Abstract
The clinical relevance of angiopoietin-2 (Ang2) in chronic lymphocytic leukemia (CLL) was previously suggested by the association between high Ang2, and shorter progression-free survival reported in small series of patients. Here, we evaluated Ang2 glycoprotein levels in plasma samples collected from a multicentric cohort of CLL patients (n = 316) using an enzyme-linked immunosorbent assay method, and we investigated its prognostic role in relation to time to first treatment (TTFT) and overall survival. Based on a cutoff equal to 2459 pg/mL, we divided our cohort in 2 subsets (high and low Ang2) composing 100 (31.6%) and 216 (68.4%) patients, respectively. High Ang2 was predictive of reduced TTFT (P < .001) and overall survival (P = .002). Multivariate analysis confirmed that high Ang2 was an independent prognosticator for TTFT (hazard ratio = 1.739; 95% confidence interval, 1.059-2.857; P = .029). Significant associations were found between high Ang2 and advanced Binet stages (P < .001), high β2-microglobulin (P < .001), unmutated variable region of immunoglobulin heavy chain gene status (P < .001), high CD38 and ζ-chain-associated protein kinase 70 expression (P < .001 and P = .003), and intermediate/high cytogenetic risk (P = .005). Moreover, Ang2 added prognostic power to other conventional prognosticators and helped to refine prognosis among CLL subsets with both high and low vascular endothelial growth factor plasma levels. Ang2 plasma level may be a useful independent prognosticator for CLL.
Introduction
The clinical course of patients with chronic lymphocytic leukemia (CLL) is extremely heterogeneous, with survival ranging from less than 1 to 2 years to more than 15 years. Some patients show aggressive clinical course and die shortly after diagnosis resulting from disease-related or treatment-related complications, whereas others experience an indolent clinical course, never require therapy, and eventually die of disease-unrelated causes. Because CLL patients who will experience adverse clinical outcome may potentially benefit from early therapy, it is crucial to assess accurately patients' prognosis at diagnosis.
Several prognostic factors are currently used for discriminating patients with rapidly evolving disease from those with indolent disease.1 A useful prognostic factor should be able to stratify patients with different risk to progressive disease and shorter survival. Its usefulness may be further increased if the prognostic factor provides a potential therapeutic target because of its involvement in disease pathobiology. Moreover, the technical approach used to assess the prognostic factor should be easy, reproducible, well standardized, and relatively inexpensive to be included in routine clinical practice.
Angiopoietin-2 (Ang2) is a 75-kDa secreted glycoprotein able to bind to the receptor tyrosine kinase Tie-2. Ang2 is a destabilizing factor able to revert vessels to a more plastic state by blocking the maturing function of Ang1. Ang2 works in concert with vascular endothelial growth factor (VEGF) to determine angiogenic remodeling and sprouting. Ang2 activates endothelial cells and destabilizes vessel structure; then VEGF promotes endothelial cell proliferation and migration allowing the formation of new vessels. Instead, in absence of VEGF, Ang2 promotes vessel regression.2,3 High levels of Ang2 were observed in highly vascularized tumors.4,5 Moreover, preliminary studies in solid tumors showed that disruption of Ang2 function can inhibit tumor vessel density and growth, suggesting a role for Ang2 in tumor angiogenesis and progression.6-8
In CLL, we have recently reported that: (1) Ang2 protein is secreted by leukemic cells, (2) CLL cells induce increased angiogenesis, and (3) angiogenesis in CLL is mediated by both leukemia-derived VEGF and Ang2.9 These data suggest that Ang2 may be involved in CLL pathobiology. In addition, elevated levels of Ang2 were reported to be associated with advanced-stage disease and shorter progression-free survival in a small number of cases.10-12 These preliminary results prompted us to further investigate the clinical significance of Ang2 plasmatic levels in a large multicentric series of 316 CLL patients.
Methods
Patients
A total of 316 CLL patients diagnosed at the Divisions of Hematology of Novara (n = 115), Modena (n = 58), Rome (n = 56), Siena (n = 52), and Ferrara (n = 35) during the years 1985 to 2008 were enrolled in this study. Diagnosis was based on the National Cancer Institute-sponsored Working Group guidelines on Chronic Lymphocytic Leukemia and reviewed according to the new International Workshop on Chronic Lymphocytic Leukemia criteria.13,14 Patients were selected according to the availability of plasma samples collected in overt disease: 274 (86.7%) cases were untreated at sample collection, whereas 42 (13.3%) patients had relapsed after previous treatment and none had received chemotherapy within 3 months before sample collection. During the course of the study, a further 53 (16.8%) patients required therapy. The median follow-up was 48 months. At the time of analysis, a total of 21 patients (6.6%) had died. All patients provided informed consent in accordance with local institutional review board requirements and the Declaration of Helsinki principles. The study was approved by the ethical committee of the University of Modena and Reggio Emilia. The clinical and biologic characteristics of our CLL cohort are summarized in Table 1.
IGHV mutational analysis
To evaluate the mutational status of immunoglobulin heavy chain genes (IGHVs) expressed by CLL clones, total RNA was extracted, reverse-transcribed, and tumor variable region of IGHV DJ rearrangement was amplified as previously described.15-17 Alternatively, IGHV DJ rearrangements were amplified starting from genomic DNA as described elsewhere.18 The tumor-specific IGHV DJ sequence was aligned to ImMunoGeneTics directories (http://imgt.cines.fr). IGHV gene sequences deviating more than 2% from the corresponding germline gene were defined as mutated.19
Immunophenotypic analyses of CD38, CD49d, and ZAP-70
All flow cytometric analyses reported in this study were performed on a FACSCalibur flow cytometer (BD Biosciences). The expression of CD38 and CD49d was analyzed by 3-color immunofluorescence, as previously described.20,21 Expression data were reported as percentage of CD5+CD19+ CLL cells displaying specific fluorescence intensity higher than the 98% to 99% of the same cell population stained with isotype- and fluorochrome-matched control immunoglobulins. The detection of ζ-chain-associated protein kinase 70 (ZAP-70) was performed as previously reported.22 ZAP-70 intracellular expression was determined as percentage of CD19+ CLL cells expressing the protein above the isotype control and/or above a marker set to include as positive all autologous T cells. The cutoff of 30% positive cells was chosen to discriminate CD38− from CD38+ CLL or CD49d− from CD49d+ CLL.15,23,24 The cutoff of 20% was instead selected to divide ZAP-70− from ZAP-70+ CLL.25
Analysis of cytogenetic aberrations
Cytogenetic abnormalities on chromosomes 11, 12, 13, and 17 were detected by interphase fluorescence in situ hybridization (FISH). The 3 locus specific probes LSI-ATM, LSI-D13S319, and LSI-p53, directly labeled with SpectrumGreen (LSI-ATM) or SpectrumOrange (LSI-D13S319 and LSI-p53), were used for chromosomes 11, 13, and 17, respectively (Vysis). An alpha satellite DNA probe CEP12, directly labeled with SpectrumGreen, was used to identify aneuploidy of chromosome 12 (Vysis). The FISH procedures used for this study were previously described.22,26
Quantification of Ang2 and VEGF plasma levels
Peripheral blood (PB) samples were collected from 316 CLL patients in the presence of overt disease and anticoagulated in ethylenediaminetetraacetic acid. To verify Ang2 level variations over time, a second blood sample was collected from 35 cases in a subsequent time of the disease (29 of 35 before treatment, 6 of 35 after treatment). Of note, all 6 previously treated patients were relapsed at sample collection, and none had received chemotherapy within 3 months before collection. Moreover, bone marrow (BM) and PB blood samples collected at the same time were available for 14 patients. Plasma samples were obtained by blood centrifugation at 1000g for 15 minutes and then stored at −20°C. Plasma samples were centralized to the Division of Hematology of Modena for Ang2 and VEGF quantification. Ang2 and VEGF plasma levels were quantified using Quantikine solid-phase enzyme-linked immunosorbent Ang2 and VEGF assay (DANG20 and DVE00, ELISA; R&D Systems). The mean minimum detectable dose was 8.3 pg/mL for Ang2 and less than 9.0 pg/mL for VEGF. Each plasma sample was tested in duplicate, and concentrations were reported in picograms per milliliter. The coefficient of variability intra-assay and interassay was less than 5% and 10%, respectively, in all experiments.
Immunohistochemical staining for Ang2
Immunohistochemistry for Ang2 was performed on CLL-infiltrated BM and lymph node (LN) biopsies collected at diagnosis from 8 patients. Briefly, tissue sections were microwave treated 3 times in citrate buffer, pH 6 (Dako Denmark), for 5 minutes. After neutralization of the endogenous peroxidase with H2O2 for 10 minutes, the slides were incubated with protein block Novocastra (Leica Biosystems) for 10 minutes. Slides were then incubated with the primary monoclonal antibody mouse anti–human Ang2 (MAB0983, R&D Systems, dilution 1:50). Normal mouse serum was used instead of the primary antibody as negative control. Slides were subsequently incubated with biotinylated link antibody and peroxidase-labeled streptavidin (Universal LSAB + System-HRP, Dako Denmark). The staining was revealed by incubation with 3-amino-9-ethylcarbazole substrate-chromogen (Dako Denmark). After counterstaining with hematoxylin (Dako Denmark), the slides were evaluated under a Leica DM3000 optical microscope (Leica Microsystems) and images were collected using a Leica DFC320 digital camera and Leica IM50 software (Leica Microsystems).
Statistical analyses
All data were analyzed using SPSS, Version 16.0 for Windows. The cutoff point for Ang2 plasma levels was selected according to receiver operating characteristic (ROC) analysis using treatment as state variable, and the Youden index was calculated using the sensitivity and specificity derived from ROC analysis. The Pearson χ2 test was used to determine significant differences in categorized variables. Furthermore, comparisons of distributions of continuous variables were performed with the Mann-Whitney test. The Spearman test was used for estimating correlations between quantitative parameters. The primary endpoints were time to first treatment (TTFT) and overall survival (OS): TTFT was defined as time from diagnosis to first treatment (event) or last follow-up, whereas OS was defined as time from diagnosis to death (event) or last follow-up. For OS analyses, all events were considered as CLL-related (ie, all deaths were considered as events independently from the cause). TTFT and OS functions were estimated using the product-limit (Kaplan-Meier) method, and the curves were compared between groups using the log-rank test. Univariate and multivariate analyses were performed using Cox models.27 Wilcoxon test and concordance correlation coefficient were used to compare Ang2 levels measured in plasma samples collected from the same patients in 2 different times of the disease.28 All tests were 2-sided. An effect was considered statistically significant at P less than or equal to .05.
Results
Ang2 plasma levels are an independent predictor of short TTFT in CLL
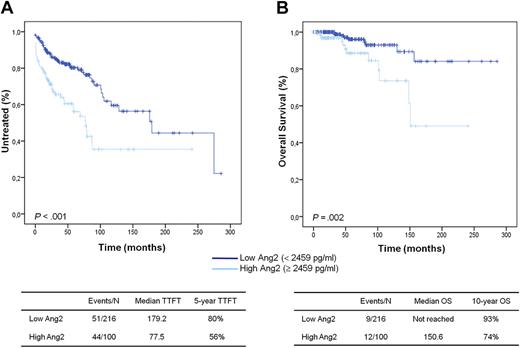
We measured Ang2 level in PB plasma samples derived from 316 CLL patients using an enzyme-linked immunosorbent assay (ELISA)-based method. Ang2 plasma levels ranged from 972 to 17 281 pg/mL with a median value of 2061 pg/mL (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The best cutoff point, generated by ROC analysis and Youden index using treating as state variable, was 2459 pg/mL. Based on the 2459 pg/mL cutoff, the CLL cohort was divided into 2 groups (high Ang2 and low Ang2) composing 100 (31.6%) and 216 (68.4%) patients, respectively. After a median observation from CLL diagnosis of 48 months, 95 of 316 patients have been treated, accounting for a median TTFT of 40 months. Cox univariate analysis identified Ang2 more than or equal to 2459 pg/mL as a predictor of reduced TTFT (hazard ratio [HR] = 2.437; 95% confidence interval [CI], 1.621-3.664, P < .001). Accordingly, patients with high Ang2 were characterized by a significantly shorter TTFT (77.5 months) compared with patients with low Ang2 (179.2 months; P < .001; Figure 1A). Other variables associated with short TTFT were advanced Binet stage, unmutated IGHV status, high CD38, ZAP-70, and CD49d expression, intermediate/high cytogenetic risk, high β2-microglobulin, and diffuse BM pattern (P < .001 in all instances; Table 2). Multivariate analysis selected Ang2 more than or equal to 2459 pg/mL as an independent predictor of TTFT (HR = 1.765; 95% CI, 1.052-2.960; P = .031) together with advanced Binet stage (HR = 5.917; 95% CI, 3.341-10.479; P < .001), intermediate/high FISH risk (HR = 3.116; 95% CI, 1.758-5.521; P < .001), and unmutated IGHV status (HR = 2.269; 95% CI, 1.282-4.015; P = .005; supplemental Table 1). Because advanced Binet stage at diagnosis may be per se an indication to first-line treatment, the analysis was repeated after removing Binet stage from the covariates. This model selected as predictors of TTFT high Ang2 level (HR = 1.739; 95% CI, 1.059-2.857; P = .029) together with intermediate/high FISH risk (HR = 3.272; 95% CI, 1.993-5.373; P < .001) and unmutated IGHV status (HR = 2.260; 95% CI, 1.340-3.812; P = .002). The same results were obtained by limiting TTFT analysis only to the cohort of 274 CLL patients who did not receive treatment before this study (supplemental Table 1).
Kaplan-Meier curves for TTFT and OS in 316 CLLs. Patients are stratified in high and low Ang2 subsets based on Ang2 levels (cutoff, 2459 pg/mL). High Ang2 CLLs display significantly shorter TTFT (A) and OS (B) compared with low Ang2 CLLs (P < .001 and P = .002, respectively; log-rank test).
Kaplan-Meier curves for TTFT and OS in 316 CLLs. Patients are stratified in high and low Ang2 subsets based on Ang2 levels (cutoff, 2459 pg/mL). High Ang2 CLLs display significantly shorter TTFT (A) and OS (B) compared with low Ang2 CLLs (P < .001 and P = .002, respectively; log-rank test).
At the time of the analysis, 21 of 316 CLL patients have died, accounting for a median survival not reached and a 10-year survival of 87.1%. Because the statistical power of survival analysis was limited by the low number of deaths in this CLL cohort, the impact of Ang2 levels on OS was only explored. Along with TTFT, Ang2 more than or equal to 2459 pg/mL was also a predictor of poor OS (HR = 3.566; 95% CI, 1.496-8.499; P = .004) in CLL (Table 2). Accordingly, patients with high Ang2 were characterized by a significantly shorter OS (10-year OS, 74%) compared with patients with low Ang2 (10-year OS, 93%; P = .002; Figure 1B).
Ang2 plasma level refines prognosis among CLL subgroups identified by conventional prognosticators
In our cohort of CLL patients, we found significant associations between Ang2 plasma levels and other previously defined prognosticators. Table 3 compares the distribution of CLL prognostic factors between low Ang2 and high Ang2 CLL subgroups. High Ang2 was associated with Binet stages B-C (P < .001), high β2-microglobulin (P < .001), unmutated IGHV status (P < .001), high CD38 expression (P < .001), elevated ZAP-70 expression (P = .003), and intermediate/high cytogenetic risk (P = .005). No significant associations were found with the extent of infiltration (P = .215) and BM pattern (P = .785).
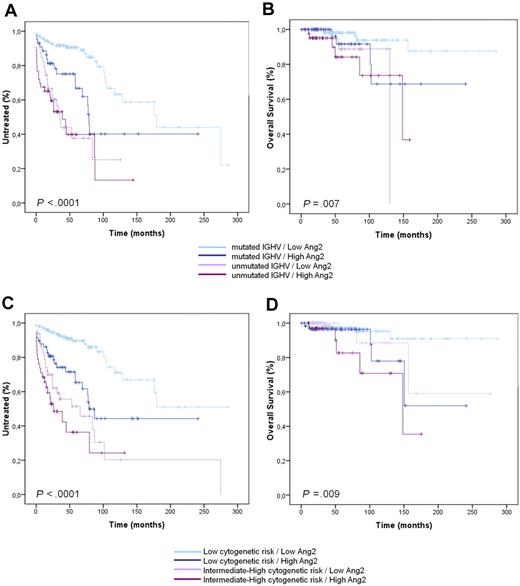
However, a consistent percentage of patients showed high Ang2 levels in the presence of favorable prognosticators and vice versa: these cases were defined as discordant and accounted for 80 of 289 (27.7%) patients for Binet stage, 83 of 268 (31.0%) for IGHV mutational status, 97 of 308 (31.5%) for CD38 expression, 100 of 264 (37.9%) for ZAP-70 expression, 64 of 164 (39.0%) for CD49d expression, 106 of 298 (35.6%) for FISH risk, and 111 of 294 (37.8%) for β2-microglobulin (Table 3). By analyzing these discordances in greater depth, we found that high Ang2 level identified a CLL subgroup displaying early need for treatment and reduced survival despite being characterized at diagnosis by favorable predictors, such as mutated IGHV status (P = .002 for TTFT and P = .05 for OS, Figure 2A-B), low CD38 expression (P = .001 for TTFT and P = .003 for OS), low ZAP-70 expression (P = .039 for TTFT), low CD49d expression (P < .001 for TTFT), low FISH risk (P < .001 for TTFT and P = .05 for OS, Figure 2C-D), and low β2-microglobulin (P = .013 for TTFT; supplemental Table 2). Moreover, low Ang2 levels identified a CLL subgroup with longer TTFT and OS despite being characterized by advanced Binet stage (P = .02 for TTFT and P = .032 for OS), high expression of CD38 (P = .018 for TTFT), ZAP-70 (P = .005 for TTFT and P = .05 for OS), and CD49d (P = .011 for OS), and high β2-microglobulin (P = .019 for OS; supplemental Table 2).
Prognostic relevance of Ang2 plasmatic level in combination with other established prognostic factors (IGHV and cytogenetic risk). Ang2 plasmatic level in combination with IGHV mutational status (A-B) and cytogenetic risk (C-D) carries a greater prognostic power in defining TTFT and OS.
Prognostic relevance of Ang2 plasmatic level in combination with other established prognostic factors (IGHV and cytogenetic risk). Ang2 plasmatic level in combination with IGHV mutational status (A-B) and cytogenetic risk (C-D) carries a greater prognostic power in defining TTFT and OS.
Ang2 level retains the prognostic value for TTFT in CLL with low and high VEGF
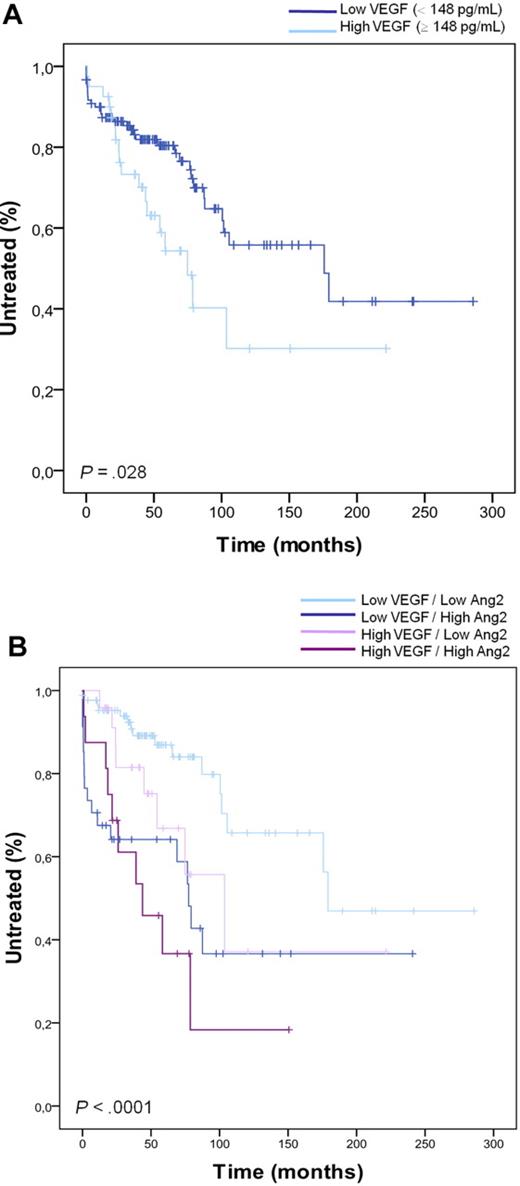
In physiologic and pathologic angiogenic processes, Ang2 is functionally associated with VEGF. We evaluated the plasma levels of VEGF protein in a subset of 160 CLL cases (supplemental Table 3). The subset of patients (cohort 2), investigated for both Ang2 and VEGF, was defined by a random selection of 40 CLL cases from each quartile of Ang2 distribution. In this way, the chosen CLL subset was representative of the whole cohort: the patients' characteristics were not statistically different, and the previously reported analyses assessing Ang2 prognostic value were confirmed in this 160 subset (data not shown). VEGF plasma levels ranged from 0 to 614 pg/mL with a median value of 52 pg/mL. No significant association was found between VEGF levels and all conventional prognostic factors. Moreover, Ang2 and VEGF plasma levels were not statistically correlated (r = 0.22, P > .05). Then, we evaluated the prognostic value of VEGF plasma levels for TTFT. We first defined the median value as a cutoff to divide CLL into high and low VEGF groups, obtaining no significant prognostic value of VEGF for TTFT (median TTFT, 179 vs 104 months in low vs high VEGF group, P = .373). Then, we tested the 75th percentile (148 pg/mL) as a cutoff, and we found that the subgroup of CLL cases with very high VEGF (≥ 148 pg/mL) had significantly shorter TTFT compared with low VEGF cases (< 148 pg/mL; median TTFT, 176 vs 75 months in low vs high VEGF group, P = .028; Figure 3A). Interestingly, Ang2 added prognostic power to both high and low VEGF subsets. In particular, high Ang2 plasma level identified CLL with short TTFT among cases with low VEGF (median TTFT, 179 vs 77 months in low vs high Ang2 group, P < .001; Figure 3B).
Prognostic value of Ang2 in combination with VEGF. (A) Patients are stratified in high and low VEGF subsets based on VEGF plasma levels (cutoff = 148 pg/mL). High VEGF CLLs have significantly shorter TTFT compared with low VEGF CLLs (P = .028, log-rank test). (B) Ang2 plasmatic level adds prognostic power in defining TTFT inside CLL subsets with high and low VEGF plasma level (P < .001). The cutoff level for Ang2 is 2459 pg/mL and for VEGF is the 75th percentile (148 pg/mL).
Prognostic value of Ang2 in combination with VEGF. (A) Patients are stratified in high and low VEGF subsets based on VEGF plasma levels (cutoff = 148 pg/mL). High VEGF CLLs have significantly shorter TTFT compared with low VEGF CLLs (P = .028, log-rank test). (B) Ang2 plasmatic level adds prognostic power in defining TTFT inside CLL subsets with high and low VEGF plasma level (P < .001). The cutoff level for Ang2 is 2459 pg/mL and for VEGF is the 75th percentile (148 pg/mL).
Ang2 is expressed by CLL cells in BM and LN compartments
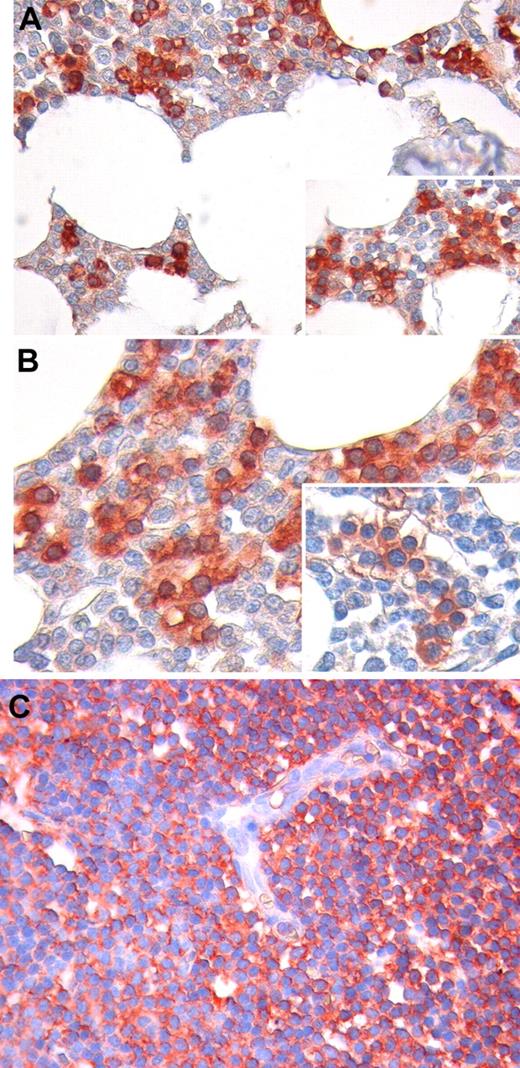
We analyzed Ang2 expression by immunohistochemistry in CLL-infiltrated BM and LN biopsies of 8 patients. In the BM compartment, we found that Ang2 staining is mainly confined to marrow infiltrating leukemic cells (Figure 4A-B). Notably, Ang2 expression was also observed in CLL cells with BM intravascular distribution. In contrast, the other BM hematopoietic cells, as well as stromal and endothelial cells, were mostly negative or slightly reactive. Similarly to BM infiltrates, neoplastic cells diffusely effacing LN architecture displayed a clear positivity to Ang2, and such a reactivity did not involve endothelia (Figure 4C).
Immunohistochemical staining for Ang2 protein in BM and LN biopsies. In BM, Ang2 immunoreactivity is mainly confined to infiltrating CLL cells (red), whereas other hematopoietic cells are mostly negative or slightly reactive (light pink; A-B). Notably, Ang2 expression is detected also in CLL cells displaying an intravascular distribution. No significant staining for Ang2 is observed on stromal and endothelial cells (A-B insets). Neoplastic cells diffusely effacing LN architecture display a clear positivity to Ang2, and such a reactivity does not involve endothelia (C). Original magnification, 200× for panels A and C, 400× for panel B.
Immunohistochemical staining for Ang2 protein in BM and LN biopsies. In BM, Ang2 immunoreactivity is mainly confined to infiltrating CLL cells (red), whereas other hematopoietic cells are mostly negative or slightly reactive (light pink; A-B). Notably, Ang2 expression is detected also in CLL cells displaying an intravascular distribution. No significant staining for Ang2 is observed on stromal and endothelial cells (A-B insets). Neoplastic cells diffusely effacing LN architecture display a clear positivity to Ang2, and such a reactivity does not involve endothelia (C). Original magnification, 200× for panels A and C, 400× for panel B.
Furthermore, we measured Ang2 levels by ELISA in both PB and BM plasma samples collected at the same time from 14 CLL patients. A strong positive correlation was found between BM and PB Ang2 levels (r = 0.802; P = .001), although slightly lower values were measured in the BM compartment compared with PB. Moreover, no correlation was found between BM Ang2 plasma levels and the degree of BM infiltration by CLL cells (r = 0.140; P = .632).
Ang2 level is not significantly modulated during disease course
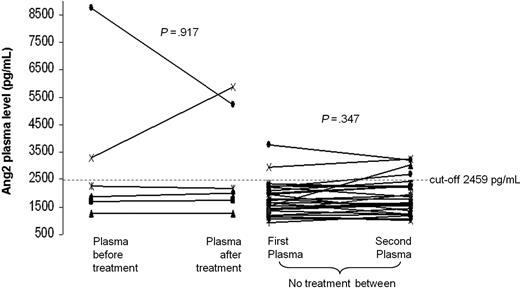
We performed comparison of Ang2 plasma levels between 2 sequential PB plasma samples collected from 35 CLL cases with median interval of 19 months (range, 3-30 months). Six cases received treatments between the 2 collections, and the second plasma sample was obtained at relapse. No significant differences (P = .376) in Ang2 levels over time in the same patient were found. Of note, Ang2 plasma levels did not significantly change among the sequential samples of both the 6 patients treated between the 2 observations (P = .917) and the 29 cases not treated between the 2 collections (P = .347; Figure 5). The overall concordance correlation coefficient between sequential Ang2 plasma levels was 0.757 (95% CI, 0.585-0.864), indicating a good stability for Ang2. In particular, only 2 of 35 (6%) patients displayed variations of Ang2 plasma levels between serial plasma samples that implied a cutoff crossing (Figure 5).
Stability of Ang2 plasma level during the course of CLL. The comparison between 2 sequential plasma samples derived from 35 CLL patients shows no statistical difference in Ang2 level both for the 6 treated cases (left) and for the 29 untreated patients (right; P = .917 and P = .347, respectively, Wilcoxon test).
Stability of Ang2 plasma level during the course of CLL. The comparison between 2 sequential plasma samples derived from 35 CLL patients shows no statistical difference in Ang2 level both for the 6 treated cases (left) and for the 29 untreated patients (right; P = .917 and P = .347, respectively, Wilcoxon test).
Discussion
Previous evidence has shown that Ang2 might play a role in the pathobiology and prognosis prediction of several solid tumors and hematologic malignancies.29-32 In particular, a strict association between high Ang2 levels and more advanced disease, unfavorable biologic prognostic parameters, and shorter progression-free survival was assessed in small series of CLL patients.10-12
On these premises, we reasoned that Ang2 would have a strong rationale for being tested as an independent prognostic factor in CLL. To test this hypothesis, we evaluated Ang2 plasma levels in a large cohort of CLL cases using a rapid and easy commercial ELISA kit. We measured variable amounts of Ang2 protein in 316 patients, and we identified Ang2 more than or equal to 2459 pg/mL as the best cutoff able to discriminate cases with shorter TTFT and reduced OS. In particular, high Ang2 level is an independent predictor of TTFT in multivariate analysis and is also a negative prognosticator for OS because the difference in OS at 10 years from diagnosis between patients with low and high Ang2 level approximated 20%.
Given the increasing availability of biologic CLL prognosticators, novel risk factors should provide information useful for refining the prognosis of risk categories identified by already existing markers. We therefore studied the association between Ang2 dosage and main established CLL prognostic markers. Patients with high Ang2 levels showed high prevalence of unfavorable prognostic parameters, but a relevant group of patients harbored high Ang2 levels together with a favorable asset of conventional markers or vice versa. In these discordant cases (∼ 30% of patients), high Ang2 level refines prognosis by identifying a subgroup of patients displaying short TTFT despite being characterized by favorable predictors, including mutated IGHV, low FISH risk, low CD38, ZAP-70, and CD49d expression, and low β2-microglobulin. Conversely, low Ang2 levels soften the unfavorable prognosis of patients with advanced Binet stage and high CD38 and ZAP-70 expression.
In multiple myeloma, serum Ang2 levels showed a trend of correlation with grade of BM involvement.33 In contrast, we found absence of correlation between Ang2 and degree/pattern of marrow infiltration in CLL, suggesting that increased Ang2 plasma levels are the direct effect of abnormal production by CLL cells that had experienced an “angiogenic switch,” not merely an index of extent of CLL cells infiltrating BM. Moreover, the study of sequential samples demonstrated that Ang2 levels are quite stable during the disease course and that current treatments do not seem able to interfere with Ang2 production by the CLL clone or do not select for subclones with different angiogenic capacities. As a consequence, higher Ang2 secretion seems to be a peculiar biologic characteristic of more aggressive CLL, already present at the beginning of the disease and maintained subsequently at relapse.
Our findings are subject to several limitations. First, the chosen cutoff has to be confirmed in an independent cohort of patients. Second, it would be important to study extensively Ang2 dosage trends in particular subsets of patients, such as those with renal impairment,34 cardiovascular disease, or essential hypertension,35 because these comorbidities may per se affect, at least in part, Ang2 levels. Finally, given the long natural history of CLL, also the observation of the stability of Ang2 levels during the disease course needs to be confirmed in a larger series with a longer follow-up.
Angiogenesis is a multistep process tightly regulated by several proangiogenic and antiangiogenic factors. Ang2 blocks the stabilizing action of the Tie-2 receptor, thus making vessels either more susceptible to the angiogenic effect of agents such as VEGF or more vulnerable to regression in absence of other angiogenic support.36 As a consequence, Ang2 works under the influence of VEGF. VEGF (both isoforms VEGF121 and VEGF165) is secreted by CLL cells and induces functional angiogenesis.37 Moreover, mean serum VEGF levels were higher in CLL compared with normal controls, even if aberrant VEGF increase was found only in a portion (∼ 18%) of patients.38,39 Importantly, CD38+ CLL cells overexpressed VEGF and appeared to preferentially use an internal autocrine VEGF survival loop.40 Conflicting results were reported about the prognostic role of plasma/serum VEGF levels.39,41-44 We evaluated plasma VEGF levels in 160 CLLs, finding that cases with very high VEGF (≥ 148 pg/mL, 75th percentile) had significant shorter TTFT compared with low VEGF cases. Interestingly, poor prognosis in patients with high Ang2 plasma levels was evident in both high and low VEGF subsets, implying that abundant Ang2 production manifests its adverse effect independently from the extent of VEGF secreted by CLL.
Ang2 seems to be an interesting molecule among other angiogenesis-related factors. The immunohistochemical study of Ang2 expression in BM and LN compartments infiltrated by CLL cells revealed that Ang2 preferentially originates from leukemic cells and not from activated endothelial or other surrounding cells. Moreover, abnormal Ang2 production seems to be a feature acquired precociously by CLL and maintained during the disease course. A genome-wide array-based methylation analysis recently found that ANG2 gene was methylated in mutated CLL while being unmethylated in unmutated CLL, underlining a role of epigenetic changes in the increased Ang2 expression by the CLL subset with poor prognosis.45 Finally, high Ang2 shows strong and independent prognostic power.
Elucidation of the underlying reasons for poor outcome of CLL patients showing high levels of Ang2 prompts further studies. The imbalance toward proangiogenic cytokine production (angiogenic switch) and, as consequence, sprouting of new blood vessels in BM and LNs may provide oxygen and metabolite supply, increase cell mobility, and enhance survival circuits, thus facilitating disease progression. Of interest, in vitro interaction between CLL cells and endothelial cells was showed to promote CLL survival.46-48 The association between Ang2 and other poor prognostic parameters (unmutated IGHV, CD38+, ZAP-70+, and CD49d+) in our study implies that the leukemic clone with these molecular properties may be characterized by favorable angiogenic milieu and increased stromal nurturing.48,49
The pathways activated by Ang2 in CLL microenvironment deserve particular attention to identify new possible pathogenetic mechanisms and related therapeutic targets. Because several molecules targeting microenvironment and angiogenesis are being developed in clinical trials for CLL, it could be also intriguing to evaluate if such drugs may modulate Ang2 secretion. Blockade of Ang2 using inhibitors of nox enzymes or using selective antibodies and peptide-Fc fusion proteins was shown to reduce vascularization and growth in mouse models of several solid tumors.6-8 Moreover, AMG 386, a selective angiopoietin inhibitor has recently been tested in patients with advanced solid tumors, and it would be interesting to know its role in CLL.50
In conclusion, we first demonstrate the prognostic role of Ang2 plasma levels in CLL, in relation to TTFT and OS. Moreover, we show that Ang2 contributes to refine the prognostic assessment of CLL when used in combination with the other prognostic markers. Our observations are supported by the finding of the stability of Ang2 dosage during the disease course and suggest an important role of this molecule inside CLL pathogenesis. This study implies a simple and reproducible ELISA method that could be therefore easily used both in clinical practice and in further studies, helping to better refine prognosis, understand CLL pathogenesis, and identify target therapies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Associazione Italiana contro le Leucemie–Linfomi e Mieloma, Modena, Italy; Programma Ricerca Regione-Università (2007-2009), Emilia Romagna, Italy; Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Modena, Italy (PRIN 2008); Novara-Linfomi e Mieloma Onlus, Novara, Italy; Progetto Integrato Oncologia, MIUR, Rome, Italy (PRIN 2008); Associazione Italiana per la Ricerca contro il Cancro, Milan, Italy; and Istituto Toscano Tumori, Florence, Italy.
Authorship
Contribution: R. Maffei, S.M., and R. Marasca designed and performed the research, analyzed data, and wrote the paper; V.C., G.L., E.S., G.M.R., and A.Z. collected and managed clinical data; S.F., M.F., and P.Z. performed laboratory molecular assays; C.G., C.T., and I.C. performed and evaluated immunohistochemical staining; D.R. and R.S. performed the statistical analysis; and A.C., V.G., G.D.P., F.F., G.G., and G.T. conceived and coordinated research and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roberto Marasca, Department of Oncology and Hematology, University of Modena and Reggio Emilia, Via Del Pozzo 71, 41124, Modena, Italy; e-mail: roberto.marasca@unimore.it.