In this issue of Blood, Milne et al1 report a developmental pathway for human Langerhans cells from circulating CD14−CD1c+ dendritic cell (DC) precursors in peripheral blood. The Langerhans cell progeny, expressing CD207 (langerin) and bearing the hallmark Birbeck granules, result from culture in granulocyte macrophage colony-stimulating factor (GM-CSF) and transforming growth factor-β1 (TGF-β1) and/or bone morphogenetic protein 7 (BMP7). A similar study by Martínez-Cingolani et al,2 also published recently in Blood, identified the same circulating CD14−CD1c+ Langerhans cell precursors. These authors used a different anti-CD1c monoclonal antibody (BDCA-1). In that study, TGF-β1 synergized with thymic stromal lymphopoietin (TSLP) to yield similar Langerhans cell progeny. Both groups used fetal calf serum (FCS), however, which affects differentiation and maturation by unknown mechanisms and is not physiologically comparable to human plasma in vivo. Controls with FCS-containing medium, but without cytokines, indicated that FCS was not the driver of the conversion. However, Milne et al1 could not generate Langerhans cell progeny by culture in X-Vivo medium without FCS, despite inclusion of GM-CSF, TGF-β1, and BMP7, all of which, together with TSLP, are epithelial cell–derived cytokines under physiologic conditions.
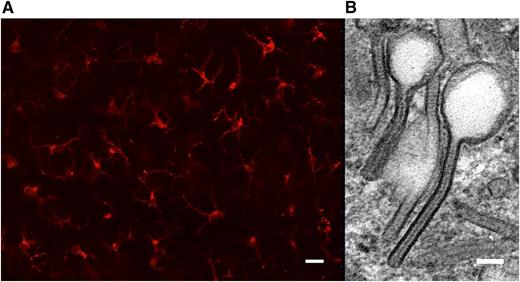
Langerin expression and Birbeck granules are hallmarks of Langerhans cells. (A) Conventional fluorescence microscopy of an epidermal sheet prepared from healthy human skin. Langerhans cells are immunolabeled with anti-CD207/langerin monoclonal antibody (Miltenyi Biotec). Scale bar, 30 µm. (B) Detail from a transmission electron micrograph of a human epidermal Langerhans cell in situ featuring 2 tennis racket–shaped Birbeck granules. Scale bar, 60 nm.
Langerin expression and Birbeck granules are hallmarks of Langerhans cells. (A) Conventional fluorescence microscopy of an epidermal sheet prepared from healthy human skin. Langerhans cells are immunolabeled with anti-CD207/langerin monoclonal antibody (Miltenyi Biotec). Scale bar, 30 µm. (B) Detail from a transmission electron micrograph of a human epidermal Langerhans cell in situ featuring 2 tennis racket–shaped Birbeck granules. Scale bar, 60 nm.
Residence in stratified epithelia, CD207 expression, and Birbeck granules all define bona fide Langerhans cells in humans (see figure). Indeed, the progeny of CD1c+ blood DCs express substantial CD207 after culture with either GM-CSF/TGF-β1/BMP71 or TGF-β1/TSLP.2 Birbeck granule morphology by electron microscopy is also convincing. Neither group evaluated the capacity to populate the epidermis, for which several methods exist using artificial skin equivalents, even though their Langerhans cell progeny express a number of skin-homing receptors like CCR6,2 EpCAM,1 or E-cadherin.1 Mouse studies have shown that Langerhans cell precursors only enter the epidermis before they express CD207, however. Hence the CD207-expressing CD1c+ progeny reported by these authors may already be too developed for such homing.
The work by Milne et al1 addresses another important issue. So-called monocyte-derived Langerhans cells, first reported by Geissmann et al,3 used negatively selected rather than positively selected precursors, which would have included the CD1c+ blood DC precursors now described by both groups. In fact, Milne et al1 demonstrate that positively selected CD14+ or CD16+ blood monocytes cultured under comparable conditions to CD1c+ DCs yield progeny with far less expression of CD1a, CD207, or Birbeck granules compared with Langerhans cell progeny derived from CD1c+ DCs. These data are also useful in explaining how a CD14− monocyte, intermediate between CD34+ hematopoietic progenitor cells and the final Langerhans cell progeny, can yield Langerhans cells. This avoids invoking a direct precursor–progeny connection with macrophages, as suggested by prior reports of CSF1 receptor (macrophage colony-stimulating factor receptor)-positive monocytic Langerhans cell precursors in mice, where the relevant cytokine proved to be interleukin-34 binding to CSF1-receptor, not macrophage colony-stimulating factor.4
Langerhans cells function as prototypic DCs, both in the induction of immunity5,6 and in the maintenance of tolerance.7 Murine Langerhans cells share a common embryonal origin from fetal liver and yolk sac with macrophages, microglia, and Kupffer cells.8 Human Langerhans cells were observed in human fetal epidermis as early as 9 weeks’ estimated gestational age.9 This is before the beginning of hematopoiesis in the bone marrow at 10.5 weeks’ estimated gestational age, suggesting that human Langerhans cells also derive from fetal hematopoietic tissues. Langerhans cells are very long-lived and maintain their steady-state population density (approximately 600/mm2) in the epidermis by low-level proliferation from local precursors.
In inflamed mouse skin, however, Langerhans cell replenishment of those that have migrated from the epidermis to draining lymph nodes occurs in 2 phases. Blood monocyte precursors provide only the first wave, whereas hematopoietic precursors comprise the second, more durable repletion of the long-lived local precursors and their Langerhans cell progeny.10 It remains elusive, however, under which circumstances CD1c+ DCs would differentiate in vivo into epidermal Langerhans cells in humans. It seems unlikely that they contribute to the initial seeding of human epidermis with Langerhans cells. Because they do not express CD14, it also seems unlikely that they correspond to the monocytic precursors in the first phase of Langerhans cell repopulation in inflamed murine epidermis.10 In contrast, the cytokines supporting human CD1c+ DC conversion into Langerhans cells are all epithelial cell derived, and the Langerhans cell progeny express skin-homing receptors. CD1c+ DCs are therefore plausible candidate precursors responsible for the second phase of epidermal repopulation by Langerhans cells after an inflammatory insult.
The identification of a phenotypically defined Langerhans cell precursor in blood could provide a more exportable methodology for generating this highly potent DC subtype in vitro, rather than the current standard methodology of starting with CD34+ hematopoietic progenitor cells.5 To this end, however, more rigorous side-by-side functional rather than phenotypic comparisons are required between different candidate Langerhans cells. Although technically and logistically demanding, Langerhans cells isolated from the epidermis will remain the “gold standard.”
Human Langerhans cells are the most potent DC subtype for the stimulation of CTLs, which they accomplish without interleukin-12p705 ; human Langerhans cells are the most potent cross-presenting DC subtype.5,6 In this respect, the 2 studies discussed here present investigators with some unresolved paradoxes. Martínez-Cingolani et al2 describe a Th2 profile stimulated by their Langerhans cells progeny, which would not support potent CD8+ CTL generation. Moreover, because CD141+ (BDCA-3+) and CD1c+ (BDCA1+) mark mutually exclusive DC subsets, in which CD141+ (BDCA3+) DCs are the most potent cross-presenting DCs, then both Milne et al1 and Martínez-Cingolani et al2 present investigators with another ambiguity. Absent functional data, it remains unclear how they would reconcile the BDCA-1+/CD1c+ (BDCA-3−/CD141−) profile of the DC precursors they describe with the resultant Langerhans cell progeny that should have potent cross-presenting capacity, nominally associated with the converse CD141+ (BDCA3+) phenotype. Detailed functional data will again therefore prove essential in extending these groups' very compelling and novel findings.
Conflict-of-interest disclosure: The authors declare no competing financial interests.