Abstract
Despite the worldwide prevalence of rare bleeding disorders (RBDs), knowledge of these conditions and their management is suboptimal; health care professionals often have little diagnostic and treatment experience with variable access to diagnostic modalities required for accurate identification. Therefore, patients often experience morbidity and mortality due to delayed diagnosis. As RBDs represent a small potential commercial market, few, if any, specific therapies exist for these conditions. As a result, affected individuals commonly face delayed diagnosis, incomplete laboratory evaluation, and limited treatment options. Standardization and customization of coagulation assays, full genome sequencing, and global clotting assays will significantly improve diagnosis of patients with RBDs. In addition, new therapeutic modalities, both recombinant and plasma derived, are emerging, at least in developed countries. Registries and clinical trials have demonstrated decreased bleeding and improved outcomes when patients are appropriately diagnosed and properly treated. Expansion and harmonization of international registries has been initiated to correlate genotype, laboratory, and clinical phenotypes including bleeding severity to improve the diagnosis and therapeutic approach. This review focuses on the latest advances in our understanding, diagnosis, and treatment of RBDs.
Introduction
Rare inherited bleeding disorders (RBDs), including deficiencies of coagulation factors fibrinogen, factor (F)II, FV, combined FV and FVIII, FVII, FX, FXI, FXIII, and congenital deficiency of vitamin K-dependent factors (VKCFDs), are transmitted as autosomal recessive conditions; some cases of FXI and dysfibrinogenemia may be autosomal dominant.1 RBDs are reported in most populations, with homozygous or a double heterozygous incidence varying from 1 in 500 000 for FVII deficiency to 1 in 2 to 3 million for prothrombin and FXIII deficiencies (Table 1).1,2 Relative frequency varies among populations, being higher where consanguineous or endogamous marriages are common, with increased frequency of specific mutant genes.3-8
General features of autosomal recessive deficiency of coagulation factors
| Deficiency . | Estimated prevalence* . | Gene (chromosome) . | Laboratory diagnosis . |
|---|---|---|---|
| Fibrinogen | 1 in 1 million | FGA, FGB, FGG (all on 4q28) | Afibrinogenemia: TT ↑↑, APTT ↑↑, PT ↑↑ |
| Dys- and Hypodisfibrinogenemia: TT ↑, APTT ↑, PT ↑↑ | |||
| Prothrombin | 1 in 2 million | F2 (11p11–q12) | TT normal, APTT ↑, PT ↑ |
| Factor V | 1 in 1 million | F5 (1q24.2) | TT normal, APTT ↑, PT ↑ |
| Combined factor V and VIII | 1 in 1 million | LMAN1 (18q21.3–q22) | TT normal, APTT ↑, PT ↑ |
| MCFD2 (2p21–p16.3) | |||
| Factor VII | 1 in 500 000 | F7 (13q34) | TT normal, APTT normal, PT ↑ |
| Factor X | 1 in 1 million | F10 (13q34) | TT normal, APTT ↑, PT ↑ |
| Factor XI | 1 in 1 million | F11 (4q35.2) | TT normal, APTT ↑, PT normal |
| Factor XIII | 1 in 2 million | F13A1 (6p24–p25) | TT normal, APTT normal, PT normal Specific assays required |
| F13B (1q31–q32.1) | TT normal, APTT normal, PT normal Specific assays required | ||
| Vitamin-K dependent coagulation factors | Reported in <50 families | GGCX (2p12) | TT normal, APTT ↑, PT ↑↑ |
| VKORC1 (16p11.2) |
| Deficiency . | Estimated prevalence* . | Gene (chromosome) . | Laboratory diagnosis . |
|---|---|---|---|
| Fibrinogen | 1 in 1 million | FGA, FGB, FGG (all on 4q28) | Afibrinogenemia: TT ↑↑, APTT ↑↑, PT ↑↑ |
| Dys- and Hypodisfibrinogenemia: TT ↑, APTT ↑, PT ↑↑ | |||
| Prothrombin | 1 in 2 million | F2 (11p11–q12) | TT normal, APTT ↑, PT ↑ |
| Factor V | 1 in 1 million | F5 (1q24.2) | TT normal, APTT ↑, PT ↑ |
| Combined factor V and VIII | 1 in 1 million | LMAN1 (18q21.3–q22) | TT normal, APTT ↑, PT ↑ |
| MCFD2 (2p21–p16.3) | |||
| Factor VII | 1 in 500 000 | F7 (13q34) | TT normal, APTT normal, PT ↑ |
| Factor X | 1 in 1 million | F10 (13q34) | TT normal, APTT ↑, PT ↑ |
| Factor XI | 1 in 1 million | F11 (4q35.2) | TT normal, APTT ↑, PT normal |
| Factor XIII | 1 in 2 million | F13A1 (6p24–p25) | TT normal, APTT normal, PT normal Specific assays required |
| F13B (1q31–q32.1) | TT normal, APTT normal, PT normal Specific assays required | ||
| Vitamin-K dependent coagulation factors | Reported in <50 families | GGCX (2p12) | TT normal, APTT ↑, PT ↑↑ |
| VKORC1 (16p11.2) |
APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time.
Including dysfunctional proteins.
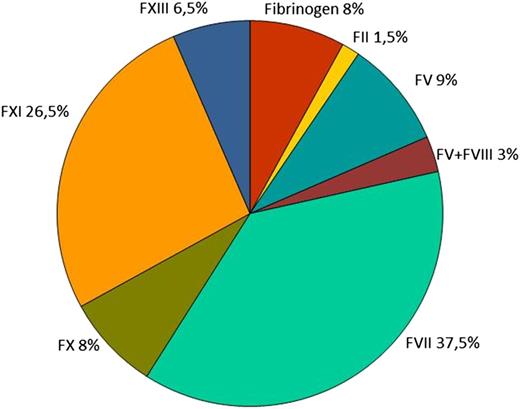
The evaluation of the worldwide RBD distribution relies on 2 large surveys that collected epidemiologic data; one is led by the World Federation of Haemophilia (WFH, http://www.wfh.org/) and the other is within the European Network of the Rare Bleeding Disorders (EN-RBD; http://www.rbdd.eu/). The WFH began RBD data collection in ∼2004, whereas the EN-RBD project began in 2007. Data from both surveys confirmed FVII and FXI deficiencies as the most prevalent, representing ∼39% and 26%, respectively, of the total affected population, followed by deficiencies of fibrinogen, FV and FX (8-9%), FXIII (∼6%), and combined FV and FVIII (∼3%); the rarest disorder was FII deficiency (prevalence, 1%; Figure 1). The WFH survey revealed that half of the available data originated from Europe, underscoring the need for increased efforts to establish accurate diagnosis and improved worldwide data collection systems.
Clinical symptoms
Clinical symptoms among RBD patients vary significantly between disorders, and patients, even when affected with the same disorder. A comparison of the most and least common symptoms among those with severe deficient RBDs is shown in Table 2.9-16 Heterozygous individuals commonly do not manifest a bleeding tendency. Mucocutaneous and surgical associated bleeding were reported in 20% of patients, whereas post-traumatic hemarthrosis and hematomas are rarely reported in FVII and FX deficiencies. Menorrhagia, spontaneous abortion, and bleeding during vaginal delivery were reported in ∼20% of women with all deficiencies.17-19
Clinical symptoms in severe RBDs
| RBD . | Afibrinogenemia; hypo- and dysfibrinogenemia . | Prothrombin deficiency . | FV deficiency . | Combined FV and FVIII deficiency . | FVII deficiency . | FX deficiency . | FXI deficiency . | FXIII deficiency . | Vitamin K-dependent factors deficiency . |
|---|---|---|---|---|---|---|---|---|---|
| Main bleeding symptoms for severe deficiencies | AFIBRINOGENEMIA: Common: Umbilical cord Epistaxis First-trimester abortion Less common: Skin GI Genito-urinary tract CNS Menorrhagia Uncommon: Musculoskeletal | Common: Subcutaneous and muscle hematomas Prolonged post-injury Mucosal tract Hemarthrosis Menorrhagia Less common: Postoperative Uncommon: CNS GI | Common: Epistaxis Menorrhagia Skin Mucosal tract Postoperative Less common: Umbilical cord Hematomas Hemarthrosis Rare: CNS GI | Common: Easy bruising Epistaxis Gingival postoperative Post-dental extraction Post circumcision Menorrhagia Post partum Uncommon: Hemarthrosis Umbilical cord Rare: CNS GI | Common: Easy bruising Epistaxis Gum bleeding Menorrhagia After surgery Less common: Hemarthrosis Hematoma Hematuria CNS GI | Common: Umbilical cord Epistaxis Menorrhagia Hemarthrosis Hematomas Post trauma Postoperative Less common: CNS GI Hematuria | Common: Oral cavity Postoperative Menorrhagia | Common: Umbilical cord CNS Ecchymosis Subcutaneous hematoma Oral cavity After trauma Menorrhagia Miscarriages and intraperitoneal Less common: Wound healing Hemarthrosis Muscle hematomas Epistaxis GI Postoperative | Common: Intracranial (at birth) Umbilical cord Retroperitoneal soft tissue Easy bruising Mucocutaneous Postoperative Children may show skeletal abnormalities |
| Risk of thrombosis | Afibrinogenemia: reported | In inherited dysprothrombinemia due to g20210a mutation and linked to slightly increased levels of circulating prothrombin, there is a significantly higher risk to develop thromboembolic diseases | — | — | Thrombotic episodes, particularly deep vein thrombosis post treatment reported (3-4% of patients) spontaneous thrombosis may occur | — | Cases of myocardial infarction and venous thrombosis reported (idiopathic or after fXI infusion) | — | Although proteins S/C levels decreased, no reports of venous/arterial thrombosis |
| Dysfibrinogenemia: reported |
| RBD . | Afibrinogenemia; hypo- and dysfibrinogenemia . | Prothrombin deficiency . | FV deficiency . | Combined FV and FVIII deficiency . | FVII deficiency . | FX deficiency . | FXI deficiency . | FXIII deficiency . | Vitamin K-dependent factors deficiency . |
|---|---|---|---|---|---|---|---|---|---|
| Main bleeding symptoms for severe deficiencies | AFIBRINOGENEMIA: Common: Umbilical cord Epistaxis First-trimester abortion Less common: Skin GI Genito-urinary tract CNS Menorrhagia Uncommon: Musculoskeletal | Common: Subcutaneous and muscle hematomas Prolonged post-injury Mucosal tract Hemarthrosis Menorrhagia Less common: Postoperative Uncommon: CNS GI | Common: Epistaxis Menorrhagia Skin Mucosal tract Postoperative Less common: Umbilical cord Hematomas Hemarthrosis Rare: CNS GI | Common: Easy bruising Epistaxis Gingival postoperative Post-dental extraction Post circumcision Menorrhagia Post partum Uncommon: Hemarthrosis Umbilical cord Rare: CNS GI | Common: Easy bruising Epistaxis Gum bleeding Menorrhagia After surgery Less common: Hemarthrosis Hematoma Hematuria CNS GI | Common: Umbilical cord Epistaxis Menorrhagia Hemarthrosis Hematomas Post trauma Postoperative Less common: CNS GI Hematuria | Common: Oral cavity Postoperative Menorrhagia | Common: Umbilical cord CNS Ecchymosis Subcutaneous hematoma Oral cavity After trauma Menorrhagia Miscarriages and intraperitoneal Less common: Wound healing Hemarthrosis Muscle hematomas Epistaxis GI Postoperative | Common: Intracranial (at birth) Umbilical cord Retroperitoneal soft tissue Easy bruising Mucocutaneous Postoperative Children may show skeletal abnormalities |
| Risk of thrombosis | Afibrinogenemia: reported | In inherited dysprothrombinemia due to g20210a mutation and linked to slightly increased levels of circulating prothrombin, there is a significantly higher risk to develop thromboembolic diseases | — | — | Thrombotic episodes, particularly deep vein thrombosis post treatment reported (3-4% of patients) spontaneous thrombosis may occur | — | Cases of myocardial infarction and venous thrombosis reported (idiopathic or after fXI infusion) | — | Although proteins S/C levels decreased, no reports of venous/arterial thrombosis |
| Dysfibrinogenemia: reported |
CNS, central nervous system; GI, gastrointestinal.
Women with RBDs require specific attention and care; in addition to the common bleeding symptoms, they may also experience gynecologic bleeding.20,21 Beyond menorrhagia, affected females are at increased risk of hemorrhagic ovarian cysts, endometriosis, endometrial hyperplasia, polyps, and fibroids. Pregnancy and childbirth pose particular clinical challenges, and miscarriages, bleeding during pregnancy and postpartum hemorrhage have been frequently reported in some deficiencies. These events impact quality of life and employment.22,23
Classification
RBD rarity has resulted in limited knowledge with decreased focus on etiologic and pathogenetic research and difficulty describing natural history and variants. Each RBD can have several bleeding symptoms ranging from minor post-traumatic to severe episodes appearing at birth or later in life. In some deficiencies, residual coagulant activity is directly related to hemorrhagic risk, yet this is not true for all. The first correlation of residual coagulant activity and clinical RBD bleeding severity was reported by the EN-RBD, based on data from 489 patients representing 13 European treatment centers.24 Clinical bleeding episodes were classified into 4 severity categories based on location, potential clinical impact, and bleeding trigger either being spontaneous, after trauma, or drug induced (Table 3). This study documented a strong association between residual coagulant activity and clinical bleeding severity for deficiencies of fibrinogen, combined FV + VIII, FX, and FXIII, with a weak association for FV and FVII deficiencies; residual FXI activity did not predict clinical bleeding severity. From the same study,24 it was documented that the minimum level to ensure complete absence of clinical symptoms is different for each disorder (see recommended EN-RBD trough levels in Table 4). Thus, RBDs cannot be considered as a single class of disorders; instead, studies should focus on evaluation of specific aspects of each individual RBD.
Categories of clinical bleeding severity
| Clinical bleeding severity . | Definition . |
|---|---|
| Asymptomatic | No documented bleeding episodes |
| Grade I bleeding | Bleeding that occurred after trauma or drug ingestion |
| Grade II bleeding | Spontaneous minor bleeding: bruising, ecchymosis, minor wounds, oral cavity bleeding, epistaxis, and menorrhagia |
| Grade III bleeding | Spontaneous major bleeding: hematomas, hemarthrosis, CNS, GI, and umbilical cord bleeding |
| Clinical bleeding severity . | Definition . |
|---|---|
| Asymptomatic | No documented bleeding episodes |
| Grade I bleeding | Bleeding that occurred after trauma or drug ingestion |
| Grade II bleeding | Spontaneous minor bleeding: bruising, ecchymosis, minor wounds, oral cavity bleeding, epistaxis, and menorrhagia |
| Grade III bleeding | Spontaneous major bleeding: hematomas, hemarthrosis, CNS, GI, and umbilical cord bleeding |
Replacement therapy for RBDs
| Deficient factor . | Plasma half-life . | Recommended trough levels2 . | On demand dosages . | Recommended trough levels to maintain asymptomatic state after publication of the EN-RBD results24 . |
|---|---|---|---|---|
| Fibrinogen | 2-4 d | 0.5-1 g/L | Cryoprecipitate (15-20 mL/kg) | 1 g/L |
| SD-treated plasma (15-30 mL/kg) | ||||
| Fibrinogen concentrate (50-100 mg/kg) | ||||
| Prothrombin | 3-4 d | 20%-30% | SD-treated plasma (15-25 mL/kg) | >10% |
| FIX concentrate and PCC (20-40 units/kg) | ||||
| Factor V | 36 h | 10%-20% | SD-treated plasma (15-25 mL/kg) | 10% |
| Factor V and factor VIII | FV 36 h | 10%-15% | As for FV | 40% |
| FVIII 10-14 h | ||||
| Factor VII | 4-6 h | 10%-15% | FVII concentrate (30-40 mL/kg) | >20% |
| PCC (20-30 units/kg) | ||||
| rFVIIa (15-30 μg/kg every 4-6 h) | ||||
| Factor X | 40-60 h | 10%-20% | SD-treated plasma (10-20 mL/kg) | >40% |
| PCC (20-30 units/kg) | ||||
| FX/FIX concentrate (10-20 units/kg) | ||||
| Factor XI | 50 h | 15%-20% | SD-treated plasma (15-20 mL/kg) | 15%-20% |
| FXI concentrate (15-20 units/kg) | ||||
| Factor XIII | 9-12 d | 2%-5% | Cryoprecipitate (2-3 bags) | 30% |
| SD-treated plasma (3 mL/kg) | ||||
| FXIII concentrate (until 50 units/kg for high hemorrhagic events) | ||||
| rFXIII-A (35 units/kg) | ||||
| Vitamin K dependent | Vitamin K (10 mg) IV or SC for minor bleeding | No data available | ||
| PCC (20-30 units/kg) with vitamin | ||||
| K (5-20 mg) for severe bleeding or major surgery | ||||
| FFP 15-25 mL/kg is an alternative to PCC |
| Deficient factor . | Plasma half-life . | Recommended trough levels2 . | On demand dosages . | Recommended trough levels to maintain asymptomatic state after publication of the EN-RBD results24 . |
|---|---|---|---|---|
| Fibrinogen | 2-4 d | 0.5-1 g/L | Cryoprecipitate (15-20 mL/kg) | 1 g/L |
| SD-treated plasma (15-30 mL/kg) | ||||
| Fibrinogen concentrate (50-100 mg/kg) | ||||
| Prothrombin | 3-4 d | 20%-30% | SD-treated plasma (15-25 mL/kg) | >10% |
| FIX concentrate and PCC (20-40 units/kg) | ||||
| Factor V | 36 h | 10%-20% | SD-treated plasma (15-25 mL/kg) | 10% |
| Factor V and factor VIII | FV 36 h | 10%-15% | As for FV | 40% |
| FVIII 10-14 h | ||||
| Factor VII | 4-6 h | 10%-15% | FVII concentrate (30-40 mL/kg) | >20% |
| PCC (20-30 units/kg) | ||||
| rFVIIa (15-30 μg/kg every 4-6 h) | ||||
| Factor X | 40-60 h | 10%-20% | SD-treated plasma (10-20 mL/kg) | >40% |
| PCC (20-30 units/kg) | ||||
| FX/FIX concentrate (10-20 units/kg) | ||||
| Factor XI | 50 h | 15%-20% | SD-treated plasma (15-20 mL/kg) | 15%-20% |
| FXI concentrate (15-20 units/kg) | ||||
| Factor XIII | 9-12 d | 2%-5% | Cryoprecipitate (2-3 bags) | 30% |
| SD-treated plasma (3 mL/kg) | ||||
| FXIII concentrate (until 50 units/kg for high hemorrhagic events) | ||||
| rFXIII-A (35 units/kg) | ||||
| Vitamin K dependent | Vitamin K (10 mg) IV or SC for minor bleeding | No data available | ||
| PCC (20-30 units/kg) with vitamin | ||||
| K (5-20 mg) for severe bleeding or major surgery | ||||
| FFP 15-25 mL/kg is an alternative to PCC |
rFVIIa, recombinant activated FVII; SD, solvent/detergent.
Laboratory diagnosis
RBD laboratory diagnosis is initially investigated via coagulation screening tests including the APTT and PT (Table 1).25 A prolonged APTT with a normal PT suggests FXI deficiency after exclusion of FVIII, FIX, and FXII deficiencies. The reverse pattern is typical of FVII deficiency, whereas the prolongation of both tests directs further analysis toward deficiencies of combined FV and FVIII, FX, FV, prothrombin, or fibrinogen. All coagulation tests depending on the formation of fibrin as the end point are necessary to evaluate fibrinogen deficiency; hence, beside the PT and APTT, the TT is performed (Table 1). Abnormal screening coagulation tests are followed by mixing studies (50:50) to exclude an inhibitor. When mixing studies correct, specific factor assays are performed to identify the deficiency. Factor antigenic assays are essential for diagnosis of quantitative deficiencies of fibrinogen or FII to appropriately classify and treat patients with dysfibrinogenemia and dysprothrombinemia, both associated with an increased thrombotic risk. The screening clotting tests (PT, APTT, fibrinogen, platelet count, and bleeding time) are normal in FXIII deficiency (Table 1); diagnosis is established via specific assays. Increased clot solubility in 5 M urea, dilute monochloroacetic, or acetic acid is not quantitative or standardized and only detects activity levels <5%, resulting in underdiagnosis. Therefore, FXIII activity should be quantitatively measured via ammonia release during the transglutaminase reaction or incorporation of radioactive amines into proteins.26 Here, plasma blanking is required to avoid the FXIIIa-independent ammonia release that could lead to incorrect results in the low-activity range (<5-10%).27 If FXIII activity is decreased, the deficiency subtype, FXIII-A or FXIII-B, is determined with an immunological FXIII antigen assay to assure appropriate classification and treatment.28
Molecular diagnosis
Molecular diagnosis is based on causative mutation identification in genes encoding corresponding coagulation factors.1 Exceptions are combined FV and FVIII deficiency, caused by mutations in genes encoding proteins involved in FV and FVIII intracellular transport (MCFD2 and LMAN1) and VKCFD, caused by mutations in genes encoding enzymes involved in post-translational modification and vitamin K metabolism (GGCX and VKOR).1 Inheritance pattern is autosomal recessive for all RBDs, except for some cases of FXI and of hypo- and dysfibrinogenemia. Information on RBD identified mutations is available through the International Society on Thrombosis and Haemostasis (ISTH) mutation database (http://www.isth.org/?MutationsRareBleedin). Missense mutations are most frequent, representing 50% to 80% of identified mutations, except for LMAN1 variants, where the most frequent mutations are insertions/deletions (50%).29 Insertion/deletion mutations represent 20% to 30% of gene variations of the fibrinogen, FV, MCFD2, and FXIII genes and <15% of remaining coagulation factor mutations. Splicing and nonsense mutations comprise 5% to 15% of coagulation factor-identified mutations, with a maximum rate of 20% in the LMAN1 gene.29 Variants located in the 3′ and 5′ untranslated regions of the genes are least frequent (<5%) and found only in fibrinogen, FVII, FXI, and FXIII.29 Despite significant advances in knowledge, 5% to 10% of affected patients with severe deficiencies have no identifiable genetic defect; here the use of next-generation sequencing, correlated with additional investigation on the deleterious/causative role of identified sequence variations, may elucidate novel genetic pathways.
Over the last 20 years, the body of literature on the molecular aspects of RBDs based on naturally occurring mutations has grown. The early work in this area, especially studies highlighting potential genotype/phenotype correlations, have largely been conducted in mouse models and revealed that complete absence of almost all of these coagulation factors resulted in a severe clinical phenotype often incompatible with life or achieving adulthood.29 These data cannot uniformly be extrapolated to RBD human expression.
Global hemostasis tests
Standard coagulation screening tests reveal the basic integrity of the coagulation process; evaluation of coagulation speed and extent are limited by test sensitivity at very low residual factor levels. Tests evaluating global hemostatic capacity (thrombin generation test and thromboelastography) may provide more accurate evaluation of in vivo hemostasis and treatment response and be better suited to predict clinical phenotype as they more effectively assess rate/total thrombin generated, whole blood clot formation, and/or fibrin polymerization. Recently, these tests have been used to evaluate hemostasis in patients with RBDs,30,31 specifically FV32,33 and FXI34 deficiency. These assays represent an emerging strategy to determine therapeutic effectiveness and to monitor RBD treatment, particularly FXI deficiency where standard assays fail to correlate with bleeding risk. Test standardization to reproduce reliable thrombin generation measurements facilitated by standardized preanalytical and analytical procedures is required before widespread clinical use.
Treatment
RBD treatment is difficult as clinical management information for specific bleeding episodes is often scarce; replacement therapy may require use of fresh frozen plasma (FFP) that may be associated with adverse events, licensed products without the specific indication (eg, prothrombin complex concentrate [PCC] for prothrombin deficiency), or unlicensed products. In some cases, specific replacement therapy is unavailable. Treatment mainstay is replacement of the deficient coagulation factor and use of adjunctive therapies (antifibrinolytics, estrogen/progestogen) where appropriate.1,2,35 Unfortunately, safety and efficacy data for the few available products are limited, as is experience in optimal use compared with hemophilia. Blood-borne infectious agent transmission avoidance is primary in replacement therapy choice. Solvent/detergent-treated plasma is an important source of replacement recommended in many RBDs; virus-inactivated concentrates, when available, are also safe yet may be cost prohibitive in developing economies.1,2,35,36 Non–virus-inactivated plasma and cryoprecipitate should be avoided when possible.1,2,35,36 Virally inactivated specific factor concentrates are available for several deficiencies and preferred when virally inactivated plasma is not available or repeated infusions required, to avoid potential fluid overload or inability to achieve a hemostatic level, especially during surgery or CNS hemorrhage.1,2 An updated registry of available clotting factor concentrates is published by the WFH (9th edition, http://www.wfh.org). The patient’s personal and family bleeding histories are important management guides. Adjuvant therapies such as antifibrinolytics alone or in combination with replacement therapy and estrogen-progestin preparations are considered for less severe mucosal tract hemorrhage or heavy menstrual bleeding and before minor surgeries.1,2,35
Most RBD treatment is “on-demand,” defined as soon as possible after bleed onset. In clinically severe cases or specific deficiency states, prophylactic treatment is considered.35 Dosage and treatment frequency depend on the required minimal hemostatic level of each coagulation factor, its plasma half-life, and the type of bleeding episode treated or to be prevented. Use of prophylaxis is related to bleeding frequency, risk of severe spontaneous bleeding, and associated long-term disability despite on-demand treatment. General treatment recommendations are summarized in Tables 4 and 5.2,35
General recommendations for long-term prophylaxis
| Deficient factor . | Recommended trough levels . | Reported dose schedule for successful long-term prophylaxis . | Notes . | ||
|---|---|---|---|---|---|
| Products . | Dose . | Frequency . | |||
| Fibrinogen | 0.5-1 g/L | Cryoprecipitate | 1 unit | 3 times/wk | Afibrinogenemic patients with recurrent life-threatening bleedings or undergoing surgeries |
| 3 units | Every 7-10 d | ||||
| Fibrinogen concentrate | 30-100 mg/kg | Every wk | |||
| Prothrombin | 20%-30% | PCC | 20-40 units/kg | 1 time/wk | — |
| Factor V | 10%-20% | SD-treated plasma | 20-30 mL/kg | 2 times/wk | — |
| Factor V and factor VIII | 10%-15% | No data | — | ||
| Factor VII | 10%-15% | SD-treated plasma | 10-15 mL/kg | 2 times/wk | Prevention of bleeding during surgery or in children with recurrent hemarthrosis or CNS |
| pdFVII | 10-40 units/kg | 3 times/wk | |||
| rFVIIa | 20-40 µg/kg | 2-3 times/wk | |||
| Factor X | 10%-20% | PCC | 20-40 units/kg | 2-3 times/wk | Patients with recurrent life-threatening bleedings or undergoing surgeries |
| FX/FIX concentrate | 20-40 units/kg | 1-2 times/wk | |||
| Factor XI | 15%-20% | No data | — | ||
| Factor XIII | 2%-5% | Cryoprecipitate | 2 units | Every 3 wk | Highly recommended in severe patients |
| SD-treated plasma | 15-20 mL/kg | Every 4-6 wk | |||
| FXIII concentrate | 10-40 units/kg | Every 4-6 wk | |||
| rFXIII-A | 35 units/kg | Every 4 | |||
| Vitamin K dependent | Vitamin K | 5-20 mg | 1 time/wk orally | — | |
| Deficient factor . | Recommended trough levels . | Reported dose schedule for successful long-term prophylaxis . | Notes . | ||
|---|---|---|---|---|---|
| Products . | Dose . | Frequency . | |||
| Fibrinogen | 0.5-1 g/L | Cryoprecipitate | 1 unit | 3 times/wk | Afibrinogenemic patients with recurrent life-threatening bleedings or undergoing surgeries |
| 3 units | Every 7-10 d | ||||
| Fibrinogen concentrate | 30-100 mg/kg | Every wk | |||
| Prothrombin | 20%-30% | PCC | 20-40 units/kg | 1 time/wk | — |
| Factor V | 10%-20% | SD-treated plasma | 20-30 mL/kg | 2 times/wk | — |
| Factor V and factor VIII | 10%-15% | No data | — | ||
| Factor VII | 10%-15% | SD-treated plasma | 10-15 mL/kg | 2 times/wk | Prevention of bleeding during surgery or in children with recurrent hemarthrosis or CNS |
| pdFVII | 10-40 units/kg | 3 times/wk | |||
| rFVIIa | 20-40 µg/kg | 2-3 times/wk | |||
| Factor X | 10%-20% | PCC | 20-40 units/kg | 2-3 times/wk | Patients with recurrent life-threatening bleedings or undergoing surgeries |
| FX/FIX concentrate | 20-40 units/kg | 1-2 times/wk | |||
| Factor XI | 15%-20% | No data | — | ||
| Factor XIII | 2%-5% | Cryoprecipitate | 2 units | Every 3 wk | Highly recommended in severe patients |
| SD-treated plasma | 15-20 mL/kg | Every 4-6 wk | |||
| FXIII concentrate | 10-40 units/kg | Every 4-6 wk | |||
| rFXIII-A | 35 units/kg | Every 4 | |||
| Vitamin K dependent | Vitamin K | 5-20 mg | 1 time/wk orally | — | |
pdFVII, plasma-derived FVII; rFVIIa, recombinant activated FVII; SD, solvent/detergent.
Although recombinant technology and viral inactivation methods have virtually eliminated the risk of blood-borne infection, other potential concentrate related adverse events persist (eg, inhibitor development, thrombosis, and hypersensitivity reactions). Cases of autoantibodies have been reported in fibrinogen, FII, FVII, FXI, and FXIII deficiencies following replacement therapy.10,11,13,15,16 In FXI deficiency, 41% of patients homozygous for null mutations developed an inhibitor following exposure to exogenous FXI (plasma, FXI concentrates, or anti-RhD immunoglobulin).15
Surveillance systems such as the Universal Data Collection by the Centers for Disease Control and Prevention in the United States37 and the European Haemophilia Safety Surveillance system in Europe38 register and monitor treatment and complications. These 2 programs highlight the importance to conduct multinational, multicenter data collection for long-term postregistration surveillance and to analyze large numbers of homogeneous/standardized data, to overcome the rarity of these disorders.
Overview of specific RBDs
Fibrinogen deficiency
Fibrinogen deficiency9,39 is heterogeneous, with 2 main phenotypes distinguished: plasma and platelet protein levels are not measurable or very low, leading to afibrinogenemia and hypofibrinogenemia, whereas low clottable fibrinogen with normal or moderately reduced fibrinogen antigen results in dys- and hypodysfibrinogenemia. Fibrinogen is hepatically produced from 3 homologous polypeptide chains, Aα, Bβ, and γ, and assembled to form a 340-kDa hexamer. The 3 genes encoding fibrinogen Bβ (FGB), Aα (FGA), and γ (FGG), ordered from centromere to telomere, are clustered in a region of ∼50 kb on chromosome 4.
Dys- and hypofibrinogenemic patients are usually asymptomatic or intermittently symptomatic, whereas those with afibrinogenemia exhibit bleeding commonly manifesting in the neonatal period, with 85% presenting with umbilical cord bleeding.40,41 Table 2 lists other less frequent symptoms. Women may experience menometrorrhagia, but some have normal menses. First-trimester abortion is common in afibrinogenemic women but less common in dysfibrinogenemia. Reports of thromboembolism in afibrinogenemia exist either with or without replacement therapy, likely related to fibrinogen’s binding of excess thrombin. Some mutations predict clinical phenotype, particularly in dysfibrinogenemia where some gene variations are associated with bleeding and others with thrombotic risk.9
Conventional treatment is on-demand, but effective long-term secondary prophylaxis with fibrinogen administration every 7 to 14 days has been described after CNS hemorrhage. High-level replacement, eg, during pregnancy, should be moderated by the reported occurrence of thromboembolic events. Depending on the country, patients may receive FFP, cryoprecipitate, or fibrinogen concentrate, with the latter being the treatment of choice.42-44
Prothrombin deficiency
Prothrombin deficiency10,39 is the rarest inherited coagulation disorder, with a prevalence of ∼1 in 2 million. Two main phenotypes are distinguished: hypoprothrombinemia with both decreased activity and antigen levels and dysprothrombinemia with normal synthesis of a dysfunctional protein. Hypoprothrombinemia associated with dysprothrombinemia was also described in compound heterozygotes. Prothrombin, a vitamin K-dependent glycoprotein that is hepatically synthesized, is the zymogen of the serine protease α-thrombin and is encoded by a gene of ∼21 kb located on chromosome 11.
Severe prothrombin deficiency (plasma levels <5%) in either homozygous or double heterozygotes is uniformly characterized by severe bleeding (Table 2). Dysprothrombinemia manifests as a variable less severe bleeding tendency, whereas heterozygous subjects are usually asymptomatic; occasionally, excessive bleeding after surgical procedures has been observed. In homozygous females, menorrhagia is frequent.
Replacement therapy is needed only in severe deficiency, for bleeding, or to ensure adequate prophylaxis before major procedures. As no prothrombin concentrate exists, FFP, PCC, or both are used. In case of severe bleeding or major surgery, higher prothrombin levels are achieved with PCCs without risk of FFP-associated volume overload.45 PCCs contain other vitamin K-dependent coagulation factors, which could potentially induce thrombotic complications; therefore, patients require close monitoring.
FV deficiency
FV11,39 has a dual role in coagulation: it is a protein cofactor required by the prothrombinase complex for thrombin generation and contributes to the proteins C/S anticoagulant pathway by downregulating FVIII activity. FV is mainly hepatically secreted, with some evidence that it is also synthesized in the megakaryocyte/platelet lineage. FV protein is encoded by a large (80 kb) and complex (25 exons) gene located on chromosome 1.
Severe deficiency typically presents early in life; nonetheless, FV deficiency is clinically heterogeneous, as despite lower FV levels, severe patients may not bleed as expected. Recent observations point to a pivotal role for platelet FV, providing new insight into this inconsistency. Megakaryocytes can synthesize FV; however, the majority of platelet FV is endocytosed from the plasma. Following endocytosis, FV is modified intracellularly; these changes appear to provide the cofactor with unique physical and functional characteristics rendering it more procoagulant compared with its plasma counterpart. Platelet degranulation and release of platelet FV at the site of vascular injury is a critical contributor to local FV concentration. Furthermore, there is evidence that platelet FV that is locally released in high concentrations is less susceptible to inhibition, supporting normal hemostasis.
Symptomatic patients usually present with umbilical stump bleeding, skin, and mucosal tract hemorrhage; epistaxis and menorrhagia are relatively frequent, even with measurable FV levels (Table 2). In patients with mild-to-moderate deficiency, therapy with antifibrinolytic agents is sufficient to control epistaxis, menorrhagia, or other non–life-threatening mucosal bleeding. Menorrhagia can also be managed using hormonal suppressive therapy, progestin-containing intrauterine devices, endometrial ablation, or hysterectomy when required.
FV replacement is accomplished through FFP, preferably virus inactivated, as currently no FV concentrate is available. A study of FV concentrate developed for clinical use in deficient patients is ongoing in preparation for orphan drug designation application to the European Medicine Agency and the Food and Drug Administration. Platelet concentrates are an alternative source of FV that have been used in combination with FFP.46
Combined FV and FVIII deficiency
Combined FV and FVIII deficiency (F5F8D)12,39 is characterized by concomitantly low levels (usually 5-20%) of both coagulant activity and antigen. Interestingly, the concomitant deficiency of these 2 coagulation factors does not enhance the hemorrhagic tendency observed in each separate defect. F5F8D is causally associated with mutations in the LMAN1 gene, encoding lectin mannose-binding protein (previously named ERGIC-53), a 53-kDa type 1 transmembrane protein that acts as a chaperone in the intracellular transport of both factors and with mutations in the MCFD2 gene, encoding multiple coagulation factor deficiency (MCFD)2 protein, which acts as a cofactor for LMAN1, specifically recruiting correctly folded FV and FVIII in the endoplasmic reticulum. Recent studies have failed to identify additional components of the LMAN1-MCFD2 receptor complex, supporting the concept that F5F8D might be limited to LMAN1 and MCFD2. Phenotypes associated with mutations in MCFD2 and LMAN1 are indistinguishable and manifested only by FV and FVIII deficiency, although a selective secretion delay of the cargo protein procathepsin C has been observed in HeLa cells overexpressing a dominant-negative form of LMAN1.
Symptoms are usually mild, with a predominance of easy bruising, epistaxis, and bleeding after dental extractions (Table 2). Menorrhagia and postpartum bleeding are reported in affected women. Because of the mild clinical pattern, treatment is usually on-demand and does not require regular prophylaxis. Affected individuals can be treated with desmopressin and FFP. More severe cases require FVIII concentrate. Sources of both FV and FVIII are required and differential plasma half-lives (FV, 36 hours; FVIII, 10-14 hours) must be considered.
FVII deficiency
FVII deficiency13,39 is the most common autosomal recessive coagulation disorder (1 in 500 000) and is typically clinically heterogeneous, ranging in severity from lethal to mild, or even asymptomatic. FVII is hepatically synthesized and encoded by the FVII gene (F7) located on chromosome 13, 2.8 kb upstream of the FX gene. Both coagulant and antigenic plasma FVII levels are influenced by genetic and environmental factors (sex, age, cholesterol, and triglyceride levels); FVII levels are also modulated by F7 polymorphisms.
FVII deficiency is phenotypically variable: some patients do not bleed despite very low FVII activity, whereas others with similar levels experience frequent bleeding. The most frequent symptoms are epistaxis and menorrhagia, with life- or limb-threatening bleeding being relatively rare (Table 2). However, CNS hemorrhage was reported to have a high incidence (16%) in a series of 75 patients.47 Thrombotic episodes have also been reported in 3% to 4% of FVII-deficient patients, particularly associated with surgery and replacement therapy, but spontaneous thrombosis may also occur.48 It can be inferred that patients with FVII deficiency are not protected against thromboembolism.
Various therapeutic options are available for FVII deficiency, including FFP and PCCs (FVII concentration in both are low and therefore they are not optimal treatments); plasma-derived FVII concentrates; and recombinant FVIIa. Recombinant FVIIa is genetically engineered and considered the optimal replacement therapy as used at a low dose (10-20 µg/kg). Prophylaxis is debated in FVII deficiency but has been used in those with severe bleeding.49 The infused FVII short half-life contributes to difficulty establishing standardized prophylactic protocols.
The occurrence of frequent menorrhagia is almost invariably associated with chronic iron deficient anemia in women with severe deficiency. Pregnancy alone does not require special precautions, and uncomplicated delivery is possible without prophylaxis. However, all cases of reported postpartum hemorrhage occurred with FVII coagulant activity <15% and no prophylaxis. Therefore, delivery should occur under the coverage of short-term replacement.50
FX deficiency
FX14,39 is a glycoprotein pivotal in the coagulation cascade, as the first enzyme in the common pathway of thrombin formation. FX is mainly hepatically synthesized and encoded by the FX gene (F10), comprising 22 kb and located on chromosome 13, a few kilobases downstream of the F7 gene.
In FX deficiency, the bleeding tendency appears at any age, although the more severely affected (<1% activity) present early in life with umbilical stump, CNS, or GI bleeding (Table 2). Patients with severe deficiencies commonly experience hemarthroses and hematomas. Common symptoms reported at all severity levels include epistaxis and menorrhagia. Heterozygous patients have been reported with postpartum bleeding requiring treatment.
Data from the United Kingdom Haemophilia Centre Doctors’ Organization registry demonstrated that the proportion of FX-deficient patients requiring treatment is higher than other RBDs. Therapy usually is administration of PCC. A recently developed freeze-dried human coagulation FIX/FX concentrate with specified FIX/X content has facilitated prophylaxis.51 Pharmacokinetics of a new high-purity FX concentrate have been recently performed (ClinicalTrials.gov identifier: #00930176) and are ongoing in children. Therapeutic options for control of menorrhagia are both medical (eg, antifibrinolytics, hormonal suppressive therapy, levonorgestrel intrauterine device, and clotting factor replacement) and surgical (eg, endometrial ablation and hysterectomy if required). Even if FX plasma concentration increases in pregnancy, women with severe deficiency and a history of adverse pregnancy outcomes (eg, abortion, placental abruption, or premature birth), may benefit from continuous replacement therapy.
FXI deficiency
The estimated prevalence of severe FXI deficiency in most populations is ∼1 in 1 million, but is higher in Ashkenazi Jews where heterozygosity approaches 8%.15,39 FXI is mainly hepatically synthesized, although small transcript quantities are detected in megakaryocytes and platelets. The protein is encoded by the FXI gene, comprising 23 kb located on chromosome 4. In some cases, missense mutations were shown to exert a dominant-negative effect through heterodimer formation between the mutant and wild-type polypeptides, resulting in dominant transmission.52 The existence of a platelet FXI transcript, originating from the skipping of exon 5, was hypothesized but subsequently not confirmed. Although recent data support FXI transcripts undergoing alternative splicing leading to FXI isoform synthesis, their physiological role and importance require elucidation.
The most frequent symptoms are oral and postoperative bleeding, occurring in >50% of patients (Table 2). FXI-deficient women are prone to menorrhagia. Case series of severely deficient women revealed that 70% of pregnancies were uneventful without prophylactic treatment.53
The relationship between plasma FXI levels and the bleeding tendency is not as clear-cut as in other RBDs. Bleeding phenotype is not correlated with genotype but rather the site of injury. Injury in an area of high fibrinolytic activity (eg, urogenital tract, oral cavity after dental extraction, or tonsillectomy) increases bleeding risk (49-67%) compared with sites with less fibrinolytic activity (1.5-40%).54 Usually, patients with severe deficiency (≤1%) are mildly affected, with most manifestations being injury related. Patients with more moderate (low but detectable) FXI levels are also mild bleeders. Therefore, phenotypes are not strikingly different in these 2 groups. Patients with similarly reduced FXI antigen and activity levels exhibit variable bleeding tendencies; some are asymptomatic even after trauma, whereas others display bleeding with trauma or delayed bleeding beginning several hours to days following injury. Neither FXI antigen nor activity correlates with clinical bleeding risk, and APTT assays are not predictive. Attempts to differentiate FXI-deficient patients with or without a bleeding tendency have focused on plasma thrombin generation characteristics; conflicting results are reported. Recently, new plasma clot formation assays were demonstrated to be useful to distinguish phenotypes, with bleeders exhibiting significantly reduced fibrin network density and clot stability, suggesting that these parameters are determinants of bleeding risk but are not wholly dependent on plasma FXI levels.55
Treatment is based on antifibrinolytic agents, FFP, and FXI concentrate. Recombinant FVIIa was successfully used in surgery. Care should be taken to reduce complications including thrombosis, especially with FXI concentrate, volume overload, and hypersensivity reactions. Inhibitory antibodies can develop in severe deficiency. Patients with FXI deficiency without a bleeding history despite appropriate challenges do not require prophylactic treatment.
FXIII deficiency
FXIII16 is a transglutaminase functioning to cross-link the α and γ fibrin chains, resulting in increased clot strength and fibrinolytic resistance. FXIII consists of 2 catalytic A subunits (FXIII-A) and 2 carrier B subunits (FXIII-B). FXIII-A is synthesized in cells of bone marrow origin, whereas FXIII-B is hepatically produced. The corresponding genes are located on chromosomes 6 and 1. FXIII deficiency, together with prothrombin deficiency, are the rarest of recessively transmitted RBDs and occur in 1 in 2 million. In inherited FXIII deficiency, FXIII-A plasma levels measured as functional activity or immunoreactive protein are usually extremely reduced, whereas the FXIII-B subunit is reduced but at measurable levels.
Patients with FXIII-A deficiency have a bleeding tendency that is usually severe, with early onset of life-threatening symptoms (eg, umbilical cord and CNS bleeding) in up to 80% and 30%, respectively.56,57 Table 2 reports other common or less frequent clinical symptoms. In women of reproductive age, miscarriage and intraperitoneal bleeding are often reported. These symptoms collectively lead to early diagnosis and prophylactic treatment. Prophylaxis is feasible, as 2% to 5% FXIII plasma levels are sufficient to prevent severe bleeding, and the long in vivo half-life, 11 to 14 days, requires infrequent replacement (1 month or longer). Importantly, the recent EN-RBD study revealed that only patients with FXIII:C > 30% remain asymptomatic. Prophylactic FXIII infusions are recommended in FXIII-A-deficient pregnant women to prevent fetal loss.58
When FXIII concentrate is not available, FFP and cryoprecipitate should be considered, the latter being preferable due to higher FXIII content.59 Plasma-derived FXIII has been used for several years and has been shown to be safe and effective; a new recombinant FXIII-A2 concentrate (rFXIII-A2) is available, and a phase 3 clinical trial (ClinicalTrials.gov identifier: #00713648) that was recently completed demonstrated that rFXIII is safe and effective in bleed prevention in congenital FXIII-A subunit-deficient patients. rFXIII was recently approved for the treatment of FXIII-A deficiency in Australia, Canada, the European Union, Switzerland, and the United States.
Only a few cases of inherited FXIII-B deficiency are reported, with 16 causative mutations identified; FXIII-B deficiency bleeding symptoms appear milder than in FXIII-A-deficient patients.
VKCFD
Vitamin K-dependent coagulation factors, FII, FVII, FIX, and FX,39,60 require glutamic acid residue γ-carboxylation at Gla domains to enable calcium binding and attachment to phospholipid membranes. The process is catalyzed by hepatic γ-glutamyl carboxylase (GGCX) and its cofactor, reduced vitamin K (KH2). During the reaction, KH2 is converted to vitamin K epoxide (KO), which is recycled to KH2 by the vitamin K epoxide reductase (VKOR) enzyme complex. Heritable dysfunction of GGCX or the VKOR complex results in the secretion of undercarboxylated vitamin K-dependent coagulation factors, leading to a combined deficiency. γ-carboxylation of glutamic acid residues is required for activity of proteins C, S, and Z; despite decreased proteins S/C levels in VKCFD, there are no reports of venous/arterial thrombosis. The effect of VKCFD is clearly hemorrhagic. The GGCX and VKOR proteins are encoded by 2 corresponding genes: GGCX (13 kb, 15 exons) located on chromosome 2 and the unusually small VKORC1 (5126 bp, 3 exons) located on chromosome 16; the latter gene was so named because of evidence suggesting that VKOR is a multi-subunit complex.
VKCFD commonly presents early in life with intracranial hemorrhage or umbilical stump bleeding (Table 2); routine vitamin K administration may delay neonatal diagnosis. Severely affected children may present with skeletal abnormalities including nasal and distal digital hypoplasia, epiphyseal stippling, and mild conductive hearing loss. Older patients can present with easy bruising and mucocutaneous or postsurgical bleeding.
Treatment with oral or parenteral vitamin K1 should be promptly started. Some patients demonstrate inadequate response. Limited data exist on the effectiveness of 10 mg vitamin K1 weekly prophylaxis. Massive parenteral doses do not always correct activity levels with persistence of undercarboxylated molecules; here factor replacement via PCC or alternatively a virally inactivated FFP could be used in acute bleeding episodes or prior to surgery.
Concluding remarks
Understanding the pathophysiology, presentation, and treatment options for RBDs is critical to facilitate genetic counseling, optimal patient management, and improved long-term outcomes. RBD rarity limits in-depth individual deficiency analysis and contributes to increased risk of misdiagnosis and poor and sometimes fatal consequences. Although many efforts have been made to address these gaps in RBD knowledge, further multinational collaborative efforts are required. Despite valuable findings obtained by current registry efforts, accurate knowledge of the true worldwide incidence of each RBD, the pattern and frequency of their bleeding episodes, and the minimum residual coagulation factor level required to ensure normal hemostasis remain. These gaps can only be addressed by international global efforts.
Ongoing efforts continue to promote international data harmonization. In the United States, the American Thrombosis and Hemostasis Network (www.athn.org) is expanding its data collection system to assure inclusion of individuals with RBDs followed through the US federal Hemophilia Treatment Center Network. In Europe, the PRO-RBDD project (http://eu.rbdd.org), through the development of an international network of care providers and a Web-based database, aims to better identify the number of affected individuals worldwide and to prospectively collect clinical and laboratory data to evaluate the frequency of clinical manifestations, their sequelae, and document consumption of treatment products and define related complications.
The Rare Coagulation Disorders Resource Room Web site39 is another step in global initiatives enhancing information dissemination, ongoing research, and registry efforts, and fosters collaboration among a growing international network. This resource provides readily available first-line education for both health care providers and affected individuals39 and will serve as a platform for worldwide researchers and clinicians to exchange information and share experiences while fostering data collection and collaboration on clinical trial design.
Participation in the PRO-RBDD Registry
PRO-RBDD is open to Hemophilia Treatment Centers worldwide; to join, visit http://eu.rbdd.org and contact info@rbdd.eu. Project staff will assist you in joining the network, including required documentation. Currently studies on fibrinogen and factor XIII deficiencies are ongoing. Participants may use the database and propose future new studies.
Acknowledgments
The authors thank Marzia Menegatti (Università degli Studi di Milano, Milan, Italy) for assistance in the preparation of the manuscript.
F.P. is the grant recipient, and serves as lead in the prospective study of rare bleeding disorders within the project “European Haemophilia Network (EUHANET)” funded by the commission of the European Union–the Executive Agency for Health and Consumers.
Authorship
Contribution: All authors contributed to the design and development of the manuscript; R.P. wrote the primary manuscript with all authors providing critical input and editing.
Conflict-of-interest disclosure: F.P. has received honoraria for participating as a speaker at satellite symposia and educational meetings organized by Alexion, Baxter, Bayer, Biotest, CSL Behring, Grifols and Novo Nordisk, she has received research grant funding from Alexion, Bayer, Biotest, Kedrion Biopharma and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Amy D. Shapiro, Indiana Hemophilia and Thrombosis Center, 8326 Naab Rd, Indianapolis, IN 46260; e-mail: ashapiro@ihtc.org.