Key Points
CD1c+ DCs differentiate into Langerhans cells in response to GM-CSF, TGFβ, and BMP7.
CD14+ monocytes express low langerin but do not make Langerhans cells under the same conditions.
Abstract
Langerhans cells (LCs) are self-renewing in the steady state but repopulated by myeloid precursors after injury. Human monocytes give rise to langerin-positive cells in vitro, suggesting a potential precursor role. However, differentiation experiments with human lineage-negative cells and CD34+ progenitors suggest that there is an alternative monocyte-independent pathway of LC differentiation. Recent data in mice also show long-term repopulation of the LC compartment with alternative myeloid precursors. Here we show that, although monocytes are able to express langerin, when cultured with soluble ligands granulocyte macrophage colony-stimulating factor (GM-CSF), transforming growth factor β (TGFβ), and bone morphogenetic protein 7 (BMP7), CD1c+ dendritic cells (DCs) become much more LC-like with high langerin, Birbeck granules, EpCAM, and E-cadherin expression under the same conditions. These data highlight a new potential precursor function of CD1c+ DCs and demonstrate an alternative pathway of LC differentiation that may have relevance in vivo.
Introduction
Langerhans cells (LCs) are antigen-presenting cells of the epidermis that are self-renewing in the steady state1 but recruited from blood-borne precursors after inflammation.2-4 Observations in humans also confirm that LCs can be self-maintained5-7 or replaced by bone marrow–derived cells in the context of transplantation and inflammation.7-10
The nature of bone marrow–derived LC precursors that repopulate the epidermis after inflammation is incompletely defined. Experiments in mice with clodronate depletion and bead-labeling suggest a monocyte origin but do not completely exclude other precursors.2 More recent observations in mice lacking LCs as a result of Id2 knockout or conditional ablation show that LC repopulation occurs in 2 waves.3,4 The epidermis is initially infiltrated by a short-term precursor with low langerin expression and features in common with monocytes, followed by a long-term precursor that acquires the full phenotype of LCs, including self-renewal capacity.3,4
In humans, langerin+ cells can be made in vitro from monocytes.11-13 Experiments with CD34+ progenitors also demonstrate the existence of an LC-differentiation pathway that appears independent of CD14+ monocytes.14,15 A previous report suggested the existence of CD3/7/14/16/19-negative LC precursors in human blood and, although noted to express CD1c, these were incorrectly described as expressing CD1a.16,17 Here we show that CD1c+ blood dendritic cells (DCs) are alternative LC precursors that achieve higher levels of langerin, CD1a, and Birbeck granules than CD14+ monocytes when exposed to soluble factors known to drive LC differentiation.
Methods
Blood mononuclear cells were obtained from healthy volunteers under local ethical approval. CD14+ monocytes, CD16+ monocytes, CD1c+ DCs, and CD123+ plasmacytoid DCs were sorted from peripheral blood mononuclear cells (PBMCs). LCs were isolated from epidermal sheets separated from whole-skin keratome sections with dispase (Invitrogen) 1 mg/mL incubated at 37°C for 90 minutes in RPMI 1640 and subsequently digested with collagenase (Worthington Type IV) 1.6 mg/mL incubated for 12 hours at 37°C in RPMI 1640 with 10% fetal bovine serum. Sorting was performed with an ARIA Fusion (Becton Dickinson) using previously described protocols (supplemental Figure 1, available on the Blood Web site).18 Ten-thousand cells were cultured in RPMI 1640 with 10% fetal bovine serum or X-Vivo in 100 μL. Supplements were added at the following concentrations: GM-CSF 50 ng/mL, TGFβ, 10 ng/mL, and BMP7 200 ng/mL. Cultures were maintained for 3 to 7 days and supplemented with fresh cytokines on day 4.
Flow cytometry analysis was performed with an FACS Canto (Becton Dickinson) using appropriate isotype controls. Antibodies were from Becton Dickinson unless stated otherwise (antigen fluorochrome clone): CD1a BV421 HI149, CD1c PeCy7 L161, CD3 FITC SK7, CD11b APC ICRF44, CD11c A700 B-ly6; CD11c APCCy7 Bu15, and CD14 BV650 M5E2 (all from Biolegend); CD14 ECD RMO52 (Beckman Coulter); CD16 APCCy7 3G8; CD19 FITC 4G7; CD34 FITC 8G12; CD20 FITC L27; CD56 FITC NCAM16.2; CD83 FITC HB15e; CD123 PerCPCY5.5 7G3; CD207 PE DCGM4 (Beckman Coulter); E-Cadherin APC 67A4 (Biolegend); EpCAM APC EBA-1; and HLA-DR V500 G46-6.
Cells were fixed for electron microscopy (EM) according to standard protocols in 2% glutaraldehyde, and then pelleted, dehydrated, and fixed in resin (reagents from TAAB Laboratory, Aldermarston, UK). Ultrathin sections were cut with a diamond knife RMC MT-XL ultramicrotome and examined with a Philips CM 100 Compustage (FEI) Transmission Electron Microscope. Images were collected with an AMT CCD camera (Deben).
Results and discussion
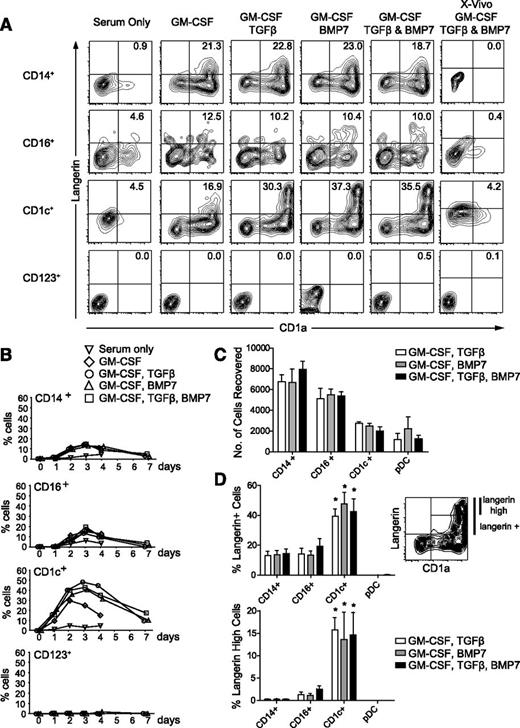
DC and monocyte fractions of human PBMCs were cultured in conditions that induce langerin in progenitor cells, including BMP7.19-23 This induced a rapid upregulation of langerin and CD1a dual expression by CD1c+ DCs, peaking within 3 days (Figure 1A-B). Langerin+ cells also appeared in CD14+ and CD16+ monocytes, but the level of expression was lower and did not increase further by day 7. Cells did not expand in culture, and approximately 60% to 70% of monocytes and 20% to 30% of CD1c+ DCs were recovered after 7 days (Figure 1C). Induction of a CD1a+ langerinhigh population was restricted to CD1c DCs treated with TGFβ, BMP7, or both (Figure 1D). No significant synergy was observed between TGFβ and BMP7, in contrast to recent results obtained with TGFβ and thymic stromal lymphopoietin (TSLP).24 No langerin induction was seen in medium with serum alone, but serum-free medium with supplements also failed to induce any expression, in contrast to results obtained with CD34+ progenitor cells.20,23 Exhaustive testing of different serum-free media was not conducted.
Expression of langerin and CD1a by monocytes and DCs. (A) Sorted cells cultured for 3 days in conditions as indicated showing expression of CD1a and extracellular langerin. The experiment was repeated 5 times except for the panels with X-Vivo, which were repeated 3 times. Four subsets of cells were collected from 1 donor, different in each experiment. (B) Time course of expression of CD1a and langerin double-positive cells showing a peak at 3 days and gradual decline in the percentage of positive cells up to 7 days of culture. Mean ± SEM of 5 experiments with different donors. (C) Recovery of viable cells under each condition at 7 days of culture, estimated by the total number of 4,6 diamidino-2-phenylindole (DAPI)–negative cells recorded when the culture was analyzed and run to dryness on the cytometer. Ten-thousand cells were added to each well as counted by the sorter but typically resulted in 6000 to 8000 viable cells at the start of the culture. There were no statistically significant differences between each condition for a given subset of cells. (D) Upper plot: percentage of langerin+ cells derived from GM-CSF+TGFβ (open bars), GM-CSF+BMP7 (gray bars), and GM-CSF+TGFβ and BMP7 (black bars) after 3 days of culture. Lower plot: percentage of langerinhigh cells derived from GM-CSF+TGFβ (open bars), GM-CSF+BMP7 (gray bars), and GM-CSF+TGFβ and BMP7 (black bars) after 3 days of culture. Mean ± SEM of 5 experiments with different donors. Gating of langerin+ and langerinhigh cells is illustrated using CD1c+ DCs incubated with GM-CSF and BMP7 as an example. There were no statistically significant differences between each condition for a given subset of cells. *P < .01 compared with corresponding CD14+ monocyte culture. Differences in langerin induction between monocyte subsets and pDCs for a given culture condition were not significant.
Expression of langerin and CD1a by monocytes and DCs. (A) Sorted cells cultured for 3 days in conditions as indicated showing expression of CD1a and extracellular langerin. The experiment was repeated 5 times except for the panels with X-Vivo, which were repeated 3 times. Four subsets of cells were collected from 1 donor, different in each experiment. (B) Time course of expression of CD1a and langerin double-positive cells showing a peak at 3 days and gradual decline in the percentage of positive cells up to 7 days of culture. Mean ± SEM of 5 experiments with different donors. (C) Recovery of viable cells under each condition at 7 days of culture, estimated by the total number of 4,6 diamidino-2-phenylindole (DAPI)–negative cells recorded when the culture was analyzed and run to dryness on the cytometer. Ten-thousand cells were added to each well as counted by the sorter but typically resulted in 6000 to 8000 viable cells at the start of the culture. There were no statistically significant differences between each condition for a given subset of cells. (D) Upper plot: percentage of langerin+ cells derived from GM-CSF+TGFβ (open bars), GM-CSF+BMP7 (gray bars), and GM-CSF+TGFβ and BMP7 (black bars) after 3 days of culture. Lower plot: percentage of langerinhigh cells derived from GM-CSF+TGFβ (open bars), GM-CSF+BMP7 (gray bars), and GM-CSF+TGFβ and BMP7 (black bars) after 3 days of culture. Mean ± SEM of 5 experiments with different donors. Gating of langerin+ and langerinhigh cells is illustrated using CD1c+ DCs incubated with GM-CSF and BMP7 as an example. There were no statistically significant differences between each condition for a given subset of cells. *P < .01 compared with corresponding CD14+ monocyte culture. Differences in langerin induction between monocyte subsets and pDCs for a given culture condition were not significant.
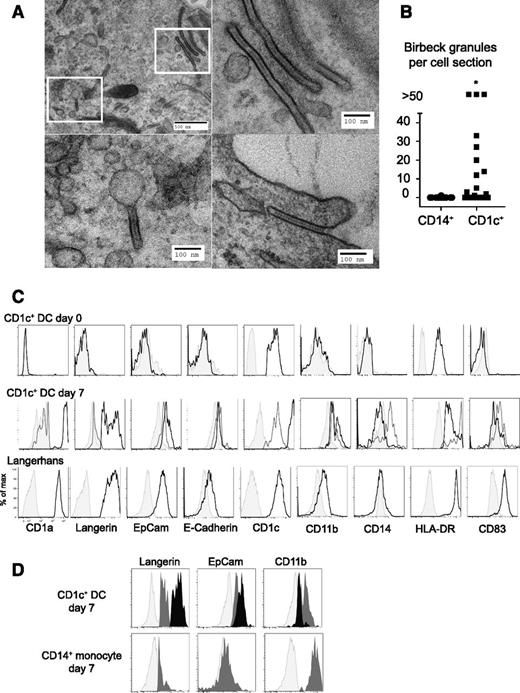
Day 7 cultures of CD1c+ DCs and CD14+ monocytes treated with GM-CSF and BMP7 were harvested for EM (all cells). Birbeck granules were observed in 12 of 20 CD1c+ ultrathin sections of CD1c+ DCs, with many sections containing >10 granules. In contrast, Birbeck granules were rarely found in cultures of CD14+ monocytes (a single granule in 20 cell sections) (Figure 2A-B). CD1c+ DCs developed similar cytologic appearance to freshly isolated LCs (supplemental Figure 2). Extended phenotyping showed induction of EpCAM and E-cadherin, and low expression of CD11b, similar to primary LCs. Langerin+ CD1c+ DCs remained immature as assessed by the expression of HLA-DR and CD83 (Figure 2C). The expression of EpCAM was highest on langerinhigh cells derived from CD1c+ DCs; in contrast, langerin+ cells derived from CD14+ monocytes had low EpCAM and higher CD11b expression (Figure 2D).
Formation of Birbeck granules in cultures of CD1c+ DCs. (A) EM images of Birbeck granules in CD1c+ DCs cultured for 7 days showing classical “tennis racket” morphology, pentalaminar structure, and formation by endocytosis. Insets in the top left panel are displayed beneath and to the right. The experiment was performed twice with 2 different donors only with GM-CSF and BMP7. The entire culture was processed for EM. (B) Comparison of the number of Birbeck granules per cell section between CD14+ monocytes and CD1c+ DC cultures. *P < .01. (C) Extended phenotype of LC-like cells (CD1c+) compared with primary Langerhans cells (Langerhans). Shaded histograms, isotype controls; gray line, CD1a and langerin double-negative cells; black line, CD1a+ langerin+ cells. Similar results were obtained with GM-CSF and TGFβ, BMP7, or a combination of both in 3 different donors; 1 of 3 experiments performed with GM-CSF, TGFβ, and BMP7 is shown. (D) Comparison of langerin, EpCAM, and CD11b expression in CD1c+ DCs and CD14+ monocytes. Light gray, isotype; midgray, CD1a+ langerinlow cells; black, CD1a+ langerinhigh cells. One of three experiments performed with GM-CSF, TGFβ, and BMP7 is shown.
Formation of Birbeck granules in cultures of CD1c+ DCs. (A) EM images of Birbeck granules in CD1c+ DCs cultured for 7 days showing classical “tennis racket” morphology, pentalaminar structure, and formation by endocytosis. Insets in the top left panel are displayed beneath and to the right. The experiment was performed twice with 2 different donors only with GM-CSF and BMP7. The entire culture was processed for EM. (B) Comparison of the number of Birbeck granules per cell section between CD14+ monocytes and CD1c+ DC cultures. *P < .01. (C) Extended phenotype of LC-like cells (CD1c+) compared with primary Langerhans cells (Langerhans). Shaded histograms, isotype controls; gray line, CD1a and langerin double-negative cells; black line, CD1a+ langerin+ cells. Similar results were obtained with GM-CSF and TGFβ, BMP7, or a combination of both in 3 different donors; 1 of 3 experiments performed with GM-CSF, TGFβ, and BMP7 is shown. (D) Comparison of langerin, EpCAM, and CD11b expression in CD1c+ DCs and CD14+ monocytes. Light gray, isotype; midgray, CD1a+ langerinlow cells; black, CD1a+ langerinhigh cells. One of three experiments performed with GM-CSF, TGFβ, and BMP7 is shown.
While this paper was under review, LC formation by CD1c+ DCs treated with TGFβ and TSLP was reported.24 Together, these results indicate that there are several pathways to the generation of langerin+ cells in humans and that, under the conditions tested, CD1c+ DCs but not CD14+ monocytes, generate langerinhigh CD1a+ EpCAM+ cells with Birbeck granules. Notably, monocytes prepared by negative selection contain CD1c+ DCs (supplemental Figure 3). It is therefore likely that CD1c+ DCs contribute significantly to LC development when these preparations are induced to differentiate.11
There are 2 limitations to this study. First, other conditions not tested here, such as Notch ligation, may be capable of inducing higher langerin in CD14+ monocytes.12,13 Birbeck granule formation was reported in monocytes exposed to Delta-1,12 although with Jagged, langerin expression did not exceed that observed with GM-CSF and TGFβ.13 Second, although the Birbeck granule is the ultrastructural hallmark of LC phenotype in vivo, the derivation of Birbeck granule–containing cells in vitro does not prove a precursor-progeny relationship. The expression of EpCAM and E-cadherin and low level of CD11b are consistent with an LC phenotype, but further expression profiling and functional studies would be required to evaluate fully the proximity of derived cells to primary LCs. It would be of interest to determine whether a similarly high capacity to crosspresent antigen is found in CD1c+ DC–derived LC-like cells, as shown by those obtained from CD34+ progenitors.21,22 However, it has been reported that crosspresentation is not as marked in freshly isolated primary LCs18 and may not be discriminatory for LC differentiation, because it is easily induced in CD1c+ DCs under other conditions.25
Although the cultures contained serum, as in other recent publications describing DC differentiation,24,26 the factors GM-CSF, TGFβ, and BMP7 are expressed by epithelial tissues under physiologic conditions.23 Previous experiments on CD34+ progenitors have highlighted the difference between a TGFβ-dependent pathway of LC generation from CD1a+ intermediates vs the TGFβ-independent generation of monocyte-derived DCs that are unable to express langerin.14,15 It is likely that this dichotomy reflects the differential ability of CD1c+ DCs and monocytes to generate LCs with high langerin, CD1a, EpCAM, and Birbeck granules that we now observe.
Recent data in mice describing 2 waves of repopulation of the LC compartment show an early monocytelike EpCAM– langerinlow wave succeeded by a myeloid precursor of unknown origin. In situ studies also show that LC repopulation after inflammation occurs with similar kinetics in humans.27 CD1c+ DCs have properties that make them candidates for the second-wave LC precursor. Potential homologs of the CD1c+ blood DC in mice such as the circulating pre-cDC may contain the long-term precursor of murine LCs.
Finally, these data explain the puzzling claim that there are CD1a+ “LC precursors” in human blood.16 Blood DCs were reported to express CD1a as a result of incorrect assignment of clone BB5 as a CD1a-specific antibody. It is now known that BB5 recognizes CD1b/c and not CD1a.17 The nonmonocyte cells in human blood that give rise to LCs in response to TGFβ and BMP7 in vitro are now clearly shown to be CD1c+ DCs.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tracey Davey and Newcastle University Electron Microscopy Research Services for assistance with electron microscopy.
This work was supported by Wellcome Trust grants WT088555MA (M.H.) and WT101155/Z/13/Z (V.B.), the Histiocytosis Research Trust, the Histiocytosis Association, and Bright Red (registered charity 1105891).
Authorship
Contribution: P.M. performed experiments and analyzed data; V.B. designed and performed experiments and analyzed data; M.G. performed experiments; M.H. designed experiments and analyzed data; and M.C. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Collin, Institute of Cellular Medicine, Newcastle University, Framlington Pl, Newcastle upon Tyne NE2 4HH, UK; e-mail: matthew.collin@ncl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal