In this issue of Blood, Gambacorti-Passerini et al describe, for the first time, mutations of an ethanolamine kinase called ETNK1, which is physiologically involved in the first step of the phosphatidylethanolamine biosynthesis pathway.1 The authors describe 2 recurrent point mutations of ETNK1 in 9% of atypical chronic myeloid leukemias (aCMLs) and show that these mutations are associated with the impaired catalytic activity of the kinase.
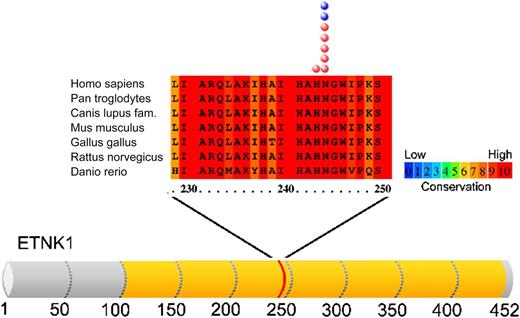
Codons of ETNK1 affected by mutations are indicated by the red and blue circles in aCML and CMML, respectively. See Figure 1A in the article by Gambacorti-Passerini et al that begins on page 499.
Codons of ETNK1 affected by mutations are indicated by the red and blue circles in aCML and CMML, respectively. See Figure 1A in the article by Gambacorti-Passerini et al that begins on page 499.
Atypical CML is an entity defined in the World Health Organization 2008 classification that belongs to the group of myeloproliferative/myelodysplastic disorders. Since 2013, next-generation sequencing approaches conducted on aCML patient samples have described recurrent mutations in CSF3R and SETBP1, genes whose mutations are able to activate cellular proliferation.
Long known in oncogenesis, the activating mutations of the granulocyte colony-stimulating factor receptor, also called CSF3R, were first described in 25% to 50% of aCML cases as well as in 3% of chronic myelomonocytic leukemia (CMML) cases in 2013.2 In aCML, a hot spot affecting codon 618 is frequently affected and could be a therapeutic target in response either to Src kinase or to Janus kinase inhibitors.2,3
Initially described in more than 90% of patients with Schinzel-Giedion syndrome,4 SETBP1 mutations were identified in 24.3% of aCML samples tested by Piazza et al.5
At the same time, SETBP1 mutations have been identified in other hematological malignancies, specifically in 4% to 14% of CMMLs, demonstrating one more time that the mutational spectrum between aCML and CMML is very close. SETBP1 mutations were characterized as conferring a proliferative advantage to mutated cells5-7 and as closely related to a negative effect on overall survival in patients with aCML. As suggested by Makishima et al,6 the presence of SETBP1 mutations could allow the use of transforming growth factor β inhibitors as a therapeutic option.
In 2013, and after all these descriptions, Gotlib et al8 tried to recapitulate the different genotypes that an aCML sample could present in comparison with chronic neutrophilic leukemia, which gave us an overview of the disease’s complexity. These mutations are not mutually exclusive; for example, concomitant mutations of CSF3R and SETBP1 could be found in 14% of the patients.
However, a complete description of the somatic mutations involved in the onset of aCML was still lacking. Using a whole-exome analysis approach on 15 aCML cases, and after their own description of SETBP1 mutation in aCML,5 Gambacorti-Passerini et al described 2 point mutations of an ethanolamine kinase called ETNK1, an enzyme that is physiologically involved in the first step of the phosphatidylethanolamine biosynthesis pathway. In a large cohort of 515 hematologic clonal disorders, the authors described recurrent ETNK1 heterozygous mutations in 9% of atypical CML and 3% of CMML samples.
The described mutations are all restricted in the kinase domain, affecting 2 hot spot codons (H243Y and mainly N244S), as shown in the figure.
The authors also showed that these mutations are associated with impaired catalytic activity of the kinase, leading to an important decrease in the intracellular phosphoethanolamine/phosphocholine ratio. These results, obtained on patient samples, were confirmed on TF1 cell lines transduced with wild-type or mutated ETNK1, suggesting that ETNK1 mutations may inhibit the catalytic activity of the enzyme.
ETNK1 is responsible for the phosphorylation of ethanolamine to phosphoethanolamine, which is involved in many biochemical processes, mainly in the definition of the membrane architecture and participating in the respiratory complexes in the inner membrane of mitochondria. Given the pleiotropic role of phosphoethanolamine, the evaluation of biological effects of the ETNK1 mutations will be a challenging task.
All these studies clearly showed that aCML and CMML share not only clinical and morphologic features but also mutant genes, especially CSF3R, SETBP1 and ETNK1, which seems to be more specific than the other mutations that affect the TET2 and SRSF2 genes and frequently occur in CMML.
In conclusion, this article by Gambacorti-Passerini et al shows for the first time evidence of recurrent somatic mutations of the ETNK1 gene in the context of myeloproliferative/myelodysplastic clonal disorders. Further studies will be required to clearly understand the functional effects of these mutations and to provide clues to define new therapeutic approaches.
Conflict-of-interest disclosure: The author declares no competing financial interests.