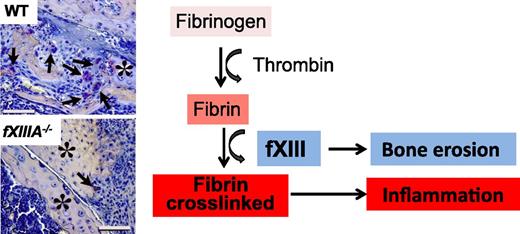
In this issue of Blood, Raghu et al provide exciting new data implicating transglutaminase factor XIII (fXIII) in rheumatoid arthritis in mice. The study presents compelling evidence demonstrating that fXIII contributes to arthritis pathogenesis through distinct mechanisms linked to inflammation and destructive bone loss.1
The coagulation fXIII is a crucial determinant of inflammation and bone erosion in rheumatoid arthritis in mice. Although reduced inflammation is a common feature between fibrinogen-deficient and fXIII-deficient mice after CIA, reduction of osteoclasts is a unique feature of fXIII-deficient mice. Histologic images are from Figure 5A in the article by Raghu et al that begins on page 427.
The coagulation fXIII is a crucial determinant of inflammation and bone erosion in rheumatoid arthritis in mice. Although reduced inflammation is a common feature between fibrinogen-deficient and fXIII-deficient mice after CIA, reduction of osteoclasts is a unique feature of fXIII-deficient mice. Histologic images are from Figure 5A in the article by Raghu et al that begins on page 427.
Components of the coagulation cascade originally studied for their role in hemostasis are now recognized as key players in inflammatory and autoimmune diseases.2,3 Fibrin, the final product of the coagulation cascade, is a result of thrombin-mediated conversion of fibrinogen to an insoluble fibrin network. A plethora of evidence from human studies and experimental animal models suggests a critical role for fibrin in inflammation.2 Fibrin drives disease pathogenesis, primarily through interactions with integrin receptors on inflammatory cells, in a variety of conditions ranging from vascular wall disease and stroke to multiple sclerosis and rheumatoid arthritis.2 Although accumulating evidence indicates that fibrin mediates, controls, and sometimes triggers immune activation, the specific roles and relative contributions of upstream components of the coagulation cascade in disease pathogenesis remain poorly understood.
In a set of well-designed and thought-provoking experiments, Raghu et al1 sought to examine the role of fXIII in rheumatoid arthritis in mice. Prior studies by Flick et al showed that genetic disruption of fibrin binding to the macrophage receptor CD11b/CD18 significantly diminishes arthritis severity.4 Moreover, prothrombin anticoagulant mutants or administration of anticoagulants that diminish fibrin formation suppresses inflammation and attenuates rheumatoid arthritis in mice.5,6 fXIII cross-links fibrin to generate fibrin clots characterized by increased stiffness and resistance to proteolytic degradation. Because fXIII contributes to the formation of stable proinflammatory fibrin matrices, Raghu et al examined the role of fXIII in inflammatory joint disease in mice using the model of collagen-induced arthritis (CIA). Mice deficient in the catalytic A subunit of fXIII (fXIIIA−/−) had significantly decreased penetrance and severity of CIA compared with control mice. Protection from CIA in fXIIIA−/− mice was accompanied by reduced inflammation and bone loss. In accordance, pharmacologic depletion of fXIII by the pan-transglutaminase inhibitor cystamine showed protection from inflammation and bone erosion. These results reveal a novel role for fXIII as a central player and therapeutic target in rheumatoid arthritis and represent perhaps the first demonstration that fXIII is required in settings outside of coagulation or wound healing.
A key question raised by these findings is, What is the mechanism of action of fXIII in inflammatory joint disease? The group sheds light on the mechanisms of action of fXIII with a set of elegant experiments. In a first set of experiments, they explored the possibility that fXIII contributes to the inflammatory process indirectly via stabilization of proinflammatory fibrin. In support of this hypothesis, fXIIIA−/− mice responded in an identical manner to fibrinogen-deficient mice in all aspects of attenuation of inflammation in the joint. Indeed, similar to fibrinogen-deficient mice, loss of fXIII was also associated with reduced infiltration by neutrophils and macrophages and reduced levels of proinflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor α after CIA. Consistent with the role of fXIII in stabilizing fibrin matrices, fXIIIA−/− mice had diffused and limited fibrin deposition in the joint after CIA. These findings provide compelling evidence that the proinflammatory effects of fXIII in rheumatoid arthritis are fibrin-dependent and suggest that fibrin cross-linking in vivo is required for the activation of the inflammatory response (see figure).
In a second set of experiments, the group made an unanticipated discovery for a fibrin-independent function of fXIII in the joint, leading to direct damage to the cartilage and bone. Surprisingly, knee joints of CIA-challenged fXIIIA−/− mice had dramatically reduced numbers of osteoclasts, which are responsible for cartilage and bone resorption (see figure). Intriguingly, osteoclast reduction associated with reduced levels of osteoclast regulators, such as receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin, was uniquely associated with fXIII deficiency because genetic loss of fibrinogen did not impact the osteoclast numbers after CIA. Osteoclast maturation of CD11b+ progenitor cells derived from fXIIIA−/− mice showed defects in osteoclast differentiation in vitro. Fibrin-induced stimulation of CD11b+ cells induces robust activation of inflammatory and oxidative stress pathways.7,8 Although the effects of fibrin on osteoclastogenesis in vitro were not tested in the study, the findings strongly support a fibrin-independent role of fXIII in bone destruction in the inflamed joint (see figure).
This exciting study raises several important questions. Does fXIII play a role in other autoimmune models of inflammation and tissue destruction? Although it is likely that fXIII regulates inflammation via stabilizing fibrin or other substrates in other disease settings, it is unknown whether its effects in tissue destruction are uniquely linked to osteoclast differentiation in the joint. Although this study supports prior findings demonstrating a critical role for coagulation in the activation of innate immunity, the role of fibrin and the coagulation cascade in the regulation of adaptive immunity remains largely unknown. Depletion of fXIII in CIA does not reduce the numbers of T and B cells in peripheral immune organs.1 However, it remains unknown whether fXIII-mediated, fibrin-induced activation of resident innate immune cells regulates local T-cell activation in tissues. Finally, this study, together with compelling evidence for the contribution of coagulation in rheumatoid arthritis patients,3 not only opens new avenues for repurposing anticoagulant therapy to limit both inflammation and bone erosion but also fuels enthusiasm for the development of new drugs to selectively target components of the coagulation cascade in autoimmune diseases.
The study by Raghu et al1 adds fXIII as a key player in rheumatoid arthritis with a dual role in inflammation and bone destruction. This exciting new finding has the potential to launch a new line of research to determine the pleiotropic effects of fXIII in inflammatory induced tissue damage and develop novel therapeutic strategies for autoimmune and inflammatory diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.