 1:1000 for immunofluorescence microscopy and western blotting. SUMO1 protein was revealed using a mouse monoclonal or a rabbit polyclonal anti-SUMO1 antibody purchased from Santa Cruz Biotechnology. Polyclonal anti-SUMO2/3 antibodies were purchased from Invitrogen or Santa Cruz. Poly-ubiquitinated proteins were revealed using the FK2 mouse monoclonal antibody from BIOMOL International. Mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase, rabbit polyclonal anti-actin, and mouse monoclonal anti-vinculin antibodies were from Sigma, and mouse monoclonal anti-GFP antibody was from Roche Applied Science. Rabbit polyclonal anti-RNF4 antibody was a gift from J. Palvimo. For confocal microscopy, primary antibodies were revealed by Alexa-Fluor 488- or 594-labeled secondary antibodies (1:500) from Molecular Probes or horseradish peroxidase-conjugated secondary antibodies from Jackson.
Immuno-precipitations and protein analyses
For immuno-precipitation of Tax conjugates, cells were washed in ice-cold phosphate-buffered saline supplemented with 10 mM N-ethylmaleimide prior to lysis in 2% sodium dodecyl sulfate and 50 mM Tris, pH 8. After brief sonication, cell lysates were diluted 10-fold in immuno-precipitation (IP) buffer containing 50 mM Tris, pH 8, 200 mM NaCl, 0.1 mM EDTA, 0.5% NP-40, 10% glycerol, and protease inhibitors. Lysates were incubated for 2 hours at 4°C with the Tax antibody (described above), followed by incubation with protein A-agarose for 2 hours. Beads were washed 3 times in the IP buffer prior to elution of immuno-precipitated proteins in sample buffer. SUMO conjugates and whole cell extracts (WCEs) were separated on 4% to 12% gradient gels (Invitrogen) or 7% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels.
Results
Tax expression is required for HTLV-1-transformed cell survival in culture
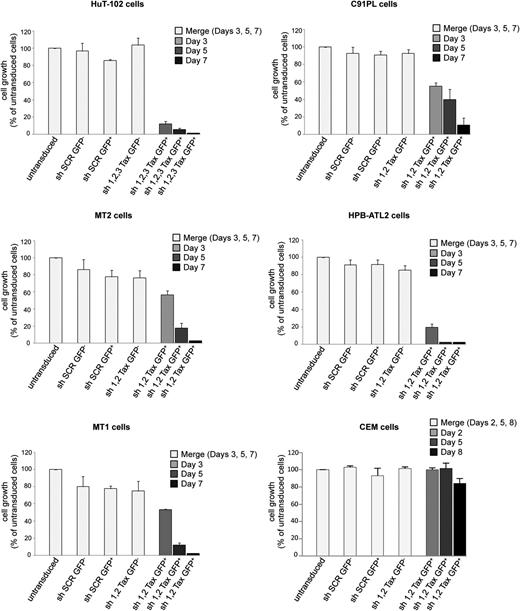
To demonstrate that HTLV-1-transformed cells depend on Tax for their survival, we transduced 5 cell lines with 2 or 3 lentiviral constructs expressing shRNAs specifically targeting Tax, one at a time. Three cell lines (HuT-102, HPB-ATL2, and MT1) were derived from ATL patients, whereas C91PL and MT2 are HTLV-1-transformed cell lines.4,22-25 They all expressed Tax transcript and displayed levels of Tax protein ranging from undetectable (MT1) to intermediate (MT2) and high (HuT-102, C91PL, HPB-ATL2) (supplemental Figure 1A-B, available on the Blood Web site; data not shown). Strikingly, extinction of Tax expression by 2 (C91PL, HPB-ATL2, MT1, and MT2) or 3 (HuT-102) independent shRNA constructs individually consistently resulted in growth arrest/apoptosis of ATL-derived and HTLV-1-transformed cells (Figure 1). Nontargeting (scrambled) sequences were ineffective, and uninfected cells (GFP negative) were unaffected (Figure 1; supplemental Figure 1C). Importantly, expression of Tax shRNAs did not affect growth of HTLV-1-negative CEM (Figure 1) and Jurkat (not shown) malignant T-cell lines, excluding off-target effects. Thus, HTLV-1-transformed cells are addicted to continuous Tax expression for their survival, indirectly implicating Tax degradation in the therapeutic efficacy of the AS/IFN combination.
Knockdown of the HTLV-1 oncoprotein Tax in HTLV-1-transformed cells abrogates proliferation. ATL-derived (HuT-102, HPB-ATL2, and MT1) or HTLV-1-transformed cell lines (C91PL and MT2) were transduced using GFP-lentiviral vectors encoding SCR shRNA or 2 (HPB-ATL2, MT1, C91PL, and MT2) or 3 (HuT-102) different shRNAs against Tax (sh1-Tax, sh2-Tax, and sh3-Tax). Growth of transduced GFP+ or untransduced GFP− sorted cells, as well as of CEM cells (HTLV-1 negative T-cell line), was assessed by cell count using trypan blue staining up to 7 days after sorting. For simplicity, the results obtained from independent experiments using different individual shRNAs were pooled together.
Knockdown of the HTLV-1 oncoprotein Tax in HTLV-1-transformed cells abrogates proliferation. ATL-derived (HuT-102, HPB-ATL2, and MT1) or HTLV-1-transformed cell lines (C91PL and MT2) were transduced using GFP-lentiviral vectors encoding SCR shRNA or 2 (HPB-ATL2, MT1, C91PL, and MT2) or 3 (HuT-102) different shRNAs against Tax (sh1-Tax, sh2-Tax, and sh3-Tax). Growth of transduced GFP+ or untransduced GFP− sorted cells, as well as of CEM cells (HTLV-1 negative T-cell line), was assessed by cell count using trypan blue staining up to 7 days after sorting. For simplicity, the results obtained from independent experiments using different individual shRNAs were pooled together.
Tax undergoes poly-sumoylation on AS/IFN exposure
Although significant degradation may be observed with arsenic alone, particularly in HPB-ATL2 cells, only the AS/IFN combination resulted in complete degradation of endogenous Tax protein in ATL-derived or HTLV-1-transformed cells (Figure 2A, upper; supplemental Figure 2A). Similarly, ectopically expressed Tax was efficiently degraded in HeLa cells exposed to both AS and IFN (Figure 2A, lower; supplemental Figure 2B). Unlike HTLV-1-transformed cells, HeLa cells transiently expressing Tax do not undergo AS/IFN-induced apoptosis (data not shown). Thus, in HTLV-1-transformed cells, Tax degradation by AS/IFN is not a mere consequence of apoptosis but most likely initiates it.
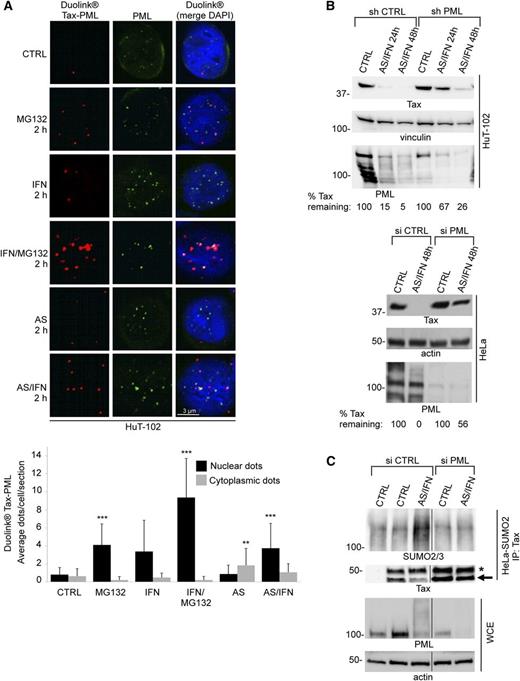
Tax undergoes poly-sumoylation on arsenic/interferon exposure. (A) AS/IFN-induced Tax degradation in (upper) HuT-102 and (lower) Tax-transfected HeLa cells. (B) AS/IFN-induced HMW modifications of Tax (bracket) transiently expressed in HeLa cells. (C) AS/IFN-triggered Tax poly-sumoylation detected by immunoprecipitation of Tax transiently expressed in (left) HeLa-SUMO1 or (right) HeLa-SUMO2 cells followed by western blot using indicated antibodies. IgG, isotypic control antibody. (D) Tax-transfected HeLa cells were cotransfected with control siRNA (siCTRL) or siRNA against SUMO1 (siS1), SUMO2&3 (siS2/3), or SUMO1&2&3 (siS1/2/3). Cells were subsequently treated with AS/IFN for 48 hours. Western blot analysis with indicated antibodies. Densitometric quantification of remaining Tax after treatment is shown. (E) (left) Endogenous Tax-SUMO1 interactions detected by Duolink in AS/IFN-treated (12 hours) HuT-102 cells. Nuclei were stained with 4,6 diamidino-2-phenylindole (DAPI; blue). (Right) Graphs show quantification of nuclear or cytoplasmic Duolink dots, indicative of interaction signals. Averages of 1 Z-section per cell from 30 different cells; significant P values are indicated by asterisks. (F) Endogenous Tax-SUMO2/3 interactions in HuT-102 cells, as in E.
Tax undergoes poly-sumoylation on arsenic/interferon exposure. (A) AS/IFN-induced Tax degradation in (upper) HuT-102 and (lower) Tax-transfected HeLa cells. (B) AS/IFN-induced HMW modifications of Tax (bracket) transiently expressed in HeLa cells. (C) AS/IFN-triggered Tax poly-sumoylation detected by immunoprecipitation of Tax transiently expressed in (left) HeLa-SUMO1 or (right) HeLa-SUMO2 cells followed by western blot using indicated antibodies. IgG, isotypic control antibody. (D) Tax-transfected HeLa cells were cotransfected with control siRNA (siCTRL) or siRNA against SUMO1 (siS1), SUMO2&3 (siS2/3), or SUMO1&2&3 (siS1/2/3). Cells were subsequently treated with AS/IFN for 48 hours. Western blot analysis with indicated antibodies. Densitometric quantification of remaining Tax after treatment is shown. (E) (left) Endogenous Tax-SUMO1 interactions detected by Duolink in AS/IFN-treated (12 hours) HuT-102 cells. Nuclei were stained with 4,6 diamidino-2-phenylindole (DAPI; blue). (Right) Graphs show quantification of nuclear or cytoplasmic Duolink dots, indicative of interaction signals. Averages of 1 Z-section per cell from 30 different cells; significant P values are indicated by asterisks. (F) Endogenous Tax-SUMO2/3 interactions in HuT-102 cells, as in E.
Molecularly, Tax degradation was accompanied by formation of high-molecular-weight (HMW) species, reminiscent of poly-ubiquitinated and/or poly-sumoylated protein conjugates (Figure 2B, bracket). To address the nature of these HMW Tax species, we immuno-precipitated ectopically expressed Tax from AS/IFN-treated HeLa cells stably expressing His-SUMO1 (HeLa-SUMO1) or His-SUMO2/3 (HeLa-SUMO2). Remarkably, in response to a short (24 hours) AS/IFN treatment, Tax underwent massive hyper-conjugation by SUMO2/3 and, to a much lesser extent, by SUMO1 (Figure 2C).
Tax lysines 7-8 and 4-8 are targets of sumoylation and ubiquitination, respectively.20 These modifications control key Tax functions, including kappa B kinase inhibitor binding, transcriptional activation, and subcellular localization.20,26 We transfected HeLa cells with wild-type Tax or 2 lysine mutants: Tax K1-3R or Tax K4-8R. Although the AS/IFN combination efficiently degraded wild-type Tax and Tax K1-3R, it failed to degrade the Tax K4-8R mutant, which is defective for both sumoylation and ubiquitination (supplemental Figure 2C). We then performed siRNA-mediated knockdown of SUMOs in HeLa cells transiently expressing Tax. Extinction of all 3 SUMO paralogs blunted the ability of the AS/IFN combination to trigger Tax degradation, whereas individual knockdown of SUMOs had only a marginal effect (Figure 2D). Conversely, stable overexpression of either SUMO1 or SUMO2/3 significantly accelerated AS/IFN-induced Tax loss (supplemental Figure 2D), suggesting that IFN-enhanced SUMO abundance contributes to accelerate Tax degradation. Altogether, these results demonstrate that the AS/IFN combination induces poly-sumoylation of Tax, which is required for subsequent Tax degradation.
We next analyzed endogenous Tax in HuT-102 ATL-derived cells using proximity ligation Duolink assays (PLA) to assess where SUMO conjugation occurs and to investigate any paralog-specific conjugation. Importantly, baseline Tax-SUMO1 interactions were detected in untreated cells, primarily in the nucleus (Figure 2E), whereas Tax-SUMO2/3 interactions were undetectable (Figure 2F). However, on AS/IFN treatment, the Tax-SUMO1 nuclear interaction was lost (Figure 2E), whereas Tax-SUMO2/3 interactions were massively increased, initially in the nucleus (12 hours) and then shifted to the cytoplasm (18 hours) (Figure 2F; supplemental Figure 2E). Identical results were obtained in C91PL cells (supplemental Figure 3A-B). Thus, endogenous Tax has a baseline SUMO1 conjugation and AS/IFN triggers a massive shift toward SUMO2/3 conjugation, as observed above for transfected Tax (Figure 2C).
PML is required for Tax sumoylation and degradation
In transient transfections, Tax bodies were often juxtaposed with endogenous PML NBs. However, the 2 completely colocalized after IFN treatment (supplemental Figure 4A). Similarly, in HuT-102 cells, PLA experiments demonstrated a basal interaction between endogenous Tax and PML, which occurred in PML NBs and was massively induced by AS/IFN (Figure 3A). Tax-PML interactions were also greatly enhanced by the proteasome inhibitor MG132, particularly in the presence of IFN, all suggesting that Tax undergoes basal catabolism within NBs (Figure 3A). Similar observations were made in C91PL cells (supplemental Figure 3C), strengthening our conclusions.
PML is required for Tax sumoylation and degradation. (A) (upper) Endogenous Tax-PML interactions detected by Duolink in HuT-102 cells treated as indicated. PML was stained with anti-PML antibodies (green), and nuclei were stained with DAPI (blue). In the quantification below, significant P values are indicated by asterisks compared with untreated samples. (B) (upper) HuT-102 cells were transduced with nontargeting control shRNA (shCTRL) or shRNA against PML (shPML) and treated with AS/IFN for up to 48 hours. Degradation of endogenous Tax was analyzed by western blot. (Lower) HeLa cells transiently expressing Tax were treated as above. Percentages show quantification of remaining Tax at each time point after normalization to actin. (C) Tax-transfected HeLa-SUMO2 cells were cotransfected with either siCTRL or siPML. Tax immunoprecipitates (IP: Tax) were blotted against SUMO2/3 and Tax. Arrow points to the band corresponding to Tax, whereas asterisk denotes IgG heavy chain. First lane shows control IP with no antibody.
PML is required for Tax sumoylation and degradation. (A) (upper) Endogenous Tax-PML interactions detected by Duolink in HuT-102 cells treated as indicated. PML was stained with anti-PML antibodies (green), and nuclei were stained with DAPI (blue). In the quantification below, significant P values are indicated by asterisks compared with untreated samples. (B) (upper) HuT-102 cells were transduced with nontargeting control shRNA (shCTRL) or shRNA against PML (shPML) and treated with AS/IFN for up to 48 hours. Degradation of endogenous Tax was analyzed by western blot. (Lower) HeLa cells transiently expressing Tax were treated as above. Percentages show quantification of remaining Tax at each time point after normalization to actin. (C) Tax-transfected HeLa-SUMO2 cells were cotransfected with either siCTRL or siPML. Tax immunoprecipitates (IP: Tax) were blotted against SUMO2/3 and Tax. Arrow points to the band corresponding to Tax, whereas asterisk denotes IgG heavy chain. First lane shows control IP with no antibody.
We then silenced PML expression to test whether PML contributes to AS/IFN-induced Tax degradation. In HuT-102 cells, shRNA-mediated PML extinction sharply delayed AS/IFN-triggered Tax degradation (Figure 3B, upper). Similar results were obtained when analyzing ectopically expressed Tax in HeLa cells (Figure 3B, lower) and endogenous Tax in C91PL cells (data not shown). Thus, Tax degradation is facilitated by PML.
We then assessed whether PML promotes Tax degradation by enhancing its SUMO2/3 conjugation by immuno-precipitating transiently transfected Tax from HeLa-SUMO2 cells, with or without PML extinction. The latter not only antagonized Tax degradation, as demonstrated above, but also greatly diminished the extent of SUMO2/3 modification (Figure 3C). Collectively, our data suggest that PML NBs promote SUMO2/3 conjugation of Tax within NBs on AS/IFN treatment.
RNF4 ubiquitinates poly-sumoylated Tax
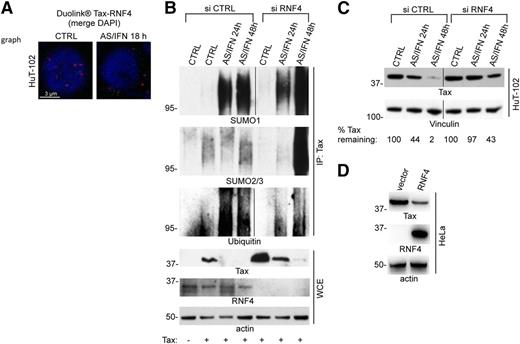
SUMO2/3 forms chains that may be recognized by SUMO-targeted ubiquitin ligases, such as RNF4,13,14 and Tax also directly binds RNF4.27 Using PLA, we indeed observed both in HuT-102 and C91PL cells, a basal nuclear RNF4 interaction with Tax (Figure 4A; supplemental Figure 3D). On AS/IFN exposure, the interaction signals became predominantly cytoplasmic (Figure 4A; supplemental Figure 3D).
RNF4 degrades poly-sumoylated Tax. (A) Endogenous Tax-RNF4 interactions in untreated or AS/IFN-treated HuT-102 cells as detected by Duolink. Nuclei were stained with DAPI (blue). (B) HeLa cells transiently expressing Tax were cotransfected with siCTRL or siRNA against RNF4 (siRNF4). (Upper) After treatment with AS/IFN for up to 48 hours, cell extracts were immune-precipitated with anti-Tax antibodies, followed by western blot with indicated antibodies. (Lower) Tax, RNF4, and actin are shown in WCE. (C) HuT-102 cells were transfected with siCTRL or siRNF4 and treated with AS/IFN for up to 48 hours. Degradation of endogenous Tax was analyzed by western blot. Vinculin serves as a loading control. Quantification in percentages shows the amount of remaining Tax after normalization. (D) HeLa cells transiently expressing Tax were cotransfected with RNF4. Western blot was performed with indicated antibodies 48 hours after transfection.
RNF4 degrades poly-sumoylated Tax. (A) Endogenous Tax-RNF4 interactions in untreated or AS/IFN-treated HuT-102 cells as detected by Duolink. Nuclei were stained with DAPI (blue). (B) HeLa cells transiently expressing Tax were cotransfected with siCTRL or siRNA against RNF4 (siRNF4). (Upper) After treatment with AS/IFN for up to 48 hours, cell extracts were immune-precipitated with anti-Tax antibodies, followed by western blot with indicated antibodies. (Lower) Tax, RNF4, and actin are shown in WCE. (C) HuT-102 cells were transfected with siCTRL or siRNF4 and treated with AS/IFN for up to 48 hours. Degradation of endogenous Tax was analyzed by western blot. Vinculin serves as a loading control. Quantification in percentages shows the amount of remaining Tax after normalization. (D) HeLa cells transiently expressing Tax were cotransfected with RNF4. Western blot was performed with indicated antibodies 48 hours after transfection.
To directly assess any role of RNF4 in promoting Tax ubiquitination, we immuno-precipitated transfected Tax from AS/IFN-treated HeLa cells, with or without RNF4 extinction. This demonstrated that AS/IFN promotes massive Tax ubiquitination (Figure 4B). RNF4 extinction antagonized Tax degradation, dramatically stabilized Tax-SUMO2/3 conjugates, and sharply reduced Tax-ubiquitin species (Figure 4B), implying that Tax is a RNF4 substrate. Indeed, extinction of RNF4 in HuT-102 cells inhibited AS/IFN-mediated degradation of endogenous Tax (Figure 4C). Conversely, RNF4 overexpression destabilized transfected Tax in HeLa cells (Figure 4D). Tax sumoylation promotes its export to the cytoplasm.21 Thus, hyper-sumoylated Tax molecules may be degraded both in NBs and in the cytoplasm (Figure 5; supplemental Figures 2B and 3E), as PLA demonstrated cytoplasmic interactions between Tax and the 20S proteasome (supplemental Figure 4B). Collectively, our studies imply that AS/IFN combination enables PML NBs to hyper-sumoylate Tax by SUMO2/3, triggering RNF4-mediated degradation.
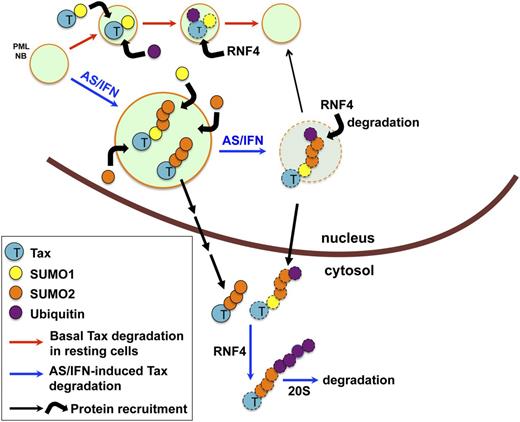
AS/IFN-induced Tax degradation involves NB-driven hyper-sumoylation. In untreated cells, Tax may be primarily SUMO1 conjugated and is mostly nuclear. Tax is rapidly turned over in NBs (upper pathway, red arrows). Treatment with AS/IFN dramatically enhances NB formation, promoting further Tax recruitment into NBs and its poly-sumoylation by SUMO2/3. SUMO2/3-conjugated Tax is poly-ubiquitinated by RNF4 and marked for degradation. SUMO-conjugated Tax can also be exported into cytoplasm, where degradation of the remaining molecules takes place (lower pathway, blue arrows).
AS/IFN-induced Tax degradation involves NB-driven hyper-sumoylation. In untreated cells, Tax may be primarily SUMO1 conjugated and is mostly nuclear. Tax is rapidly turned over in NBs (upper pathway, red arrows). Treatment with AS/IFN dramatically enhances NB formation, promoting further Tax recruitment into NBs and its poly-sumoylation by SUMO2/3. SUMO2/3-conjugated Tax is poly-ubiquitinated by RNF4 and marked for degradation. SUMO-conjugated Tax can also be exported into cytoplasm, where degradation of the remaining molecules takes place (lower pathway, blue arrows).
Discussion
That continuous Tax expression is required to sustain the established HTLV-1-transformed cells remains controversial.28,29 Here, we demonstrate in several cell lines that HTLV-1-transformed cells remain strictly dependent on continued Tax expression for their survival in culture. Extinction of HTLV-1 HBZ in SLB-1 T cells modestly reduces their proliferation,30 whereas Tax silencing induces cell death (Figure 1). Such addiction to Tax implies that, similar to the effect of AS on PML/RARA in APL, AS/IFN-initiated Tax degradation is responsible for its clinical efficiency in ATL.5,7,10,31-33 Remarkably, the biochemical pathways involved in therapy-induced Tax and PML/RARA degradation are highly similar.
Evidence has been presented that Tax protein expression is no longer detectable in established ATLs.4 However, with regulatory proteins that are expressed at very low levels, sensitivity of the detection method always remains an issue. For example, in Tax-transgenic mice, although Tax mRNA is present, the protein remains undetectable in the transformed T cells. Similarly, in MT1 cells, Tax protein is undetectable, although the mRNA is clearly expressed (supplemental Figure 1A-B). Importantly, extinction of Tax impairs MT1 cell survival (Figure 1), suggesting that even extremely low Tax protein expression suffices to sustain HTLV-1-transformed cell survival. Tax expression in ATL is also suggested by the existence of Tax specific antibodies, as well as the presence of Tax-specific CD8+ T cells, in ATL patients.34,35 It is possible that in vivo Tax is only expressed in a subset of ATL cells, possibly those in niches protected from the immune system, providing a constant reservoir of leukemia-initiating cells (LICs). Indeed, although Tax protein is not readily detectable in many freshly harvested cells from HTLV-1-infected patients, it is significantly upregulated on culture.36 Genetic studies suggested that 30% primary ATL cells cannot express Tax due to deletions or premature stop codons.4 Although the virus may circumvent this by aberrant splicing or creation of altered Tax proteins that retain some biological function, some of these ATL cells may have actually evolved to Tax independence and are expected to be resistant to AS/IFN therapy.
Because Tax degradation by the AS/IFN combination drives ATL clinical response, unraveling its biochemical mechanism is important. PML NBs function as in situ partner sumoylation and degradation machineries.18,37 IFN not only induces PML expression and NB biogenesis, but also dramatically enhances SUMO availability.19 Arsenic triggers PML NB aggregation, followed by sequential partner recruitment, sumoylation, and degradation.11-13,17,38 We proposed that IFN and AS could cooperate to promote NB-associated degradation of partner proteins.39,40 Neurotoxic poly-glutamine proteins were indeed recently shown to be highly sensitive to this degradation pathway.41 Moreover, the corresponding neurological disease is partially IFN reversible in mice, strongly suggestive for in vivo activation of this catabolic machinery.42,43 Here we demonstrate that Tax is also degraded by this SUMO/PML/RNF4/proteasome pathway. Tax/PML PLA interactions in HTLV-1-transformed cells are massively increased on proteasome inhibition, strongly suggestive of basal Tax turnover within NBs. On treatment with AS/IFN, Tax is recruited onto PML NBs, most likely through its 4 SUMO interaction motifs (Figure 5; supplemental Figure 4C).18 Tax recruitment onto NBs results in a PML-dependent increase in SUMO2/3 conjugation (Figures 2F and 3C), RNF4 recruitment, and degradation (Figure 5). It is particularly interesting that IFN and arsenic promote a shift of Tax conjugation from SUMO1 to SUMO2/3, a point that deserves further mechanistic exploration. However, both SUMO1 and SUMO2/3 appear necessary for Tax catabolism, possibly through PML or RNF4 SUMO1 conjugation. In any case, our results draw a striking parallelism between Tax and PML/RARA degradation.
When used in ATL patients after chemotherapy, the combination of AS and IFN converted partial response to durable complete responses (Ambroise Marçais, Felipe Suarez and Olivier Hermine, unpublished data, 2014). This suggests that, as demonstrated in mice, AS/IFN targets ATL LICs in ATL patients.5 In APL, we recently demonstrated the key role of PML NB reformation in enforcing p53 activation and a senescence-like process, which is ultimately responsible for cure.31 Interestingly, p53 mutations were associated to therapy resistance in ATL patients treated with IFN,44 supporting the idea that therapy may first degrade the driving Tax oncoprotein but may also activate this PML/p53 axis to eradicate the disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Beirut laboratory was supported by the American University of Beirut Medical Practice Plan and University Research Board, the Lebanese National Council for Scientific Research, the Qatar National Research Fund, and the Lady Tata Memorial Trust. The Paris laboratory was supported by the Ligue Nationale contre le Cancer, INSERM, Centre National de la Recherche Scientifique, University Paris Diderot, Collège de France, Institut Universitaire de France, Institut National du Cancer, the Association pour la Recherche contre le Cancer (Prix Griffuel), and the European Research Council (Senior Grant 268729–STEMAPL) (to H.d.T.). U.S. was supported by a fellowship from the Fondation pour la Recherche Médicale.
Authorship
Contribution: Z.D., U.S., H.E.H., and F.J. performed experiments; Z.D., U.S., H.E.H., F.J., Y.K., V.L.-B., O.H., H.d.T., and A.B. analyzed results; Z.D. and U.S. made the figures; A.B. and H.d.T. designed the research; and U.S., A.B., and H.d.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Bazarbachi, American University of Beirut, Medical Center, PO Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb; H. de Thé, INSERM U944 Hôpital St. Louis 1, Avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: hugues.dethe@inserm.fr; or U. Sahin, INSERM U944 Hôpital St. Louis 1, Avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: umut.sahin@inserm.fr.
References
Author notes
Z.D and U.S. contributed equally to this work.
H.d.T. and A.B. contributed equally to this work.