Key Points
IL-33 and ST2 expression are increased post-conditioning and with GVHD, resulting in increased T-cell activation via the IL-33/ST2 axis.
Infusion of ST2-Fc protein exploits sST2’s function as a negative regulator of acute GVHD inhibiting pro-inflammatory cytokines.
Abstract
Interleukin (IL)-33 binding to the receptor suppression of tumorigenicity 2 (ST2) produces pro-inflammatory and anti-inflammatory effects. Increased levels of soluble ST2 (sST2) are a biomarker for steroid-refractory graft-versus-host disease (GVHD) and mortality. However, whether sST2 has a role as an immune modulator or only as a biomarker during GVHD was unclear. We show increased IL-33 production by nonhematopoietic cells in the gastrointestinal (GI) tract in mice post-conditioning and patients during GVHD. Exogenous IL-33 administration during the peak inflammatory response worsened GVHD. Conversely, GVHD lethality and tumor necrosis factor-α production was significantly reduced in il33−/− recipients. ST2 was upregulated on murine and human alloreactive T cells and sST2 increased as experimental GVHD progressed. Concordantly, st2−/− vs wild-type (WT) donor T cells had a marked reduction in GVHD lethality and GI histopathology. Alloantigen-induced IL-18 receptor upregulation was lower in st2−/− T cells, and linked to reduced interferon-γ production by st2−/− vs WT T cells during GVHD. Blockade of IL-33/ST2 interactions during allogeneic-hematopoietic cell transplantation by exogenous ST2-Fc infusions had a marked reduction in GVHD lethality, indicating a role of ST2 as a decoy receptor modulating GVHD. Together, these studies point to the IL-33/ST2 axis as a novel and potent target for GVHD therapy.
Introduction
Interleukin (IL)-33, a member of the IL-1 superfamily, is a multifunctional protein with immune modulating roles as an alarmin and pleiotropic cytokine. IL-33 is expressed predominantly in fibroblasts,1 endothelial cells,2 and epithelial cells3; however, upon inflammatory stimuli, its production is increased and expression has been seen in myeloid cells.4-6 Unlike other members of the IL-1 superfamily, IL-33 is cleaved by caspase-3 and caspase-7 into a biologically inactive form during apoptosis, but is functional when released in its full-length form by necrosis.7-9 IL-33 binds to its receptor suppression of tumorigenicity 2 (ST2), which has 3 known isoforms: a membrane bound, soluble, and variant form.10,11 IL-33 affects a variety of innate and adaptive cell types including natural killer (NK) cells, mast cells, myeloid antigen presenting cells, T helper (Th)2 cells, and regulatory T cells (Tregs) that express the membrane bound ligand receptor (ST2L), on the cell surface.5,12-22 Depending on the target tissue and cell types expressing ST2L, IL-33/ST2 signaling has established dual roles in promoting pro-inflammatory responses and conversely resolving inflammatory processes, similar to its family member IL-18.14,22-25
IL-33/ST2L signaling has an established role in immune cell polarization and mobilization.5,15,26,27,29,30 IL-33 can augment interferon (IFN)-γ production by NK cells, especially in the presence of IL-12 to promote Th1 immunity.24,31 Conversely, activation of ST2L induces the production of cytokines such as IL-4, IL-5, and IL-1332-34 by T cells, or IL-6 and tumor necrosis factor (TNF)-α by mast cells,15,17,35,36 polarizing CD4+ T-cell differentiation toward Th2 immunity. IL-33 has also been shown to mediate anti-inflammatory events by expanding suppressive CD11b+Gr-1int myeloid cells and ST2+ Foxp3+ Tregs, resulting in the cardiac allograft survival.22,37 Recently, IL33 has been shown to play a prominent role in regulation of ST2+ Treg function and maintenance in the gut under homeostatic conditions.38 IL-33 also promotes the chemotaxis of dendritic cells (DC)23 and neutrophil granulocytes.39 The soluble form of the receptor, sST2, acts as a decoy receptor blocking IL-33 signaling25,40 and inhibiting lipopolysaccharide-induced cytokine production.41
Previous observations in other models of inflammation have shown IL-33 to be either pro-inflammatory or anti-inflammatory depending on the disease entity. A pro-inflammatory role for IL-33 was found in respiratory syncytial virus infection,42 and neutralizing antibodies against ST2 attenuated lung inflammation.35,43 A pro-inflammatory role for IL-33 was also reported in the pathogenesis of rheumatoid arthritis,44 mast cell-induced airway inflammation,12 allergic sensitization,23 and inflammatory bowel disease.26,45 Conversely, IL-33 was shown to protect against atherosclerosis through the induction of IL-5,46 was cardioprotective,1 promoted cardiac allograft survival,22 and had either no effect on experimental autoimmune encephalomyelitis or even reduced its severity.47 Elevated levels of the soluble form of the IL-33 receptor, sST2, have been shown in several pathologies, including asthma,48,49 cardiovascular disease,50-52 and autoimmune disorders.53
Recent evidence suggests a new role for IL-33 in graft-versus-host-disease (GVHD), wherein the donor T cells cause destruction of recipient tissues after allogeneic hematopoietic cell transplantation (allo-HCT). Acute GVHD (aGVHD) is characterized by damage to the skin, liver, and gastrointestinal (GI) tract. These mucosal barrier tissues store large amounts of IL-33 that may be released upon tissue injury.25 Recently, elevated levels of sST2 as early as day 14 post-conditioning prior to the development of GVHD has been identified as a biomarker for increased mortality and steroid-refractory GVHD.54 Yet, little is known of the role of IL-33 in the immune responses shaping GVHD. In this study, we explore the potential role of IL-33, ST2L, and sST2 in aGVHD pathogenesis.
Methods
Human subjects
We collected all of the samples after obtaining approval by the Ethics Committee of the Albert-Ludwigs-University Freiburg, Freiburg, Germany (Protocol #480/11) and after obtaining written informed consent in accordance with the Declaration of Helsinki. Intestinal tissue biopsies were collected in a prospective manner from individuals undergoing allo-HCT with or without GVHD. The grading of human GVHD was performed on the basis of histopathology.
Mice
Wild-type (WT) mice were purchased from several different vendors depending on the university conducting the experiment. BALB/c and C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD) or The Jackson Laboratories (Bar Harbor, ME). In addition, C57BL/6 (H-2b, Thy-1.2) and BALB/c (H-2d, Thy-1.2) mice were purchased either from the Charles River Laboratory (Sulzburg, Germany) or from the local stock of the animal facility at Freiburg University. The st2−/− mice were on different backgrounds, the German strain on 129 and the US strain on BALB/c background. The 129 st2−/− mice were purchased from European Mouse Mutant Archive, the il-33−/− mice were generated as described elsewhere,35 and obtained from RIKEN, Japan (Account #CDB0631K, http://www.cdb.riken.jp).35 BALB/c st2−/− were obtained from Dr Andrew Mckenzie.55 Recipients between 6 and 12 weeks of age were used for transplant experiments. All mice were housed in a specific pathogen-free environment. All breeding and experimental procedures were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota, the University of Pittsburgh, and the University of Michigan. The animal protocols (G-12/34, X-10/13H) were approved by the University Committee on the Use and Care of Laboratory Animals at Albert-Ludwigs-University Freiburg, Germany.
Bone marrow transplantation (BMT) model and GVHD histopathology
Procedures for BMT experiments differed based on laboratory. In Germany, BMT experiments were performed as previously described.56 Briefly, recipients were IV injected with 5 × 106 bone marrow (BM) cells after lethal irradiation with 900 cGy for BALB/c recipients and 1000 cGy for C57BL/6 and 129 recipients. To induce a GVHD myeloablative conditioning, on day 0, by IV, the enriched (>90% purity) CD4+/CD8+ T cells were given the numbers shown dependent on the model, as follows: C57BL/6→BALB/c (3 × 105), BALB/c→C57BL/6 (8 × 105), BALB/c→129 (1 × 105), and 129→BALB/c (3 × 105). T-cell doses were previously titrated to numbers that reliably induce GVHD in each strain combination. Slides of liver, and small and large intestine samples were stained with hematoxylin and eosin and scored by experienced pathologists (A.S.-G.) blinded to the treatment groups on the basis of a published histopathology scoring system.57 In the United States, recipients were lethally irradiated (ie, either split dose of 800 cGy for BALB/c and 1000 cGy for C57BL/6, or single dose of 700 cGy for BALB/c and 1100 cGy for C57BL/6 dependent on the laboratory) by a radiograph source 1 day prior to transfer of either 5 × 106 or 1 × 107 BM cells alone as controls or with purified T cells to induce GVHD: C57BL/6→BALB/c (0.5 × 106) BALB/c→C57BL/6 (2.5 × 106 or 5 × 106). T cells were isolated from lymph nodes and spleens, and purified by incubation with phycoerythrin-labeled antibodies to B220, CD19, γδ-TCR, and DX5, or NK1.1 (eBioscience); incubated with anti-phycoerythrin beads; and depleted on magnetic column (Miltenyi Biotec). Survival, weight, and manifestations of clinical GVHD were monitored.58
For all other methods, see supplemental Methods, available on the Blood Web site. The complete gene expression data are available at: ArrayExpress, Accession #E-MEXP-3954 (Experiment name: The role of IL33-ST2 in aGVHD) and E-MTAB-1566.
Results
Increased IL-33 expression in nonhematopoietic cells in the GI tract post-radiation conditioning and during GVHD
We first assessed whether IL-33 was upregulated post-conditioning and during GVHD. Examination of gene expression of cells located in the GI tract of mice, a known site for IL-33 production and a critical GVHD target organ, found significant upregulation of il-33 at 24 and 48 hours after total body irradiation (TBI) (Figure 1A,B). Similar levels of IL-33 protein were seen in the small intestine and colon of mice during GVHD compared with those receiving only BM after TBI; however, both had greater expression of IL-33 than untreated mice and il-33−/− negative controls (supplemental Figure 1A-C). These data suggest that initial insult from TBI creates sufficient tissue injury to trigger increased IL-33 expression. We hypothesize the increase in IL-33 production resulting from tissue damage is sufficient stimulus to drive st2+/+ T-cell responses, which in turn amplifies the signal. Most IL-33 protein localized with nonhematopoietic (CD45−Vimentin+ and CD45−Vimentin−) cells rather than hematopoietic cells (CD45+Vimentin−), indicating that nonhematopoietic cells were the major IL-33 producers (Figure 1C,D). Since many patients receive chemotherapy-based conditioning, levels of IL-33 protein were examined in mice treated with 1 of 3 common chemotherapy-based conditioning regimens (busulfan/cyclophosphamide, treosulfan/cyclophosphamide, or thiotepa/cyclophosphamide). Chemotherapy-based conditioning regimens yielded increased levels of IL-33 protein in the GI tract similar to that caused by TBI (Figure 1E), corroborating the data from Figure 1B and supplemental Figure 1A that increased IL-33 production is primarily due to tissue injury caused by conditioning. Consistent with the findings in mice, the expression of IL-33 in the intestinal tract of patients with grade 4 GVHD was increased in comparison with the GI tract of patients without GVHD (Figure 1F-G). This cohort of patients received mainly reduced intensity conditioning, with only 3/26 patients receiving Bu/Cy conditioning (patient characteristics are listed in supplemental Table 1).
IL-33 expression in the murine small intestine increases upon TBI and in the colon of stage IV GVHD patients. (A-B) After TBI of BALB/c mice with 9 Gy, RNA expression levels of multiple genes in the small bowel were analyzed using microarray-based analysis. This experiment was performed once. (A) Shown is the tile display for the most significantly regulated genes encoding for cytokines, expressed by Robust Multichip Average (RMA). Signal values of 4 individual samples in the following groups are shown: untreated, 24 hours, and 48 hours after TBI. Green rectangle: IL-33 is the second most significantly regulated gene. (B) Each RMA value for IL-33 is shown, including the P values. (C-D) Gut tissue from control (day 0), lethally-irradiated (day 2 post-irradiation), and WT or C57BL/6 recipients, that had received either 2.5 × 106 T cells and 1 × 107 BM cells, or BM only from BALB/c donors in GVHD studies (day 7 or day 14) were stained for IL-33, CD45, vimentin, and 4,6 diamidino-2-phenylindole (DAPI). This experiment was performed once. Images are representative of n = 3 mice/group and graphs were generated by averaging 2 to 3 fields (IL-33/DAPI–20×; IL-33/CD45, or IL-33/vimentin–20×) per animal. Fluorescence area/intensity reported as arbitrary units. *P < .05; ***P < .001. This experiment was performed once. (E) Gut tissue from BALB/c mice 7 days after transplantation with 5 × 106 BM cells and 3 × 105 T cells from C57Bl/6 mice. Prior to transplantation on day 0, mice received either TBI (2× 4,5 Gy), busulfuan/cyclophosphamide (Bu/Cy: busulfan day −7 to day −4 [dose: 10 mg/kg]), cyclophosphamide day −3 and day −2 (dose 100 mg/kg), treosulfan/cyclophosphamide (Treo/Cy: treosulfan day −6 to day −4 [dose: 1.5 g/kg]), cyclophosphamide day −3 and day −2 [dose 100 mg/kg]), or thiotepa/cyclophosphamide (Thio/Cy: thiotepa day −6 to day −4 [dose: 10 mg/kg], cyclophosphamide day −3 and day −2 [dose 100 mg/kg]) conditioning regimens. Fluorescence area/intensity reported as in (C-D). (Ei) Bar diagram for the indicated groups. (Eii) Representative tissue sections. Images are representative of n = 2 to 3 mice per group and graphs were generated by averaging 3 to 6 fields (IL-33/DAPI–20×) per animal. This experiment was performed once. (F-G) The amount of IL-33 in human colon biopsies was quantified by immunohistochemistry as shown for one representative section per group ([Fi] no GVHD; [Fii] GVHD IV° of the intestines) and for multiple patients (G). Samples were taken at different time points after allo-HCT in patients without GVHD or with GVHD grade 3/4. Patients’ characteristics, time of biopsy, conditioning, and immunosuppression data are shown in supplemental Table 1.
IL-33 expression in the murine small intestine increases upon TBI and in the colon of stage IV GVHD patients. (A-B) After TBI of BALB/c mice with 9 Gy, RNA expression levels of multiple genes in the small bowel were analyzed using microarray-based analysis. This experiment was performed once. (A) Shown is the tile display for the most significantly regulated genes encoding for cytokines, expressed by Robust Multichip Average (RMA). Signal values of 4 individual samples in the following groups are shown: untreated, 24 hours, and 48 hours after TBI. Green rectangle: IL-33 is the second most significantly regulated gene. (B) Each RMA value for IL-33 is shown, including the P values. (C-D) Gut tissue from control (day 0), lethally-irradiated (day 2 post-irradiation), and WT or C57BL/6 recipients, that had received either 2.5 × 106 T cells and 1 × 107 BM cells, or BM only from BALB/c donors in GVHD studies (day 7 or day 14) were stained for IL-33, CD45, vimentin, and 4,6 diamidino-2-phenylindole (DAPI). This experiment was performed once. Images are representative of n = 3 mice/group and graphs were generated by averaging 2 to 3 fields (IL-33/DAPI–20×; IL-33/CD45, or IL-33/vimentin–20×) per animal. Fluorescence area/intensity reported as arbitrary units. *P < .05; ***P < .001. This experiment was performed once. (E) Gut tissue from BALB/c mice 7 days after transplantation with 5 × 106 BM cells and 3 × 105 T cells from C57Bl/6 mice. Prior to transplantation on day 0, mice received either TBI (2× 4,5 Gy), busulfuan/cyclophosphamide (Bu/Cy: busulfan day −7 to day −4 [dose: 10 mg/kg]), cyclophosphamide day −3 and day −2 (dose 100 mg/kg), treosulfan/cyclophosphamide (Treo/Cy: treosulfan day −6 to day −4 [dose: 1.5 g/kg]), cyclophosphamide day −3 and day −2 [dose 100 mg/kg]), or thiotepa/cyclophosphamide (Thio/Cy: thiotepa day −6 to day −4 [dose: 10 mg/kg], cyclophosphamide day −3 and day −2 [dose 100 mg/kg]) conditioning regimens. Fluorescence area/intensity reported as in (C-D). (Ei) Bar diagram for the indicated groups. (Eii) Representative tissue sections. Images are representative of n = 2 to 3 mice per group and graphs were generated by averaging 3 to 6 fields (IL-33/DAPI–20×) per animal. This experiment was performed once. (F-G) The amount of IL-33 in human colon biopsies was quantified by immunohistochemistry as shown for one representative section per group ([Fi] no GVHD; [Fii] GVHD IV° of the intestines) and for multiple patients (G). Samples were taken at different time points after allo-HCT in patients without GVHD or with GVHD grade 3/4. Patients’ characteristics, time of biopsy, conditioning, and immunosuppression data are shown in supplemental Table 1.
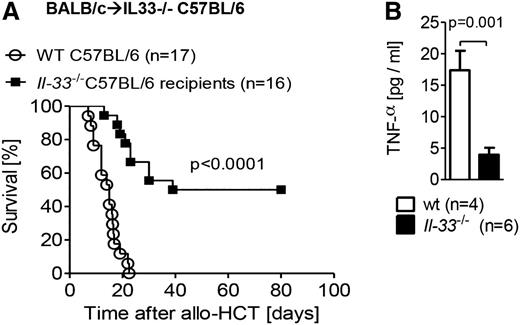
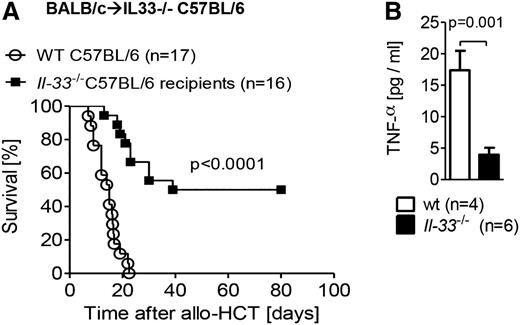
We further defined the important cellular sources of IL-33 following TBI and HCT by reconstitution of IL-33–deficient (il-33−/−) or WT recipients with allogeneic WT BM and T cells. IL-33–deficient recipients had significantly reduced GVHD lethality (Figure 2A) and lower production of the pro-inflammatory cytokine TNF-α (Figure 2B) as compared with WT recipients, indicating that radioresistant host nonhematopoietic cells are the principal source of IL-33. Additionally, the reconstitution of WT recipients with either allogeneic il-33−/− T cells and WT BM (supplemental Figure 2A) or il-33−/− BM and WT T cells (supplemental Figure 2B) demonstrated no protection from GVHD, providing further supportive evidence for nonhematopoietic cells as the most influential source of IL-33.
IL-33 deficiency in the host nonhematopoietic cells decreases GVHD severity. (A) Survival of C57BL/6 mice (WT or IL-33 deficient) after TBI (1000 cGy) and transplantation of 8 × 105 T cells and 5 × 106 BM from BALB/c mice. Data pooled from 3 independent experiments. (B) Serum was taken from recipient mice on day 7 post–allo-HCT, and TNF-α production was measured by enzyme-linked immunosorbent assay (ELISA). Data from a single experiment.
IL-33 deficiency in the host nonhematopoietic cells decreases GVHD severity. (A) Survival of C57BL/6 mice (WT or IL-33 deficient) after TBI (1000 cGy) and transplantation of 8 × 105 T cells and 5 × 106 BM from BALB/c mice. Data pooled from 3 independent experiments. (B) Serum was taken from recipient mice on day 7 post–allo-HCT, and TNF-α production was measured by enzyme-linked immunosorbent assay (ELISA). Data from a single experiment.
IL-33/ST2 engagement in the donor T cells augments aGVHD
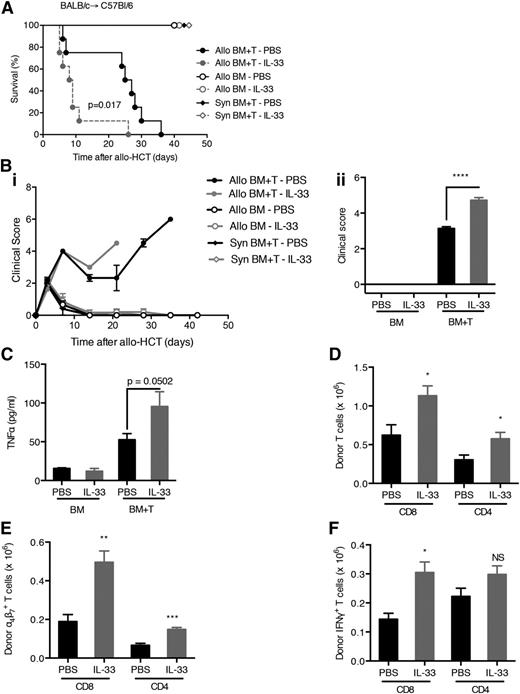
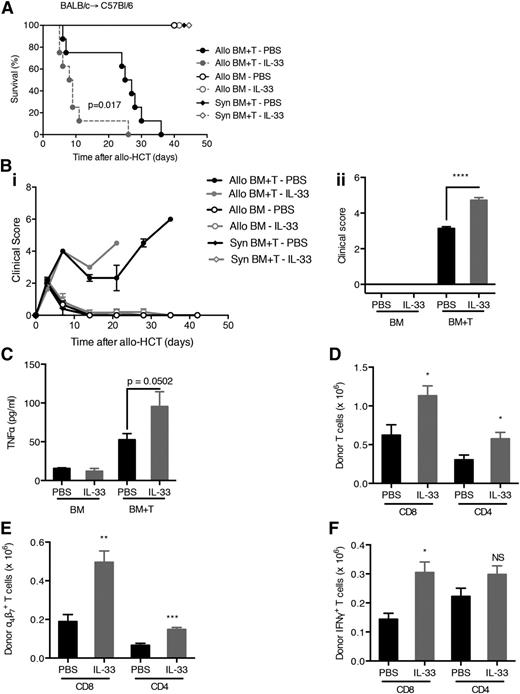
Because host IL-33 is increased upon tissue injury, we examined whether the addition of exogenous IL-33 post–allo-HCT would exacerbate the inflammatory response resulting in increased GVHD. Mice treated with IL-33 on days 3 to 7 after allo-HCT died more rapidly from severe aGVHD compared with mice receiving only the vehicle (Figure 3A-B). Comparable results were seen with recipients receiving IL-33 treatment regimen on either days 0 to 10 or days 3 to 7 (supplemental Figure 3A-C) in 2 different strain combinations. Concordant with enhanced GVHD severity, IL-33 treatment increased levels of TNF-α (Figure 3C and supplemental Figure 3D) and resulted in expansion of the donor T-cell compartment (Figure 3D-E), specifically IFN-γ+CD8+ T cells (Figure 3F). Together, these data indicate that exogenous IL-33 administration contributes to Th1 responses and GVHD severity when administered after the inflammatory response is initiated via allo-HCT. IL-33 administration after syngeneic HCT showed no toxicity (supplemental Figure 3E).
Administration of IL-33 post–allo-HCT increases the severity of GVHD. (A) C57BL/6 recipient mice of either 5 × 106 BALB/c (allo) or C57BL/6 (syngeneic) BM and 5 × 106 T cells (CD90-sorted) were treated with recombinant IL-33 (from day 3 to day 7 after BMT; 0.4 μg/dose) or phosphate-buffered saline (PBS) as control and monitored for survival. Allo BM+T-IL-33 vs Allo BM+T-PBS. P = .017. (B) Clinical GVHD scores were monitored throughout the experiment (Bi) and statistical differences were assessed on day 8 after allo-HCT (Bii) for the experiment shown in (A). (C) Concentrations of TNF-α were measured on day 8 after allo-HCT in the serum of C57BL/6 allogeneic recipients (received BM or BM plus T cells from BALB/c mice) treated with IL-33 (5 × 0.2 μg) or PBS. (D-F) Number of BALB/c donor T cells (D), a4β7+ T cells (E), or IFN-γ+ T cells (F) isolated from lamina propria of the small intestine of C57BL/6 allogeneic recipients treated with IL-33 (5 × 0.2 μg) or PBS on day 8 after allo-HCT. NS, not significant.
Administration of IL-33 post–allo-HCT increases the severity of GVHD. (A) C57BL/6 recipient mice of either 5 × 106 BALB/c (allo) or C57BL/6 (syngeneic) BM and 5 × 106 T cells (CD90-sorted) were treated with recombinant IL-33 (from day 3 to day 7 after BMT; 0.4 μg/dose) or phosphate-buffered saline (PBS) as control and monitored for survival. Allo BM+T-IL-33 vs Allo BM+T-PBS. P = .017. (B) Clinical GVHD scores were monitored throughout the experiment (Bi) and statistical differences were assessed on day 8 after allo-HCT (Bii) for the experiment shown in (A). (C) Concentrations of TNF-α were measured on day 8 after allo-HCT in the serum of C57BL/6 allogeneic recipients (received BM or BM plus T cells from BALB/c mice) treated with IL-33 (5 × 0.2 μg) or PBS. (D-F) Number of BALB/c donor T cells (D), a4β7+ T cells (E), or IFN-γ+ T cells (F) isolated from lamina propria of the small intestine of C57BL/6 allogeneic recipients treated with IL-33 (5 × 0.2 μg) or PBS on day 8 after allo-HCT. NS, not significant.
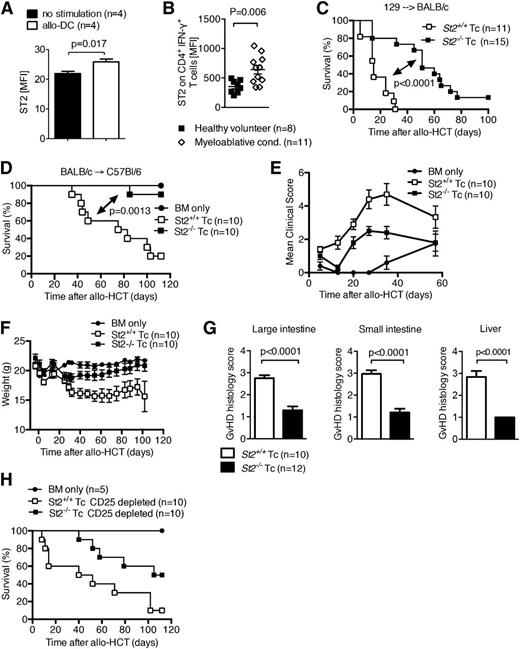
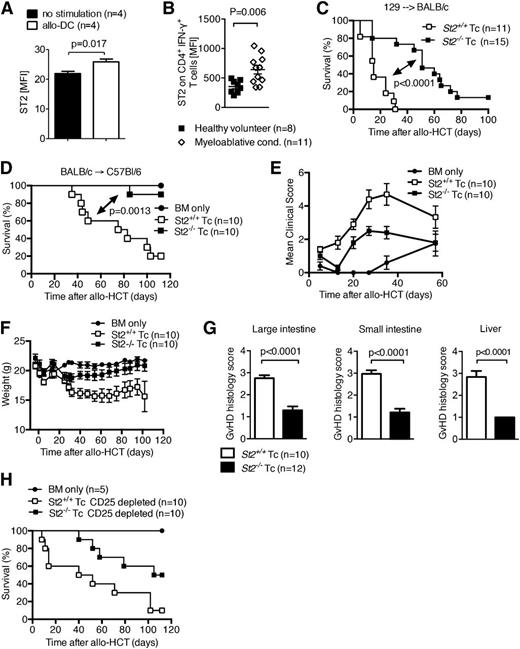
The expression of the cell surface IL-33 receptor, ST2L, increases on both donor CD4+ and CD8+ T cells after allo-stimulation in 2 strain combinations (Figure 4A and supplemental Figure 4A). Similar upregulation of ST2L occurred in CD4+IFN-γ+ cells in patients with GVHD (Figure 4B). Specificity of the anti-ST2 antibody was evaluated and controlled for by using WT and st2−/− splenocytes (supplemental Figure 4B-C). Given the increased expression of ST2L in both clinical and experimental GVHD, we tested the hypothesis that IL-33 binding to its membrane bound receptor on donor T cells worsens GVHD. Irradiated recipients were reconstituted with allogeneic WT BM and T cells were isolated from the spleens of either ST2-deficient (st2−/−) or WT (st2+/+) donor mice. Recipients receiving st2−/− donor T cells had a significant reduction in GVHD lethality compared with recipients receiving WT (st2+/+) T cells in 2 donor/recipient strain combinations (Figure 4C-D). Consistent with improved survival, GVHD induced by st2−/− donor T cells caused less clinical (Figure 4E-F) and histologic (Figure 4G) GVHD than by WT T cells. In preliminary studies, the frequencies of splenic and colonic Tregs after transfer of either donor WT or st2−/− T cells were similar (supplemental Figure 5A-B) and the beneficial effect of ST2-deficiency on donor T cells was not abrogated by removal of donor Tregs by CD25 magnetic bead depletion (Figure 4H). These data indicate that the dominant role of ST2L during GVHD is full activation of donor T cells. The addition of exogenous IL-33 to WT C57BL/6 recipients reconstituted with allogeneic WT BM and either T cells isolated from the spleens of ST2-deficient (st2−/−) or WT (st2+/+) donor mice did not exacerbate GVHD in mice receiving st2−/− T cells, corroborating the findings that IL-33 is mediating its effect through donor T cells (supplemental Figure 6).
Increased expression of ST2 on T cells augments T-cell function during aGVHD. (A) ST2 was measured by flow cytometry on murine CD4+ T cells isolated from naïve mice after in vitro stimulation with allogeneic DC. Data are representative of 2 experiments. (B) ST2 expression on human CD4+ IFN-γ+ T cells in the peripheral blood analyzed by flow cytometry. Each data point represents the MFI for ST2 of an individual patient or healthy volunteer donor. Myeloablative conditioning (cond.) was performed with busulfan/cyclophosphamide or fludarabin/BCNU/melphalan, and samples were collected within the range of 5 to 10 days after allogeneic HCT. This experiment was performed once. (C) Survival of mice after allo-HCT performed as described for the 129 into BALB/c combination with 5 × 106 BM cells and 3 × 105 WT T cells, or st2−/− T cells as indicated. The experiment was performed 3 times and the data were pooled. (D-F) Survival (D), clinical GVHD scores (E), and weight (F) of mice after allo-HCT was performed as described for the BALB/c into C57BL/6 combination with 1 × 107 BM cells and 2.5 × 106 WT BALB/c T cells, or st2−/− T cells as indicated. This experiment was performed twice and representative data shown. (G) Histopathologic GVHD severity of the intestines and liver isolated on day 8 from mice treated as described under (C) when quantified as mean + SEM. Data are pooled from 2 independent experiments. (H) Survival of WT C57Bl/6 mice after allo-HCT with 1 × 107 BM cells and 2.5 × 106 WT BALB/c T cells, or st2−/− T cells, performed as described under (D) with prior deletion of CD25+ cells within the transferred donor T cells. This experiment was performed once (n = 10). MFI, mean fluorescence intensity.
Increased expression of ST2 on T cells augments T-cell function during aGVHD. (A) ST2 was measured by flow cytometry on murine CD4+ T cells isolated from naïve mice after in vitro stimulation with allogeneic DC. Data are representative of 2 experiments. (B) ST2 expression on human CD4+ IFN-γ+ T cells in the peripheral blood analyzed by flow cytometry. Each data point represents the MFI for ST2 of an individual patient or healthy volunteer donor. Myeloablative conditioning (cond.) was performed with busulfan/cyclophosphamide or fludarabin/BCNU/melphalan, and samples were collected within the range of 5 to 10 days after allogeneic HCT. This experiment was performed once. (C) Survival of mice after allo-HCT performed as described for the 129 into BALB/c combination with 5 × 106 BM cells and 3 × 105 WT T cells, or st2−/− T cells as indicated. The experiment was performed 3 times and the data were pooled. (D-F) Survival (D), clinical GVHD scores (E), and weight (F) of mice after allo-HCT was performed as described for the BALB/c into C57BL/6 combination with 1 × 107 BM cells and 2.5 × 106 WT BALB/c T cells, or st2−/− T cells as indicated. This experiment was performed twice and representative data shown. (G) Histopathologic GVHD severity of the intestines and liver isolated on day 8 from mice treated as described under (C) when quantified as mean + SEM. Data are pooled from 2 independent experiments. (H) Survival of WT C57Bl/6 mice after allo-HCT with 1 × 107 BM cells and 2.5 × 106 WT BALB/c T cells, or st2−/− T cells, performed as described under (D) with prior deletion of CD25+ cells within the transferred donor T cells. This experiment was performed once (n = 10). MFI, mean fluorescence intensity.
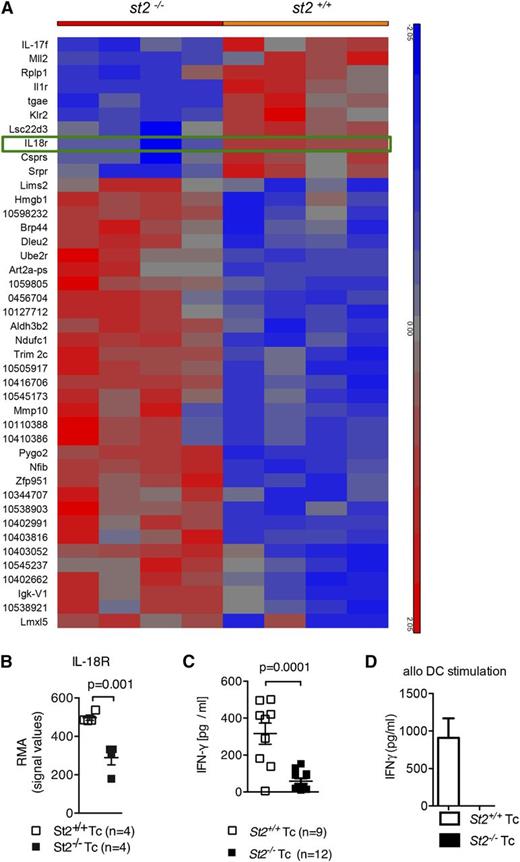
ST2 deficiency in T cells during alloantigen activation reduces upregulation of IL-18R expression and IFN-γ production
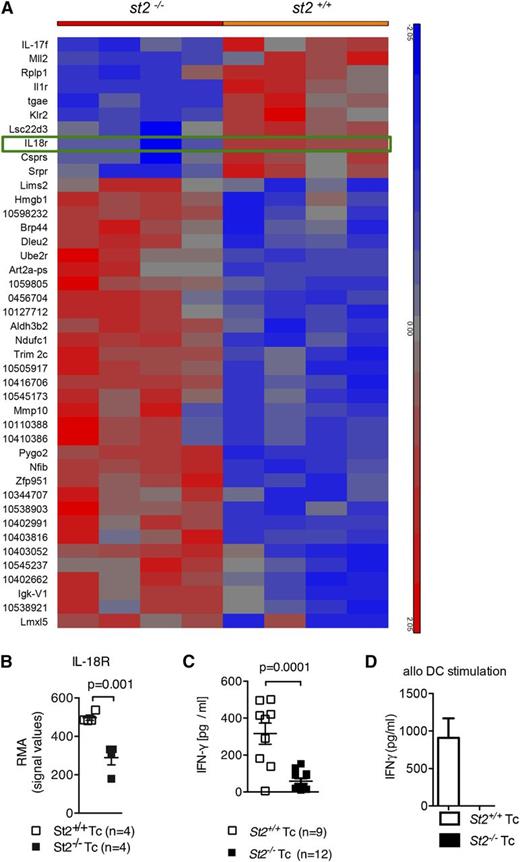
Although ST2 deficiency in T cells resulted in functional differences compared with WT T cells, the mechanism(s) by which this occurred was not fully understood. To understand the differences in GVHD biology between ST2-deficient and WT T cells, splenic CD3+ T cells were isolated from st2−/− or WT mice (H-2b) and then stimulated with allogeneic DCs (H-2kd). Gene expression was analyzed in RNA from T cells re-isolated after 48 hours. In comparison with WT T cells, st2−/− T cells exhibited less IL-18 receptor messenger RNA expression (Figure 5A-B), which was confirmed on the protein level (supplemental Figure 7A-B). The IL-33/ST2 and IL-18/IL-18R complexes are structurally similar heterodimers that share analogous downstream signaling pathways.4,7-9 Similar to IL-33, IL-18 induces production of IFN-γ,10,11,59 a cytokine known to contribute to GVHD severity.5,14-21,60 IFN-γ production was reduced in st2−/− T cells compared with WT T cells (supplemental Figure 8A-B), which translated into lower IFN-γ serum levels in recipients of ST2-deficient T cells in comparison with recipients of WT T cells (Figure 5C). Concordantly, st2−/− CD4+ T cells secreted less IFN-γ than WT CD4+ T cells after stimulation with allogeneic DCs (Figure 5D); interestingly, intracellular IL-4 and IL-17A levels were increased (supplemental Figure 8C). These findings indicate that ST2 deficiency results in decreased IL-18R upregulation and is associated with a profound defect in production of IFN-γ, a cytokine known to contribute to GVHD severity.60
Upregulation of IL-18R and IFN-γ production in response to alloantigen is reduced in st2−/− T cells. (A-B) Gene expression was quantified in WT and st2−/− T cells on the RNA level by microarray analysis. WT or st2−/− T cells were exposed to allogeneic irradiated DC for 48 hours. The tile display for the most significantly regulated genes expressed by RMA signal values of 4 individual samples in each group shown at the RNA level. (C) The values of individual mice for serum IFN-γ is shown on day 8 after allo-HCT. The experiment was performed twice and the data were pooled. (D) WT and st2−/− CD4+/CD8+ T cells stimulated with allo–BM-DCs (2:1 ratio). ELISA for IFN-γ was performed after 24 hours of exposure. The experiment was performed twice with similar results.
Upregulation of IL-18R and IFN-γ production in response to alloantigen is reduced in st2−/− T cells. (A-B) Gene expression was quantified in WT and st2−/− T cells on the RNA level by microarray analysis. WT or st2−/− T cells were exposed to allogeneic irradiated DC for 48 hours. The tile display for the most significantly regulated genes expressed by RMA signal values of 4 individual samples in each group shown at the RNA level. (C) The values of individual mice for serum IFN-γ is shown on day 8 after allo-HCT. The experiment was performed twice and the data were pooled. (D) WT and st2−/− CD4+/CD8+ T cells stimulated with allo–BM-DCs (2:1 ratio). ELISA for IFN-γ was performed after 24 hours of exposure. The experiment was performed twice with similar results.
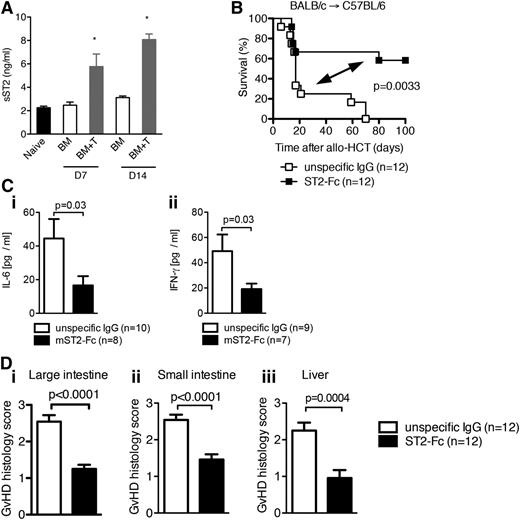
Elevated levels of sST2 function as a decoy receptor to decrease aGVHD
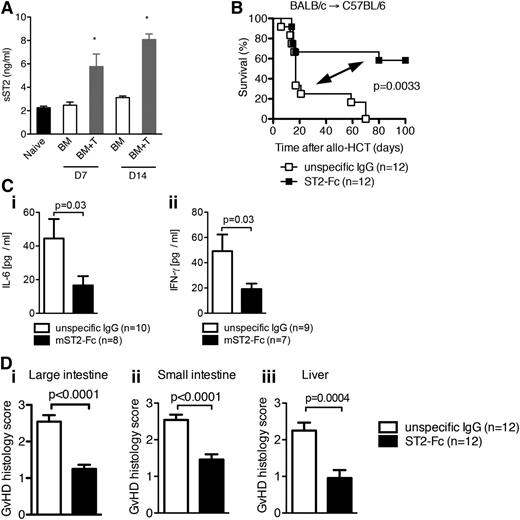
Given the increased levels of sST2 prior to the development of and during GVHD in humans, we examined sST2 levels in the serum of mice after allo-HCT. Levels of sST2 in the serum of mice were increased as GVHD progressed (Figure 6A and supplemental Figure 9A). However, onset of the elevation of sST2 levels did not occur until after at least 2 days posttransplantation (supplemental Figure 9A). We hypothesized that sST2 not only indicates tissue injury of GVHD but functions as a decoy receptor to regulate GVHD severity. To test this hypothesis, allo-HCT WT recipients were given exogenous soluble ST2 (sST2)-Fc fusion protein known to block the binding of IL-33 to the membrane bound form of ST2.61 WT recipients given infusions to block ST2/IL-33 interaction had significantly reduced GVHD lethality (Figure 6B) and lower IL-6 and IFN-γ serum levels without effects on other pro-inflammatory mediators such as IL-12, TNFα, or anti-inflammatory IL-10 (Figure 6C and supplemental Figure 9B). Concordantly, gut and liver GVHD histopathology was significantly diminished in mice receiving ST2-Fc compared with mice receiving nonspecific IgG (Figure 6D). Together, these data indicate that elevated levels of sST2 function to ameliorate tissue injury created by conditioning and GVHD.
Increased sST2 levels decrease GVHD-associated mortality. (A) Lethally irradiated C57BL/6 mice (1000 cGy) received 5 × 106 BM cells from BALB/c donors (BM) or 5 × 106 BM cells plus 5 × 106 purified CD90.2+ T cells from BALB/c donors (BM+T), and sST2 concentration in the serum was measured on day 7 and day 14 after allo-HCT by ELISA. *P < .05. (B) Survival of WT C57BL/6 mice after allo-HCT with 5 × 106 BM cells and 8 × 105 WT T cells from BALB/c recipients performed as described. Mice received sST2-Fc or isotype control IgG as indicated. The experiment was performed twice and the data were pooled. (C) The mean value ± SEM for (i) serum IL-6 and (ii) IFN-γ detected by ELISA on day 8 after allo-HCT. The experiment was performed twice and the data were pooled. (D) Histopathologic GVHD severity of the intestines (i, ii) and liver (iii) isolated on day 8 from mice treated as described under panel (B) when quantified as mean + SEM. Data are pooled from 2 independent experiments.
Increased sST2 levels decrease GVHD-associated mortality. (A) Lethally irradiated C57BL/6 mice (1000 cGy) received 5 × 106 BM cells from BALB/c donors (BM) or 5 × 106 BM cells plus 5 × 106 purified CD90.2+ T cells from BALB/c donors (BM+T), and sST2 concentration in the serum was measured on day 7 and day 14 after allo-HCT by ELISA. *P < .05. (B) Survival of WT C57BL/6 mice after allo-HCT with 5 × 106 BM cells and 8 × 105 WT T cells from BALB/c recipients performed as described. Mice received sST2-Fc or isotype control IgG as indicated. The experiment was performed twice and the data were pooled. (C) The mean value ± SEM for (i) serum IL-6 and (ii) IFN-γ detected by ELISA on day 8 after allo-HCT. The experiment was performed twice and the data were pooled. (D) Histopathologic GVHD severity of the intestines (i, ii) and liver (iii) isolated on day 8 from mice treated as described under panel (B) when quantified as mean + SEM. Data are pooled from 2 independent experiments.
Discussion
The recent identification of elevated sST2 as a biomarker of steroid-refractory GVHD and mortality54 has raised interest in the role of IL-33 and its receptor after allo-HCT. We found reproducible results in 4 different laboratories despite differences known to affect GVHD models, such as presumed differences in microbiota between murine facilities or vendors.62 Our results demonstrate that the IL-33/ST2 axis was pro-inflammatory in the immediate period after allo-HCT. The IL-33/ST2 interaction on T cells elicits increased IFN-γ production, upregulation of IL-18R, and cell proliferation resulting in augmenting GVHD severity (supplemental Figure 10A). Blocking IL-33 interaction with ST2 by increasing levels of the sST2 receptor decreased pro-inflammatory cytokine production, tissue injury, and GVHD lethality (supplemental Figure 10B). In mice, sST2 acted as a negative regulator scavenging free IL-33 and preventing it from exerting its pro-inflammatory function. This may seem contradictory to the correlation of elevated sST2 with increased GVHD severity seen in patients, however, the high levels of sST2 in patients may be the reaction of the immune system to counteract IL-33–mediated inflammation. We favor the explanation that sST2 release into the serum comes too late in the inflammatory response leading to sST2 being overwhelmed by IL-33, resulting in its inability to quell the IL-33–mediated inflammatory effects.
IL-33 has a paradoxical role on immune responses dependent on the disease model, cell type, and inflammatory milieu present. Recent evidence has shown a role for IL-33 in reducing intestinal damage from inflammation by increasing the stability and function of colonic ST2+ Treg in a colitis model.38 However, the survival advantage associated with blocking the IL-33/ST2 axis on T cells during GVHD doesn’t appear to be dependent on the presence of Treg (Figure 4H), despite the susceptibility of the colon to GVHD. Perhaps this is due to the decreased presence of thymic-derived or inducible ST2+ Treg in the colon after GVHD as compared with homeostatic conditions in nontransplanted mice (supplemental Figure 11). IL-21, IL-6, and IL-23 are markedly increased in the colon during GVHD, and inhibit the induction of Tregs by driving Th17 differentiation and function resulting in negligible frequencies of induced Tregs.63-67 Signaling blockade of each of these ILs results in the increase of Tregs and attenuation of GVHD. Additionally, Schiering et al demonstrated that the effects of IL-33 on ST2+ Tregs are inhibited in the colon in the presence of IL-23.38 Our findings showing that IL-33 is a pro-inflammatory alarmin after allo-HCT is consistent with the reported increased IL-23 levels stated for mice developing aGVHD.65 Therefore, it is likely that the presence of IL-23 is preventing the tolerogenic effects of IL-33 on Tregs during GVHD, while not affecting the IL-33–induced activation of effector T cells in the colon.
Our finding that IL-18R was downregulated in ST2-deficient T cells compared with WT T cells, which was connected to reduced GVHD severity caused by these cells, cannot be directly compared with previous studies using IL-18–deficient mice, IL-18R–deficient mice, or treatment with IL-18, because the experimental approaches are different.68-70 However, consistent with our findings that the ST2-deficient T cells with lower IL-18R expression cause less GVHD, the report by Min et al70 showed that IL-18R–deficient donor CD8 T cells caused significantly less GVHD compared with WT T cells. Conversely, this effect was not seen when suing CD4 T cells deficient for IL-18R.70
IL-33 signaling through ST2 and IL-1 receptor accessory protein dimers leads to the recruitment of the myeloid differentiation primary-response protein 88 complex, which through a yet unclear mechanism activates at least 2 independent pathways: the phospholipase D–sphingosine kinase pathway with subsequent activation of the transcription factor nuclear factor-κB15,24 and the mitogen-activated protein kinase (MAPK) pathway,30,32 which is mediated by the activation of extracellular signal-regulated kinase, p38, and JUN N-terminal kinase.32-34,71 Downregulation of IL-18R in ST2-deficient T cells may be due to an impaired MAPK-dependent pathway,15,17,32 since the regulation of the receptor has been shown to be MAPK-dependent.22,36,37 Also, the activation of stress-activated protein kinases such as MAPK72 and ERK73 were previously shown to be relevant for the development of aGVHD.
Endogenous IL-33 protein production was increased in the GI tract of GVHD patients and in the nonhematopoietic cells within the GI tract of GVHD mice. The increase in IL-33 in our model was not alloantigen-related but in response to tissue damage from TBI, as mice that received only BM as well as those given T cells for the induction of GVHD increased IL-33 production equivalently. The sustained production of IL-33 after allo-HCT likely requires nonhematopoietic cells that are radioresistant. However, we cannot exclude that host hematopoietic cells may contribute some IL-33 production early post–allo-HCT. To our knowledge, this is the first demonstration of the effects of radiation and chemotherapy conditioning on IL-33 production. The allo-HCT recipients receiving BM only recover from the initial tissue injury, whereas in mice developing GVHD, IL-33 promotes Th1 polarization of the transferred T cells contributing to GVHD. NK, NK T cells, and CD8+ T cells upregulate ST2 expression following IL-12 exposure, which can occur during GVHD. IL-12 and IL-33 can act synergistically on the transferred CD8+ T cells to promote IFN-γ production, polarizing the immune response toward Th1-mediated immunity.24,31,74 Our finding that the IL-33/ST2 axis is linked to increased IFN-γ production in vivo during GVHD is compatible with previous studies in other disease models showing that IL-33 can induce IFN-γ in murine CD8+ T cells and NK T cells.74,75 Although the literature indicates a preferential expression of ST2 on Th2 cells, our in vitro and in vivo data indicate that ST2 expression is readily detectable on activated CD8+ T cells using our conditions for activation, source of antibody, and detection protocol (supplemental Figure 12A-D). However, this may differ depending on disease model. We describe a connection of IL-33 and IFN-γ in T cells in vivo, which is functionally dependent on both ST2 and IL-18R expression. However, we cannot fully dismiss the possibility that the decreased IFN-γ production in st2−/− T cells could be the result of an intrinsic defect that may be independent of IL-33. In human T cells, IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures.76 Importantly, although alloreactive T cells deficient in IL-33 signaling produced less IFN-γ and were less pathogenic, their cytotoxic activity important for graft vs leukemia responses was intact (supplemental Figure 13).
Our findings have potential to be exploited therapeutically to improve GVHD in patients. Manipulation of the IL-33/ST2 axis on T cells either by induction of endogenous sST2 earlier in the immune response (without inducing tissue injury) or the addition of exogenous agents resulting in IL-33/ST2 axis blockade may be an important target in GVHD prevention. However, there are still many important unknown determinants that need to be examined before such interventions can be considered. It will be important to identify the major cellular sources of sST2 after allo-HCT. It will also be critical to understand if interference of IL-33/ST2 engagement results in differences dependent on conditions of the disease model or the target of the intervention: sST2 blockade, blockade of the membrane bound receptor, or its ligand (IL-33). Further investigation into the mechanism by which the IL-33/ST2 axis exerts its effect in T cells will also be valuable. The genetic deficiency of ST2 in the T-cell compartment with insensitivity toward IL-33 reduced the induction of Th1 in pathogenic T cells during GVHD. ST2 deficiency in T cells correlated with lower IL-18R expression and IFN-γ production. Increasing the levels of sST2 post–allo-HCT led to reduced GVHD lethality and GVHD-associated tissue damage. These findings indicate that ST2-Fc treatment beginning early post-HCT has the potential to be used as a therapeutic intervention in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Novartis Institutes for Biomedical Research, Novartis Pharma AG (Basel, Switzerland) for valuable suggestions and for providing essential reagents (recombinant IL-33 and ST2-Fc antibody fragment). There was no financial support provided by Novartis Institutes for Biomedical Research, Novartis Pharma AG. The authors are grateful to Jürgen Finke for providing the clinical information on patients who had undergone allo-HCT.
This study was supported in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R01 AI34495 (B.R.B.), and the National Heart, Lung, and Blood Institute grants R01 HL56067 (B.R.B.) and T32 HL007062 (D.K.R). It was also supported in part by the Deutsche Forschungsgemeinschaft, Germany, SFB620 fellowship (V.S.), Heisenberg Professorship (DFG ZE 872/3-1), and DFG Einzelantrag (ZE 872/1-2) (R.Z.). Additional grant support was provided by the National Institutes of Health, National Heart, Lung, and Blood Institute grant R00 HL097155 (H.R.T.) and the National Institute of Allergy and Infectious Diseases grant T32AI074490 (B.M.M.). Further funding was derived from an American Society of Transplantation/Pfizer Basic Science Faculty Development Grant (H.R.T.).
Authorship
Contribution: D.K.R. designed and performed experiments, analyzed data, and wrote the manuscript; V.S., B.M.M., V.T., and E.L. designed and performed experiments, analyzed data, and edited the paper; Q.L., B.H.K., P.A.T., A.S.-G., D.P., G.P., H.D., N.S., and Y.B. designed and performed experiments, and edited the paper; T.J. and M.W. contributed vital new reagents, discussed experimental design, and edited the paper; S.N., M.F., T.W., L.S., J. Devlin, S.C.W., and J. Duyster discussed experimental design and analysis; J.L.M.F., H.R.T., R.Z., and B.R.B. designed and analyzed experiments, and edited the paper.
Conflict-of-interest disclosure: T.J. and M.W. are employees of Novartis Pharma AG. The remaining authors declare no competing financial interests.
Correspondence: Heth R Turnquist, Department of Surgery and Immunology, University of Pittsburgh School of Medicine, Thomas E. Starzl Transplantation Institute, 200 Lothrop St, BST E1554, Pittsburgh, PA 15213; e-mail: het5@pitt.edu; and Robert Zeiser, Department of Hematology and Oncology, University Medical Center Freiburg, Albert-Ludwigs-University, Hugstetterstrasse 55 Freiburg 79106, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de.
References
Author notes
D.K.R., V.S., and B.M.M. are co-first authors.
H.R.T., R.Z., and B.R.B. are co-senior authors.

![Figure 1. IL-33 expression in the murine small intestine increases upon TBI and in the colon of stage IV GVHD patients. (A-B) After TBI of BALB/c mice with 9 Gy, RNA expression levels of multiple genes in the small bowel were analyzed using microarray-based analysis. This experiment was performed once. (A) Shown is the tile display for the most significantly regulated genes encoding for cytokines, expressed by Robust Multichip Average (RMA). Signal values of 4 individual samples in the following groups are shown: untreated, 24 hours, and 48 hours after TBI. Green rectangle: IL-33 is the second most significantly regulated gene. (B) Each RMA value for IL-33 is shown, including the P values. (C-D) Gut tissue from control (day 0), lethally-irradiated (day 2 post-irradiation), and WT or C57BL/6 recipients, that had received either 2.5 × 106 T cells and 1 × 107 BM cells, or BM only from BALB/c donors in GVHD studies (day 7 or day 14) were stained for IL-33, CD45, vimentin, and 4,6 diamidino-2-phenylindole (DAPI). This experiment was performed once. Images are representative of n = 3 mice/group and graphs were generated by averaging 2 to 3 fields (IL-33/DAPI–20×; IL-33/CD45, or IL-33/vimentin–20×) per animal. Fluorescence area/intensity reported as arbitrary units. *P < .05; ***P < .001. This experiment was performed once. (E) Gut tissue from BALB/c mice 7 days after transplantation with 5 × 106 BM cells and 3 × 105 T cells from C57Bl/6 mice. Prior to transplantation on day 0, mice received either TBI (2× 4,5 Gy), busulfuan/cyclophosphamide (Bu/Cy: busulfan day −7 to day −4 [dose: 10 mg/kg]), cyclophosphamide day −3 and day −2 (dose 100 mg/kg), treosulfan/cyclophosphamide (Treo/Cy: treosulfan day −6 to day −4 [dose: 1.5 g/kg]), cyclophosphamide day −3 and day −2 [dose 100 mg/kg]), or thiotepa/cyclophosphamide (Thio/Cy: thiotepa day −6 to day −4 [dose: 10 mg/kg], cyclophosphamide day −3 and day −2 [dose 100 mg/kg]) conditioning regimens. Fluorescence area/intensity reported as in (C-D). (Ei) Bar diagram for the indicated groups. (Eii) Representative tissue sections. Images are representative of n = 2 to 3 mice per group and graphs were generated by averaging 3 to 6 fields (IL-33/DAPI–20×) per animal. This experiment was performed once. (F-G) The amount of IL-33 in human colon biopsies was quantified by immunohistochemistry as shown for one representative section per group ([Fi] no GVHD; [Fii] GVHD IV° of the intestines) and for multiple patients (G). Samples were taken at different time points after allo-HCT in patients without GVHD or with GVHD grade 3/4. Patients’ characteristics, time of biopsy, conditioning, and immunosuppression data are shown in supplemental Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-606830/4/m_3183f1.jpeg?Expires=1770048122&Signature=A3OLag-iscwOXuQyoNTYnHte3KNLv61BJ1BE6bm6u299bK9gn8iR3w25zxn4GARugzn04C1CtqM0DCTFiOmbB--SDoSMMRSt~U0qR0p7NbIfO6p0H4BB~KR091J0mYsJy5cywM3MnfLyukjMH48dP92Pzxrf1~TySiRVS2a9L3V0cQMqpgnvQ~3b0HoNTso1wlW~laMe8ZusQ1BV75VGJWOMKDPYT8vp-iXufzMpGGYOvIvs57Jp5HIfdZso1myLwel1cank0cxp6yzhI6oTthTSLxBpA8Cb4SjGcnKEpp4lsIBzo47sbcnI5YwxeZpuJHObC-WIHpUCXXYlnZf2Sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. IL-33 expression in the murine small intestine increases upon TBI and in the colon of stage IV GVHD patients. (A-B) After TBI of BALB/c mice with 9 Gy, RNA expression levels of multiple genes in the small bowel were analyzed using microarray-based analysis. This experiment was performed once. (A) Shown is the tile display for the most significantly regulated genes encoding for cytokines, expressed by Robust Multichip Average (RMA). Signal values of 4 individual samples in the following groups are shown: untreated, 24 hours, and 48 hours after TBI. Green rectangle: IL-33 is the second most significantly regulated gene. (B) Each RMA value for IL-33 is shown, including the P values. (C-D) Gut tissue from control (day 0), lethally-irradiated (day 2 post-irradiation), and WT or C57BL/6 recipients, that had received either 2.5 × 106 T cells and 1 × 107 BM cells, or BM only from BALB/c donors in GVHD studies (day 7 or day 14) were stained for IL-33, CD45, vimentin, and 4,6 diamidino-2-phenylindole (DAPI). This experiment was performed once. Images are representative of n = 3 mice/group and graphs were generated by averaging 2 to 3 fields (IL-33/DAPI–20×; IL-33/CD45, or IL-33/vimentin–20×) per animal. Fluorescence area/intensity reported as arbitrary units. *P < .05; ***P < .001. This experiment was performed once. (E) Gut tissue from BALB/c mice 7 days after transplantation with 5 × 106 BM cells and 3 × 105 T cells from C57Bl/6 mice. Prior to transplantation on day 0, mice received either TBI (2× 4,5 Gy), busulfuan/cyclophosphamide (Bu/Cy: busulfan day −7 to day −4 [dose: 10 mg/kg]), cyclophosphamide day −3 and day −2 (dose 100 mg/kg), treosulfan/cyclophosphamide (Treo/Cy: treosulfan day −6 to day −4 [dose: 1.5 g/kg]), cyclophosphamide day −3 and day −2 [dose 100 mg/kg]), or thiotepa/cyclophosphamide (Thio/Cy: thiotepa day −6 to day −4 [dose: 10 mg/kg], cyclophosphamide day −3 and day −2 [dose 100 mg/kg]) conditioning regimens. Fluorescence area/intensity reported as in (C-D). (Ei) Bar diagram for the indicated groups. (Eii) Representative tissue sections. Images are representative of n = 2 to 3 mice per group and graphs were generated by averaging 3 to 6 fields (IL-33/DAPI–20×) per animal. This experiment was performed once. (F-G) The amount of IL-33 in human colon biopsies was quantified by immunohistochemistry as shown for one representative section per group ([Fi] no GVHD; [Fii] GVHD IV° of the intestines) and for multiple patients (G). Samples were taken at different time points after allo-HCT in patients without GVHD or with GVHD grade 3/4. Patients’ characteristics, time of biopsy, conditioning, and immunosuppression data are shown in supplemental Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-606830/4/m_3183f1.jpeg?Expires=1770048123&Signature=BZitlatFU3wlQ15dgnEOYsGMJtM2gSCYeFEY9D2KcEAvhAnbzu-~P8kGEqpB3M57NLbsCIovw7WCNdioxwEVwi~sX7SjoxuZhQ8SWFhiYe8bpk2IuF1ZkYb5MHEAcnq~Oo7MzzrBuEgenrWq2yOXyrammHKu2grPS0~yVbkfh0gfHmq-6ujdZvBDiJQDoyBBCI9SBOyUN5jey0LuC3bhdN6cD1~VQITflPQPSIB5XRM6wrlyl6Dxk-n~G1r5BmlMcGYaIVgJdg3~ULQh7yuLa7S323EhPp-kBsT5TEdRYbl67gz9g1egM0fcZkD3HGnq0uBq3p3~Mg9Zs7r5iPK6vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)