Introduction
von Willebrand disease (VWD) is one of the most common inherited bleeding disorders, with a prevalence of symptomatic disease of ∼1 in 10 000. Given the complexity of the disease, the ability to accurately and appropriately diagnose individuals with VWD continues to be an important and much discussed topic of interest. In this review, we highlight the current status of clinical testing and diagnostic classifications that are useful to the clinician while also underscoring the current limitations of the existing tests.
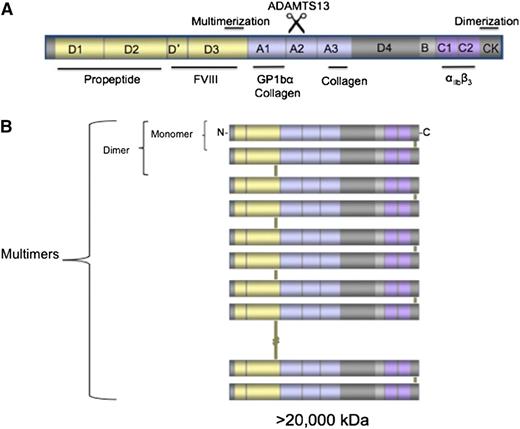
VWD, first described by Erik von Willebrand in a Scandinavian family,1 is characterized by abnormal quantity or quality of von Willebrand factor (VWF), a large glycoprotein synthesized by megakaryocytes and endothelial cells and released into the circulation through a constitutive pathway and also upon stimulation. VWF is the product of the VWF gene (VWF) on the short arm of chromosome 12 and is highly conserved across multiple species. The mature released molecule is 2050-aa long; in the intracellular space, it also has a 741-aa propeptide that is synthesized, cleaved, and released in equimolar concentrations with the VWF monomer making it a useful marker for VWF clearance.2 The VWF monomer has multiple domains that deliver its unique hemostatic abilities, including the localization of platelet-binding sites in the A1, C1, and C2 domains, collagen binding in the A1 and A3 domains, and a FVIII-binding site in the D′, D3 domains. VWF has a remarkable ability to multimerize via disulfide bonds at cysteine residues allowing for a range of VWF complexes that span from protomers (dimers) to multimers that contain >40 subunits3 (Figure 1). The high-molecular-weight multimers are the most effective forms of VWF at mediating platelet adhesion at sites of vascular injury.4,5 The biologic modulator of VWF is ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif), which can cleave VWF in the A2 domain when the VWF complexes are unraveled under shear conditions.6,7 The cleavage of ultra-large-molecular-weight multimers leads to production of smaller, lower-molecular-weight multimers that are inherently less hemostatically active.
VWF structure. (A) Graphic representation of the VWF monomer with its different domains and their binding ligands. Note that the D1 and D2 domains (propeptide) are cleaved upon released of VWF from the endothelial cell. (B) Depiction of the ability of VWF monomers to dimerize through the C terminus and of those dimers to multimerize via disulfide bonds at the D3 domain, reaching sizes >20 000 kDa.
VWF structure. (A) Graphic representation of the VWF monomer with its different domains and their binding ligands. Note that the D1 and D2 domains (propeptide) are cleaved upon released of VWF from the endothelial cell. (B) Depiction of the ability of VWF monomers to dimerize through the C terminus and of those dimers to multimerize via disulfide bonds at the D3 domain, reaching sizes >20 000 kDa.
VWD is traditionally classified into type 1 and type 3, which describe mild to moderate and severe quantitative deficiencies of VWF, respectively, and type 2, which is characterized by qualitative deficits in VWF. The prevalence of VWD in published studies ranges from 1 in 100 to 1 in 10 000 depending on the method used to identify patients, either by population screening of persons at risk or actual patients seen at medical centers8-11 ; therefore, it is reasonable to infer that the prevalence of individuals with clinically significant bleeding is likely in the range of 1:10 000.8 VWD affects individuals of all ethnic backgrounds, and the clinical symptoms can present at any age.
A large number of patients with VWD inherit the disease according to classic Mendelian genetics. Although VWD type 1 and 2 are generally inherited in an autosomal-dominant pattern, VWD type 3 is inherited in an autosomal-recessive pattern. Interestingly, not all individuals that have mutations in VWF exhibit clinical symptoms (a phenomenon known as incomplete penetrance), and although there is often a significant family history of bleeding associated with the diagnosis of VWD, many family members with low VWF levels may exhibit a range of bleeding symptoms (known as variable expressivity). Additionally, because mild bleeding is common in the general population and many individuals will have low to borderline levels of VWF, the association of these 2 traits may occur by chance in many occasions. The ability to identify a causative mutation in patients with VWD, particularly with type 1, is sometimes difficult. There are many patients, particularly with VWD type 1, for whom there is no causative mutation identified and genetic modifiers outside of the VWF gene may play significant roles in the modulation of VWF quantity, function, and multimer status. Therefore, a significant number of patients diagnosed with VWD type 1 may have a complex genetic disorder in which >1 gene coupled with environmental stressors is responsible for the phenotype.
Screening evaluation for VWD
Mucocutaneous bleeding
VWD exhibits a classic pattern of mucocutaneous bleeding in affected individuals. In particular, epistaxis, gum bleeding, and significant bruising are common complaints for individuals with VWD. Many young women are first diagnosed with VWD during their menarche, and menorrhagia represents one of the most significant morbidities of VWD. In 2005, the International Society on Hemostasis and Thrombosis: VWF SSC Subcommittee on VWF (ISTH SSC:VWF) published standardized guidelines on bleeding symptoms and family history.12 The clinical bleeding in VWD can range from bleeding usually associated with hemostatic challenges, such as trauma or surgical procedures to that triggered by mild activity or can even be spontaneous. Specifically, bleeding in VWD type 3 can present in a manner similar to hemophilia A or B with significant hemarthrosis and deep muscular hematomas due to very low plasma levels of coagulation factor VIII.
As patients with VWD often present with significant bleeding history, there have been many attempts to use questionnaire-based surveys to assess for the potential of VWD or related bleeding disorders. These questionnaires (known as bleeding assessment tools or BATs) are not universally sensitive and specific, and the receiver operating characteristic curves demonstrated significant tradeoffs in the sensitivity and specificity when a cutoff score is chosen.13,14 Although the published data support that bleeding scores may be useful for the identification of patients at risk for clinical bleeding,13,15 they demonstrate wide variation in their ability to define types of VWD16 and are likely not effective at making a sound diagnosis of VWD. Additionally, the overall time required to gather bleeding information with these BATs represents an obstacle for their use in real-time clinical decision-making.17 A condensed version of the BAT is available18 and although it shows significant promise, it is not universally specific in its ability to diagnosis VWD. In the presence of hemostatic challenges, such as menses or a tooth extraction, current data suggest that a normal bleeding score rules out the presence of a bleeding disorder. However, the lack of hemostatic challenges early in life can make the bleeding score age-dependent. Therefore, when positive, the use of these bleeding questionnaires should be followed by an evaluation by a hematologist to assess for the need for further laboratory-based screening to confirm the diagnosis of VWD.15
Laboratory screening
The prothrombin time and the partial thromboplastin time are not reliable tests in screening for a diagnosis of VWD. Although the partial thromboplastin time may be prolonged in specific types of VWD, such as VWD type 3 or type 2N (as being a proxy for the degree of factor VIII activity), it is not traditionally used as a screening test for VWD because in most cases it will be normal. Clinically, the greatest benefit of the PTT is the ability to screen for non-VWD causes of bleeding that would prolong clotting times such as factor XI deficiency. A normal PTT coupled with a normal score in a BAT have been demonstrated to increase the negative predictive value of a mild bleeding disorder in some bleeding evaluation algorithms.14 The complete blood count is neither sensitive nor specific in the diagnosis of VWD, and abnormalities are usually only seen in the setting of significant bleeding leading to iron-deficiency anemia or in specific types of VWD associated with thrombocytopenia, such as VWD type 2B. The bleeding time is historically an important test for mucocutaneous-specific bleeding and was traditionally used to screen for bleeding disorders related to platelets and VWF. However, the bleeding time is rarely used in current practice. The sensitivity and specificity of the test have been largely questioned due to significant differences reported among different centers and practitioners.19 Finally, the Platelet Function Analyzer (PFA 100) is a relatively novel test that measures both platelet adhesion to collagen and platelet aggregation, thus being VWF-sensitive. Although the PFA 100 has shown promise in evaluating patients with VWD, it is more commonly used to screen for platelet abnormalities and has been shown to lack a uniform high sensitivity and specificity for VWD.19-24 Therefore, given the inability of each of these screening tests to accurately diagnose VWD or even to guide the diagnosis, it is essential to conduct VWF-specific testing if VWD is highly suspected due to a significant bleeding history.
Laboratory testing for VWD
Current standard practice in the laboratory diagnosis for VWD relies on 2 VWF-specific tests that evaluate (1) the quantity of VWF that is present in plasma, and (2) the efficacy of this plasma VWF in its ability to bind platelets in the presence of the antibiotic ristocetin. A combination of these tests, plus the measurement of coagulation factor VIII (FVIII:C), allows for the classification of patients into the classic groupings of VWD type 1, type 2, or type 3. Although there have been many novel additions to these tests, these basic VWF assays remain essential to making an accurate diagnosis.
VWF:Ag
The VWF antigen level (VWF:Ag) is typically conducted using an enzyme-linked immunosorbent assay (ELISA)–based immunoassay method, allowing for the quantification of VWF present in patient plasma. The normal range of VWF:Ag value varies with each laboratory, but is generally accepted to be between 50 and 200 IU/dL. Levels below 50 are considered to be low, although the degree to which these levels may be depressed on a population-wide basis generates controversy in the diagnosis of VWD. It is well known that individuals with blood group type O exhibit a 25% decrease in VWF levels when compared with individuals with blood type A.25
VWF:RCo
The VWF ristocetin cofactor assay (VWF:RCo) takes advantage of the ability of ristocetin to promote platelet agglutination in the presence of VWF. Initially discovered as an antibiotic, ristocetin has been used for decades to evaluate the glycoprotein 1bα (GP1bα)–mediated binding role of VWF, being considered a qualitative test for VWF. Much like the VWF:Ag, the normal range of VWF:RCo is 50 to 200 IU/dL. Several alternative assays for ristocetin cofactor have been developed by using bound GPIbα in an ELISA platform with or without ristocetin; these assays show promise to perhaps replace the VWF:RCo assay in the near future.26-28 The VWF:RCo has several limitations including the fact that it can be affected by VWF sequence variations; in particular, an A1 domain single nucleotide polymorphism (D1472H) affects VWF binding to ristocetin without altering in vivo activity. This has no clinical significance but can lead to a misdiagnosis of VWD.29
Ratio of VWF:RCo/VWF:Ag
In order to differentiate VWD type 1 and 2, the ratio of VWF:RCo and VWF:Ag can be used. Because VWD type 1 commonly results in a concomitant depression in both VWF:RCo and VWF:Ag, the ratio of these 2 values tends to remain around 1. Conversely, in VWD type 2, where the function of VWF is decreased to a greater extent that the amount of protein, the VWF:RCo decrease is disproportionate to the VWF:Ag level. Thus, a ratio of <0.6 is often indicative of VWD type 2.30-32
FVIII:C
FVIII:C levels are an important diagnostic evaluation in the diagnosis of VWD. Individuals with type 1 or type 2 may have lower levels of FVIII:C, as VWF is necessary to stabilize and chaperone the FVIII protein in plasma. In VWD type 3, the severe absence of VWF leads to a dramatic decrease in FVIII:C that may approximate levels of moderate-severe hemophilia. There is also a significant decrease of FVIII:C in VWD type 2N, where a mutation in VWF abrogates FVIII binding, resulting in decreased FVIII levels in plasma due to accelerated proteolysis, although in this case the VWF:Ag levels are not as severely depressed as in VWD type 3.
VWF multimers
High-molecular-weight multimers are the most hemostatically active.4,5 Given the differential effectiveness of VWF multimers, it is not surprising that variations in the distribution of multimer sizes in patient’s plasma can alter their hemostatic phenotype. Multimer analysis is commonly done via sodium dodecyl sulfate agarose gels with various agarose gel concentrations to resolve multimer patterns. A normal pattern of VWF multimers shows a relatively even proportion of multimer sizes ranging from large to small. Although many specialized hemostasis centers use them as “first-line” testing along with the initial panel of laboratory tests, in common clinical practice the multimer pattern is used as essential “second-line” testing after the initial VWF:Ag and VWF:RCo because it is especially useful in differentiating between different subtypes of VWD type 2. One limitation of the analysis is the inability to accurately quantify multimers. Ongoing efforts by investigators are attempting to address this issue.33 The specific utility of multimer analyses is discussed in the next section in the context of the different types of VWD. It is important to mention that multimer analysis is dependent on several preanalytical variables and therefore proper collection and processing methods should be followed.
It is important to mention that these tests can be used to determine response to therapeutic interventions such as desmopressin.
Specialized laboratory testing
Once the diagnosis of VWD is made, clinicians have a battery of tests available, mostly in specialized reference laboratories, which will provide additional information for the diagnosis of different subtypes of VWD. These tests may confirm a rare subtype that will determine therapeutic alternatives.
VWF:collagen-binding capacity
Several assays have been developed to measure plasma VWF binding to collagen and because they are sensitive to the presence of high-molecular-weight multimers, these assays have been proposed as replacements of the VWF:RCo. Most collagen-binding assays use collagens type 1 and 3, but recently the use of collagen type 6 has allowed for the identification of potential VWF-binding defects not identified by other types of collagen.32,34 More recently, families with abnormalities in VWF due to mutations in VWF that decrease the ability of VWF to bind to various types of collagen32,35 have been described.
VWF propeptide
Several laboratories around the world offer quantitation of the VWF propeptide (VWFpp). Because the propeptide of VWF is synthesized and released on a 1:1 basis with the VWF monomer, quantification of this protein is useful in characterizing clinical situations in which VWF shows accelerated clearance (because pathological clearance mechanisms only affect VWF:Ag and not VWFpp). By using the VWFpp/VWF:Ag ratio, it is possible to identify individuals with markedly elevated VWF clearance.2,36
Low-dose ristocetin-induced platelet aggregation
Low-dose ristocetin-induced platelet aggregation (LD-RIPA) is a test that uses a lower dose of ristocetin than that for the VWF:RCo assay in order to detect hyperreactivity of VWF as seen in VWD type 2B or platelet-type pseudo-VWD.37,38 The source of platelets and plasma (patient plasma or platelets vs fixed platelets or standard plasma) allows for the determination of the molecule responsible for the hyperresponse: the mutated VWF or the mutated GP1bα.
VWF:FVIII-binding capacity
The VWF:FVIII-binding assay measures the ability of patient’s VWF to bind recombinant FVIII in vitro in an ELISA. This assay allows for the identification of patients with defects in FVIII binding such as the ones seen in VWD type 2N.39
Genetic testing
Genetic testing for VWD is not indicated except for specific cases in which the results of the test would make a difference in the patient's therapeutic management or counseling. There are several complicating factors that make genetic testing difficult for VWD. VWF is a very large gene that spans 178 kb and contains 52 exons. The presence of a highly homologous partial pseudogene in chromosome 22 makes the sequencing and its interpretation particularly difficult. Additionally, the gene is also highly polymorphic with >300 single nucleotide polymorphisms reported. Recently, the presence of common variants that affect the VWF:RCo assay have been described, however, they do not appear to increase the risk of bleeding.29,40 Therefore, genetic testing should be limited to very specific situations. For example, it might be important to identify large deletions in patients with VWD type 3 that would place them at higher risk of developing neutralizing antibodies and anaphylactic reactions upon treatment or to provide genetic counseling for patients. Also, genetic testing may also be justified in cases in which the molecular diagnosis will help to guide treatment, such as type 2N and 2B VWD. In these particular cases, mutations are usually clustered in specific areas of VWF, making sequencing and interpretation of the data less cumbersome.
Quantitative defects of VWF
VWD type 1
VWD type 1 is the most common form of VWD. It affects ∼80% of all individuals diagnosed with VWD and is traditionally characterized by reduction of functionally normal VWF in the presence of mucocutaneous bleeding. The VWF:RCo/VWF:Ag ratio is >0.7.41,42 Most reported mutations associated with VWD type 1 are missense causing a dominant-negative effect. Additionally, several mechanisms have been shown to cause low VWF levels mostly related to decreased cellular secretion of VWF, including mutations that affect gene expression,43 protein trafficking,44 or mild increases in VWF clearance.45 Typical levels are notable for an absolute value of VWF:Ag below 40 IU/dL with a normal multimer distribution and normal proportion of high-molecular-weight multimers (although multimers are usually faint because there is a decreased amount of protein). Once the diagnosis of VWD type 1 is made based on clinical history and laboratory testing, no further specialized testing is usually required.
VWD type 1C
In comparison with the classic VWD type 1, where there is an alteration in the production of VWF or mild increases in clearance, VWD type 1C (C for clearance) is characterized by a significant increase of VWF clearance, leading to low VWF levels and a higher than normal response to desmopressin: 1-desamino-8-D-arginine vasopressin (DDAVP) but significantly shortened half-life. Patients with VWD type 1C are identified by an abnormal ratio of VWFpp to VWF:Ag because only the mature VWF molecule is cleared more rapidly. The ratio of VWFpp to VWF:Ag is significantly elevated in the plasma of patients with VWD type 1C, proving that the defect is in abnormally accelerated clearance of VWF as opposed to reduced synthesis.2,46 In some patients with type 1C, multimer analysis often reveals the presence of ultra-large-molecular-weight multimers likely explained by high turnover of VWF which does not give enough time for ADAMTS13 to cleave the VWF, resulting in the presence of recently secreted ultra-large-molecular-weight multimers in plasma. Recently, a higher proportion of patients with type 1 were found to have abnormal VWFpp:VWF:Ag ratios, suggesting that VWD type 1C is more common than previously suspected.47
Making a diagnosis of VWD type 1C has significant therapeutic implications because these patients may not be good candidates for desmopressin, due to the decreased half-life of their native VWF.
Low VWF levels
Perhaps no group engenders more controversy in the diagnosis of VWD than the patients who have VWF levels in the 40 to 50 IU range and present with mild mucocutaneous bleeding. Although this value is technically below the normal level for VWF:Ag or VWF:RCo in a number of clinical laboratories, it is also known that 14% of individuals with blood type O (a known modifier of VWF levels) will have VWF levels ≤50 IU and that other genetic variants in multiple loci can affect VWF levels.48,49 In 2008, the National Institutes of Health published guidelines (www.nhlbi.nih.gov/health-pro/guidelines/current/von-willebrand-guidelines/full-report/4-management-of-vwd.htm) in which the suggested level for the designation of type 1 VWD was a VWF:Ag or VWF:RCo level of <30 IU/dL50 and “low VWF levels” refer to individuals with VWF:Ag levels between 30 and 50 IU/dL. It is becoming increasingly evident that borderline normal plasma VWF levels represent 1 among several risk factors for bleeding and cannot be assigned as the only cause for mucocutaneous bleeding in these patients. Regardless of the label giving to the clinical condition, patients who demonstrate significant bleeding associated with low VWF levels are often treated as VWD type 1 patients as they still may benefit from therapy such as desmopressin to raise the level of plasma VWF.
VWD type 3
VWD type 3 is a severe deficiency of VWF, usually due to autosomal-recessive or compound heterozygote inheritance of a null VWF allele.51 This leads to not only low VWF:Ag and VWF:RCo levels, but also low FVIII:C levels as FVIII is not stabilized in the plasma by VWF. Because FVIII:C levels are usually <10 IU/dL, the bleeding patterns of these patients are severe and can mimic hemophilia, including the occurrence of hemarthrosis given the extremely low values of FVIII:C.
Disorders of VWF function: VWD type 2
VWD type 2 is characterized by qualitative defects in VWF, usually manifesting in asymmetric decreases in VWF:RCo and VWF:Ag, leading to a VWF:RCo/VWF:Ag that is lower than 0.7.41,42 The specific subtypes of VWD type 2 are due to mutations in VWF that cause abnormalities in the interactions of VWF with its ligands leading to functional defects and requiring specialized testing such as LD-RIPA or FVIII-binding capacity for an accurate diagnosis (Figure 2).
Mechanisms for VWD type 2. Schematic representation of VWF and its main interactions with platelets and FVIII, and how these interactions, when affected, cause VWF functional deficits and subsequent clinical bleeding.
Mechanisms for VWD type 2. Schematic representation of VWF and its main interactions with platelets and FVIII, and how these interactions, when affected, cause VWF functional deficits and subsequent clinical bleeding.
VWD type 2A
VWD type 2A is the most common form of type 2 disease, and is characterized by preferential loss of intermediate- and high-molecular-weight VWF multimers, usually through abnormal synthesis or packaging of VWF prior to endothelial cell release45 or to increased susceptibility to ADAMTS13 after exocytosis.52 Although the underlying pathophysiology can be heterogeneous, the resultant loss of multimers causes a moderate to severe qualitative defect due to diminished platelet binding. Given similarities with type 2B, the LD-RIPA can be helpful to differentiate type 2A (normal LD-RIPA) from type 2B.
VWD type 2B
In contrast to type 2A, in VWD type 2B there is significantly increased VWF-platelet GP1bα binding due to gain-of-function mutations in VWF.53 This type is classically diagnosed with an increased LD-RIPA because of the enhanced ability of VWF to bind its platelet receptor GP1bα. The increased binding of VWF (especially the high-molecular-weight multimers) to platelets under physiologic shear form aggregates that are rapidly cleared from the circulation, resulting in a decreased total amount of VWF, loss of high-molecular-weight multimers, and often thrombocytopenia. It is important to mention that thrombocytopenia is not always present in VWD type 2B.54
In patients diagnosed with VWD type 2B that do not respond well to VWF concentrate infusion, it is critical to differentiate VWD type 2B from platelet-type pseudo-VWD because they are clinically indistinguishable, but the therapeutic approach is different. Platelet-type pseudo-VWD is due to mutations in GP1BA, the gene that encodes the GP1bα receptor (instead of mutations in VWF) such that it binds to VWF spontaneously and at a much higher rate than the normal receptor.55 The response of the LD-RIPA with either patient’s plasma and fixed platelets, or patient’s platelets and normal plasma, allows for the identification of the defect. Although the treatment of bleeding in VWD type 2B is with VWF concentrates, in the platelet-type pseudo-VWD, platelet transfusions are the treatment of choice.
VWD type 2M
VWD type 2M is due to mutations in VWF that cause decreased interaction of VWF with GP1bα; therefore, the VWF:RCo assay is reduced, leading to a low VWF:RCo/VWF:Ag ratio (<0.7). In this case, the distribution of multimers is normal, but they are intrinsically dysfunctional because they cannot bind platelets properly. Therefore, a low VWF:RCo/VWF:Ag ratio in the presence of normal multimers strongly suggests the diagnosis of VWD type 2M.
VWD type 2N
VWD type 2N is a rare defect which results in the inability of VWF to bind FVIII56 leading to accelerated clearance of unbound FVIII and therefore disproportionate lower levels of FVIII:C when compared with VWF:Ag. Affected individuals are usually homozygous or compound heterozygotes for a VWF mutation in the FVIII-binding site (D′D3)56 or, alternatively, heterozygotes in the presence of a VWD type 1 allele. Therefore, patients with type 2N disease manifest not only with low VWF:RCo levels, but also with lower FVIII:C levels that can mimic the presentation of mild hemophilia. Type 2N should be suspected in individuals diagnosed with mild hemophilia who belong to families that have affected males and females or who do not respond adequately to FVIII infusions. The definitive diagnosis of VWD type 2N is made by measuring the VWF:FVIII-binding capacity and eventually can be confirmed with genetic testing. A simple algorithm that can be used as a guide to the diagnosis of the several subtypes of VWD is presented in Figure 3.
Diagnostic algorithm. Algorithm of the diagnostic approach to mucocutaneous bleeding with a high clinical suspicion for VWD. The initial diagnosis is based on VWF:Ag, VWF:RCo, and FVIII:C whereas different types are diagnosed based on specialized tests. Specific groupings are based on the underlying pathophysiology of different VWD types. RIPA, ristocetin induced platelet aggregation.
Diagnostic algorithm. Algorithm of the diagnostic approach to mucocutaneous bleeding with a high clinical suspicion for VWD. The initial diagnosis is based on VWF:Ag, VWF:RCo, and FVIII:C whereas different types are diagnosed based on specialized tests. Specific groupings are based on the underlying pathophysiology of different VWD types. RIPA, ristocetin induced platelet aggregation.
Advances and limitations in the current clinical/laboratory tests available for the diagnosis of VWD
Although widely used and important in the diagnosis of VWD, the currently available clinical evaluations and laboratory tests are not infallible; often indeterminate or unreliable results can lead to confusion in the diagnosis of VWD. Perhaps most obviously, the discrepancy in cutoff normal values for VWF:Ag, VWF:RCo, and other specialized tests among laboratories can lead to a wide variation in the diagnosis of VWD type 1.57,58 The VWF:RCo, used primarily to determine abnormalities in function of VWF, has a high coefficient of variation between laboratories, and this variation increases significantly when the level of VWF:RCo is below 15 IU/dL.59,60 Novel automated systems that test VWF activity can minimize operator imprecision61-63 and provide timely results useful in therapeutic trials64 but may still show relatively wide coefficients of variation.65 Standardization of all assays for VWF is an ongoing effort66 led by the ISTH. It is still recommended that all laboratories establish their own normal ranges and follow strict quality control procedures.
Another critical issue is that although VWF:Ag levels generally have low coefficients of variation, they can vary widely in the same individual due to several environmental factors including stress, exercise, and inflammatory states. Therefore, if VWD is highly suspected, laboratory tests may be repeated at a different time to either refute or confirm the diagnosis. In addition, many preinstrumental variables can play a role in the determination of VWF. Improperly isolated plasma can be contaminated by platelets, leading to protease-induced alterations and causing increased VWF levels but paradoxically decreased activity. Additionally, the refrigeration of whole blood prior to plasma separation may alter VWF multimeric structure.
It is becoming more evident that the discrepancies in test values among clinical laboratories as well as the use of more specialized tests can have a significant impact on the diagnosis of VWD. Large population-based studies have demonstrated that a significant number of patients who had been initially diagnosed with VWD type 1 had multimer distributions compatible with VWD type 2 upon further investigation with high-resolution gels.18,67,68 The need for highly specialized hemostasis laboratories to conduct tests such as multimer analysis, VWF:collagen-binding capacity, and VWF:FVIII binding presents another challenge in the accurate diagnosis of different types of VWD (Table 1).
Conclusions
Perhaps the 2 most significant limitations of current testing for VWD are (1) the inability to simulate the function of VWF under conditions of physiologic flow and (2) the limitations to adequately correlate current clinically available and research-based testing with clinical bleeding. Presently, all of the standard and more advanced laboratory tests are performed under static conditions. However, novel examination of the ability of VWF to bind platelets and collagen under flow conditions may reveal abnormal phenotypes in function that are not able to be assessed with current tests. Novel flow-based research assays have been recently developed. These assays may be able to identify not only those individuals at high risk for bleeding, but also, hopefully, safely predict those individuals for whom the risk of clinical bleeding is low.69-71 It is expected that in the future, with better understanding of the genetics and biochemistry of VWF as well as other modifying factors for bleeding, investigators and clinicians will be able to more accurately diagnose VWD and eventually develop novel therapies for the disease.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01 HL084086 (J.D.P.) and the Postle Chair of Pediatric Cancer and Blood Disorders (J.D.P.). C.N. is a recipient of the National Hemophilia Foundation Baxter Clinical Fellowship Award and the Hemostasis and Thrombosis Research Society Mentored Research Award.
Authorship
Contribution: C.N., D.G.M., and J.D.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge Di Paola, 12800 East 19th Ave, Aurora, CO 80045; e-mail: jorge.dipaola@ucdenver.edu.