Abstract
Using a combination high-throughput screening technology, multiple classes of drugs and targeted agents were identified that synergize with dexamethasone (Dex) in multiple myeloma (MM) cells. Performing combination screening with these enhancers, we discovered an unexpected synergistic interaction between adenosine receptor agonists and phosphodiesterase (PDE) inhibitors that displays substantial activity in a panel of MM and diffuse large B-cell lymphoma (DLBCL) cell lines and tumor cells from MM patients. We have used selective adenosine receptor agonists, antagonists, and PDE inhibitors as well as small interfering RNAs targeting specific molecular isoforms of these proteins to dissect the molecular mechanism of this synergy. The adenosine A2A receptor and PDE2, 3, 4, and 7 are important for activity. Drug combinations induce cyclic AMP (cAMP) accumulation and up-regulate PDE4B. We also observe rigorous mathematical synergy in 3-way combinations containing A2A agonists, PDE inhibitors, and Dex at multiple concentrations and ratios. Taken together, these data suggest that A2A agonist/PDE inhibitor combinations may be attractive as an adjunctive to clinical glucocorticoid containing regiments for patients with MM or DLBCL and confer benefit in both glucocorticoid-sensitive and -resistant populations.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy, comprising almost 40% of all lymphomas.1 Multiple myeloma (MM) is the second most prevalent B-cell malignancy with approximately 20 000 new patients and 10 000 deaths each year in just the United States alone.2 There have been considerable advances in the understanding of these diseases and the development of treatment options. For example with MM, before the option of chemotherapy, the median survival was 7 months.3 With the use of melphalan and prednisolone in the 1960s, median survival was increased to 24 to 30 months.3,4 In the past few years, important clinical advances have been the Food and Drug Administration (FDA) approval of bortezomib (2003), thalidomide/lenalidomide (2006), and the combination of bortezomib and liposome-encapsulated doxorubicin (2007). These new therapeutic options have dramatically improved the clinical response rate and lifespan of patients.5 Of particular note, use of agents in combination appears to have the most benefit. For example, bortezomib and liposome-encapsulated doxorubicin, approved for the treatment of relapsed/refractory MM, significantly extends time to progression versus bortezomib alone (9.3 vs 6.5 months6 ). The combination of lenalidomide, bortezomib, and dexamethasone (Dex) has significantly enhanced clinical activity versus the individual agents.7 The response rate is better for melphalan/prednisone/bortezomib versus melphalan/prednisone.8 With such encouraging results, combination treatment is being aggressively explored in the clinic and even better combination regimens will undoubtedly be discovered. However, while drugs such as Dex/prednisone, melphalan, bortezomib, lenalidomide, and liposome-encapsulated doxorubicin have efficacy in the clinic, patients eventually relapse and are frequently resistant to agents to which they initially responded. In addition, many patients experience significant dose-limiting side effects requiring dose reductions or cessation of therapy. Although average survival time has overall improved, MM remains an incurable disease and new therapies are in demand.
Glucocorticoids (GCs) have historically been a key component of combination therapy regimens in B-cell and other hematologic malignancies. GCs are standard treatment for childhood acute lymphoblastic leukemia (ALL), invariably in combination with other chemotherapeutic agents, and the use of GCs is a key reason remission rates are more than 95% with an overall cure rate of 80%.9,10 Prednisone is a constituent of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone, a commonly used cancer combination chemotherapy), standard of care for DLBCL, the most common type of non-Hodgkin lymphoma in adults. In MM, Dex and prednisone remain prominent in 2-, 3-, and 4-way combination drug regimens used clinically. Importantly, GC use comes with substantial dose-limiting toxicities and long-term use of GCs can contribute to bone loss, elevated glucose levels, and other intolerable side effects. The ability to enhance GC-dependent effects while sparing GC-induced toxicities may have important implications for therapeutic interventions in MM and other B-cell malignancies.
We have developed a combination high-throughput screening (cHTS) platform that systematically and effectively identifies drugs that have synergistic activity when used in combination.11-13 Synergistic combinations have been identified where the result is an efficacy boost (increase in the maximal effect achieved), increase in compound potency (dose-sparing), and/or conversion of cells from chemoresistance to a chemosensitive state. These features are quite attractive for oncology indications, as it is important to maximize tumor cell killing and reduce the dose of agent(s) producing adverse effects. Here, we have deployed cHTS to identify compounds that synergize with GCs to inhibit the proliferation of the MM cells. This screen has led to the identification of 2 classes of targeted agents that synergize with GCs and quite strongly with each other. Using chemical genetics and small interfering RNA (siRNA), the molecular targets have been identified as the adenosine receptor subtype A2A and a specific subset of the phosphodiesterase gene family. Synergy is observed broadly across a large panel of MM and DLBCL cell lines. Exposure of MM or DLBCL cells to A2A agonists and specific phosphodiesterase (PDE) inhibitors results in rapid synergistic elevation of cyclic AMP (cAMP), inhibition of proliferation, and induction of apoptosis. The concentrations at which combination activities are observed suggest that these agents have potential value in a clinical setting.
Methods
Cell lines
All cell lines were obtained from ATCC, DSMZ, or Lonza except MM.1S (Dr Stephen Rosen, Northwestern University, Evanston, IL), MM.1R and INA-6 (Dr Constantine Mitsiades, Dana-Farber Cancer Institute, Boston, MA), ANBL-6 (Dr Robert Orlowski, M. D. Anderson Cancer Research Center, Houston, TX), and SU-DHL4, SU-DHL6, SU-DHL-7, OCI-ly1, OCI-ly3, OCI-ly4, OCI-ly10, and OCI-ly19 (Dr Margaret Shipp, Dana Farber Cancer Institute, Boston, MA). All cell lines were cultured in RPMI 1640 with 10% fetal bovine serum and 1% penicillin/streptomycin except OCI-ly1, 3, 4, and 19 which were cultured in Iscove modified Dulbecco medium (IMDM), 10% fetal bovine serum, and 1% penicillin/streptomycin. OCI-ly10 was cultured in IMDM, 20% human serum, and 1% penicillin/streptomycin. The ANBL-6 and INA-6 cell line media was supplemented with 10 ng/mL IL-6 (R&D Systems).
Compounds
All compounds were obtained from Sigma-Aldrich, EMD Chemicals, or Biomol and dissolved in dimethyl sulfoxide (DMSO) to make 1000× stock solutions.
Proliferation assays
Cells were seeded at 1000 to 1500 cells per well (384-well plate) and cultured 18 to 24 hours before compound addition. Upon addition of drug, cells were incubated for 72 hours and effects on proliferation were determined using ATPlite 1 Step (PerkinElmer) using conditions suggested by the manufacturer. Combination screening was performed as described.11-13 Synergy was determined by comparing a combination's response to Loewe additivity.11,13 Activity in excess of the dose-additivity model was calculated (subtraction of dose-additive values from the observed data) and assigned a synergy score. The synergy score S = ln fX ln fY Σdoses max(0,Zdata) (Zdata − Zmodel) sums up the excess inhibition over the model with weights to account for drug dilution factors fX,fY, and favor synergy at high inhibition levels. Z = the measured dose matrix inhibitory values (see Lehár et al14 for additional details).
Patient samples
All experiments involving patient samples were approved by the Dana-Farber Cancer Institute ethical review board. Following informed consent in accordance with the Declaration of Helsinki, bone marrow aspirates from MM patients were collected, mononuclear cells were separated by Ficoll (Amersham Biosciences) gradient centrifugation, and MM cells were purified using CD138 microbeads (Miltenyi Biotec). Purified MM patient cells were plated at a density of 10 000 cells per well, treated, and cultured for 48 hours. After incubation at 37°C, CellTiterGlo (CTG; Promega) was added and incubated for 30 minutes. Plates were then read using a Luminoskan luminometer (Labsystems).
Apoptosis assays
MM1.S cells were plated at 4 × 105 cells/mL on a 6-well tissue-culture dish. Cells were either untreated or treated with 50nM CGS-21680, 2μM trequinsin, or a combination of both drugs and harvested at 24 hours and 48 hours. Apoptosis analysis was performed using the Annexin-V–Fluos Staining Kit according to the manufacturer's instructions (BD Biosciences), followed by flow cytometric analysis using a BD Biosciences FACSCalibur.
cAMP assays
cAMP measurements were done using the MM.1R cell line and Perkin Elmer LANCE cAMP 384 Kits. The assays were performed using conditions described by the manufacturer. Cells were treated with compounds for 1 hour in 384-well plates using 5000 cells in 6 μL of kit stimulation buffer. Plates were read using an EnVision plate reader with an excitation of 340 nm and an emission of 665 nm.
siRNA transfections
Two different siRNA were used per target. The siRNA with the best silencing activity (as determined by polymerase chain reaction [PCR]) were from QIAGEN (Hs_ADORA1_4, Hs_ADORA2A_1, Hs_ADORA2B_6, Hs_ADO26_5, and PDE2A_5) and Dharmacon (J-007646-08 [PDE3B], J-007647-14 [PDE4A], J-007648-07 [PDE4B], J-004657-17 [PDE4D], and J-003818-15 [PDE7A]). siGLO (QIAGEN) was used as a control siRNA. A total of 2 × 106 MM.1R cells were electroporated using 100 μL of solution V nucleofection reagent containing 50nM siRNA using program S-20 (Amaxa). As determined using 50nM siGLO and fluorescence-activated cell sorting (FACS) analysis, transfection efficiency was 87% and viability was 89% 24 hours posttransfection. At 24 hours posttransfection, cells were seeded at 1500 cells per well and at 48 hours, drugs were added to cultures. Some cells were harvested at the time of drug addition to isolate RNA for PCR assessment of gene knockdown, using primers, reagents, and protocols supplied by Applied Biosystems.
Results
A steroid enhancer screen reveals novel glucocorticoid-dependent and -independent synergies
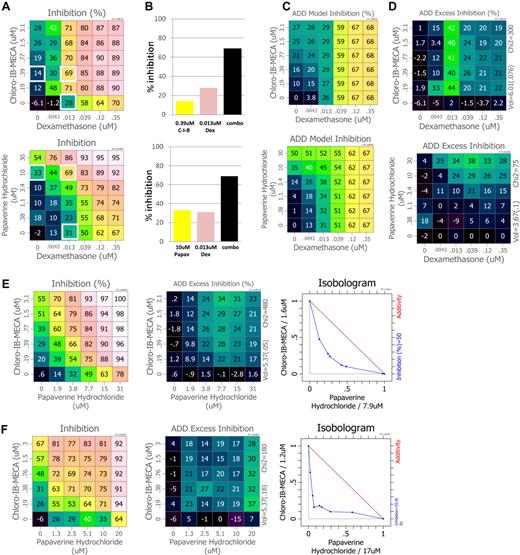
GCs have been part of MM therapy for many years and most effective when deployed in combination with other drugs.15 To discover novel GC combinations, we deployed cHTS to identify compounds that synergize with the GC Dex to inhibit MM cell proliferation. We evaluated a defined set of combinations of approved drugs and molecular probes in 4 MM cell lines. A total of 2841 unique combinations were evaluated, 648 of which were assayed in all 4 cell lines. Multiple compounds were identified that synergize with Dex, including known anticancer drugs and combinations of targets not previously studied in MM. Two compounds of particular interest, Chloro-IB-MECA and papaverine, potentiate the antiproliferative effect of Dex at various concentrations and ratios in RPMI-8226 cells (Figure 1A). A small sampling of single agents and combinations (white boxes) are graphed for comparative purposes, highlighting combination effects and the breadth of combination data captured using a full-dose matrix (Figure 1B). Using the Loewe additivity model which predicts additive effects (Figure 1C), we demonstrate that both Chloro-IB-MECA and papaverine strongly synergize with Dex (see Figure 1D and figure legend). These synergistic effects are not observed using the steroid-insensitive cell line MM.1R,16 confirming the importance of GC signaling for activity for these combinations (data not shown). Dex activity was also potentiated by sirolimus (rapamycin) and retinoid adapalene (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Chemical probes for these targets have been reported to synergize with Dex to inhibit MM cell growth, thus validating the use of this screen to identify CG potentiators.17,18
Identification of novel synergistic antiproliferative multiple myeloma drug combinations using cHTS. A select set of approved drugs, emerging therapeutics, and research probes were combined with the glucocorticoid dexamethasone using our high-throughput combination screening platform and antiproliferative activity was determined using the multiple myeloma cell lines RPMI-8226, MM.1S, and MM.1R. (A) A 6 × 6 dose matrix was generated for each combination. The matrix samples all mixtures of 2 serially diluted single-agent concentrations. Phenotypic assay measurements of inhibition over the test matrix are visualized using a color scale. Shown are the combination activities for dexamethasone combined with Chloro-IB-M ECA, an adenosine receptor agonist and papaverine, a PDE inhibitor. (B) For comparative purposes, a small sampling of single agents and combinations are graphed at concentrations where combination activities are more than additive (synergistic). Single-agent and combination activities used are from the matrix shown in panel A and are highlighted by white boxes. Comparison of panels A and B demonstrate how the dose-matrix format captures the breadth of combination activities at multiple ratios over a wide concentration range. (C) To assess synergy, each observed point is compared with the dose-additive model (expectation for a drug crossed with itself) that is calculated at every test point in the matrix using the single-agent responses at the edges (see “Proliferation assays” for more information). (D) Activity in excess of the dose-additivity model is calculated (subtraction of dose-additive values from the observed data) and assigned a synergy score (see “Proliferation assays” for more information). (E) Dose-matrix analysis, dose-additivity excess, and isobologram analysis for Chloro-IB-MECA combined with papaverine in the MM.1S cell line and (F) the MM.1R cell line. The isobologram error bars are the result of comparison of drug-treated cells versus untreated controls.
Identification of novel synergistic antiproliferative multiple myeloma drug combinations using cHTS. A select set of approved drugs, emerging therapeutics, and research probes were combined with the glucocorticoid dexamethasone using our high-throughput combination screening platform and antiproliferative activity was determined using the multiple myeloma cell lines RPMI-8226, MM.1S, and MM.1R. (A) A 6 × 6 dose matrix was generated for each combination. The matrix samples all mixtures of 2 serially diluted single-agent concentrations. Phenotypic assay measurements of inhibition over the test matrix are visualized using a color scale. Shown are the combination activities for dexamethasone combined with Chloro-IB-M ECA, an adenosine receptor agonist and papaverine, a PDE inhibitor. (B) For comparative purposes, a small sampling of single agents and combinations are graphed at concentrations where combination activities are more than additive (synergistic). Single-agent and combination activities used are from the matrix shown in panel A and are highlighted by white boxes. Comparison of panels A and B demonstrate how the dose-matrix format captures the breadth of combination activities at multiple ratios over a wide concentration range. (C) To assess synergy, each observed point is compared with the dose-additive model (expectation for a drug crossed with itself) that is calculated at every test point in the matrix using the single-agent responses at the edges (see “Proliferation assays” for more information). (D) Activity in excess of the dose-additivity model is calculated (subtraction of dose-additive values from the observed data) and assigned a synergy score (see “Proliferation assays” for more information). (E) Dose-matrix analysis, dose-additivity excess, and isobologram analysis for Chloro-IB-MECA combined with papaverine in the MM.1S cell line and (F) the MM.1R cell line. The isobologram error bars are the result of comparison of drug-treated cells versus untreated controls.
We hypothesized that compounds which synergize with Dex may target distinct molecular mechanisms important for cell proliferation and/or survival and that combining Dex enhancers may reveal synergistic, nonsteroid pathway interactions with therapeutic potential. We therefore combined the enhancers with each other using MM.1S cells. This cell line is Dex-sensitive and deployed because of the availability of the derivative MM.1R, which is no longer sensitive to Dex and can be used as a counterscreen. Shown in Figure 1E is the activity of one combination, Chloro-IB-MECA and papaverine. This combination demonstrates robust inhibition of MM.1S cell proliferation and strong synergy at multiple concentrations and ratios. Importantly, the combination of Chloro-IB-MECA and papaverine also synergistically inhibited the proliferation of the MM.1R GC-insensitive cell line (Figure 1F) indicating that although originally identified as Dex enhancers, these compounds probe pathways independent of steroids.
Combinations of adenosine A2A receptor agonists and PDE inhibitors demonstrate synergistic inhibition of proliferation
Papaverine is reported to be a PDE inhibitor selective for PDE7.19 Chloro-IB-MECA is an adenosine receptor agonist reported to be selective for adenosine A3 receptors over the A1, A2A, and A2B receptor subtypes.20 To better understand the cellular targets required for the synergistic effect, we examined the activity of 5 adenosine receptor agonists with preferential selectivities to adenosine receptor subtypes combined with 20 PDE inhibitors of known potencies and selectivities,19,21 which represent chemical inhibitors of the 11 members of the PDE superfamily (Table 1 and supplemental Figure 2). Synergistic interactions were quantified using a modification of the Loewe additivity method to calculate a synergy score for each pairing. The synergy score takes into account both the excess inhibition over the Loewe additivity model as well as the overall effect level achieved (see Figure 1 legend and “Proliferation assays”). Synergistic inhibition of proliferation was observed in pairings that included inhibitors of PDE 2 (BAY 60-7550), PDE 3 (cilostamide, cilostazol, siguazodan, milrinone), PDE 4 (R-(-)rolipram, RO-20-1724, roflumilast), and PDE 7 (papaverine, BRL-54680) as well as with compounds that inhibit multiple members of the group of PDE 2, 3, 4, and 7 (ibudilast, zardaverine, and trequinsin). Notably, no synergistic effects were observed when adenosine receptor agonists were combined with inhibitors of PDE 1, 5, 6, 10, and 11 (vinpocetine, zaprinast, sildenafil, vardenafil).
Synergy scores for adenosine receptor/PDE inhibitor combinations
| PDE inhibitor and selectivity . | Adenosine receptor agonist and selectivity . | |||||
|---|---|---|---|---|---|---|
| A1 > A3 ≫ A2A = A2B . | A1 = A2A = A3 > A2B . | A2A > A3 > A1 ≫ A2B . | A3 > A1 > A2A ≫ A2B . | A3 > A1 > A2A ≫ A2B . | ||
| (S)-ENBA . | HE-NECA . | CGS-21680 . | Chloro-IB-MECA . | IB-MECA . | ||
| pan | IBMX | 0.09 | 0.27 | 0.38 | 0.34 | 0.11 |
| pan | Pentoxifylline | 0.50 | 0.28 | 0.23 | 0.06 | 0.09 |
| pan | Dipyridamole | 0.17 | 0.08 | 0.10 | 0.20 | 0.07 |
| 1 | Vinpocetine | 0.26 | 0.27 | 0.29 | 0.13 | 0.21 |
| 2 | BAY 60-7550 | 2.82* | 3.56* | 3.84* | 2.09* | 1.80* |
| 2,3,4 | Trequinsin | 4.53* | 10.34* | 6.96* | 5.91* | 6.38* |
| 3 | Cilostamide | 1.15* | 1.88* | 0.88 | 1.10* | 1.46* |
| 3 | Cilostazol | 1.61* | 4.07* | 4.08* | 1.30* | 1.55* |
| 3 | Siguazodan | 0.40 | 0.73 | 1.44* | 0.42 | 0.61 |
| 3 | Milrinone | 1.31* | 0.33 | 0.70 | 0.26 | 0.40 |
| 3,4 | Zardaverine | 3.25* | 4.40* | 5.12* | 3.82* | 4.90* |
| 4 | R-(-)-Rolipram | 1.89* | 4.38* | 1.93* | 1.65* | 2.69* |
| 4 | RO-20-1724 | 0.74 | 3.52* | 0.90 | 1.60* | 1.82* |
| 4 | Roflumilast | 2.19* | 3.24* | 4.39* | 2.85* | 3.21* |
| 3,4,10,11 | Ibudilast | 0.67 | 0.98 | 2.21* | 0.74 | 1.16* |
| 1,5,6,10,11 | Zaprinast | 0.13 | 0.09 | 0.05 | 0.07 | 0.03 |
| 1,5,6 | Sildenafil | 0 | 0 | 0.07 | 0 | 0 |
| 5 | Vardenafil | 0 | 0 | 0 | 0 | 0 |
| 6,7,10 | Papaverine | 1.95* | 2.07* | 0.95 | 2.32* | 1.68* |
| 7 | BRL-50481 | 1.23* | 1.41* | 1.98* | 1.89* | 1.51* |
| PDE inhibitor and selectivity . | Adenosine receptor agonist and selectivity . | |||||
|---|---|---|---|---|---|---|
| A1 > A3 ≫ A2A = A2B . | A1 = A2A = A3 > A2B . | A2A > A3 > A1 ≫ A2B . | A3 > A1 > A2A ≫ A2B . | A3 > A1 > A2A ≫ A2B . | ||
| (S)-ENBA . | HE-NECA . | CGS-21680 . | Chloro-IB-MECA . | IB-MECA . | ||
| pan | IBMX | 0.09 | 0.27 | 0.38 | 0.34 | 0.11 |
| pan | Pentoxifylline | 0.50 | 0.28 | 0.23 | 0.06 | 0.09 |
| pan | Dipyridamole | 0.17 | 0.08 | 0.10 | 0.20 | 0.07 |
| 1 | Vinpocetine | 0.26 | 0.27 | 0.29 | 0.13 | 0.21 |
| 2 | BAY 60-7550 | 2.82* | 3.56* | 3.84* | 2.09* | 1.80* |
| 2,3,4 | Trequinsin | 4.53* | 10.34* | 6.96* | 5.91* | 6.38* |
| 3 | Cilostamide | 1.15* | 1.88* | 0.88 | 1.10* | 1.46* |
| 3 | Cilostazol | 1.61* | 4.07* | 4.08* | 1.30* | 1.55* |
| 3 | Siguazodan | 0.40 | 0.73 | 1.44* | 0.42 | 0.61 |
| 3 | Milrinone | 1.31* | 0.33 | 0.70 | 0.26 | 0.40 |
| 3,4 | Zardaverine | 3.25* | 4.40* | 5.12* | 3.82* | 4.90* |
| 4 | R-(-)-Rolipram | 1.89* | 4.38* | 1.93* | 1.65* | 2.69* |
| 4 | RO-20-1724 | 0.74 | 3.52* | 0.90 | 1.60* | 1.82* |
| 4 | Roflumilast | 2.19* | 3.24* | 4.39* | 2.85* | 3.21* |
| 3,4,10,11 | Ibudilast | 0.67 | 0.98 | 2.21* | 0.74 | 1.16* |
| 1,5,6,10,11 | Zaprinast | 0.13 | 0.09 | 0.05 | 0.07 | 0.03 |
| 1,5,6 | Sildenafil | 0 | 0 | 0.07 | 0 | 0 |
| 5 | Vardenafil | 0 | 0 | 0 | 0 | 0 |
| 6,7,10 | Papaverine | 1.95* | 2.07* | 0.95 | 2.32* | 1.68* |
| 7 | BRL-50481 | 1.23* | 1.41* | 1.98* | 1.89* | 1.51* |
MM.1S cells were incubated with adenosine receptor agonists and PDE inhibitors alone and in combination in multiple doses and ratios in a 6 × 6 dose matrix (as described in Figure 1). The synergy scores were determined as detailed in the legend for Figure 1. The activity of 5 adenosine receptor agonists and 20 PDE inhibitors was examined. The selectivity of each of these agents is shown. All of the adenosine receptor agonists target the A2A receptor but differ in potency, with selectivity being concentration dependent.20 PDE inhibitor specificity is as described.19,21
PDE indicates phosphodiesterase; and IBMX, 3-isobutyl-1-methylxanthine.
Adenosine receptor agonist/PDE inhibitors combinations with synergy scores > 1.
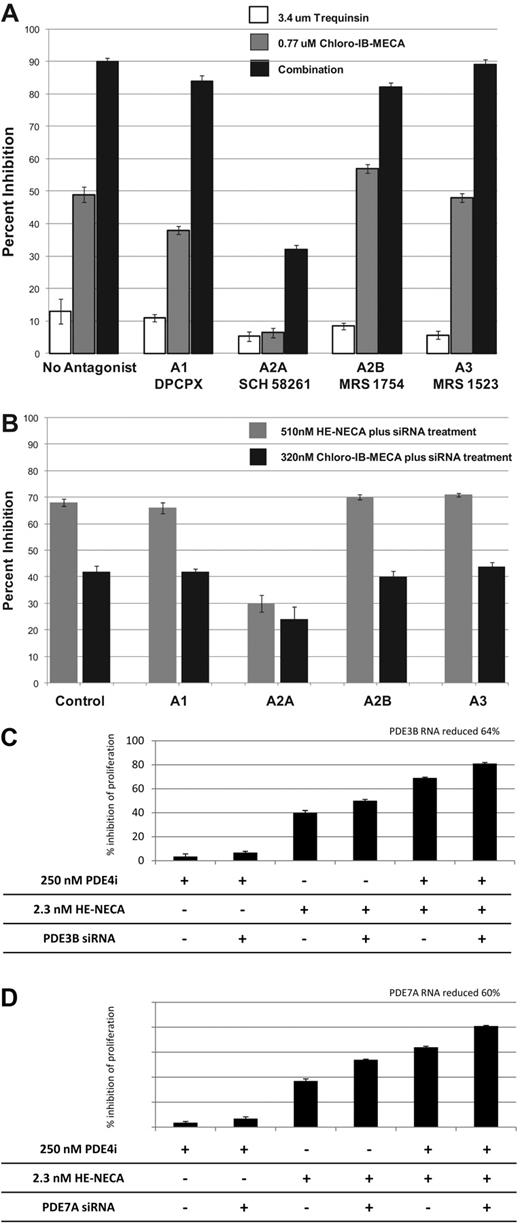
To identify the adenosine receptor subtype responsible for synergy, we examined the activity of adenosine receptor agonists in the presence of selective adenosine receptor subtype-specific antagonists (Figure 2A). The single agent and combination activity of Chloro-IB-MECA was dramatically inhibited by the A2A antagonist SCH 58261 but not by antagonists of A1 (DPCPX), A2B (MRS 1754), or A3 (MRS 1523). The ability of the A2A antagonist SCH58261 to block antiproliferative effects was not a general phenomenon, as MM.1S sensitivity to trequinsin (Figure 2A) and other anitproliferative agents such as Dex, sirolimus, topotecan, and vinorelbine was unchanged in the presence of this agent (data not shown). As a complementary approach, we also evaluated the activity of adenosine receptor agonists after specific knockdown of receptor subtypes using siRNA (Figure 2B). Cells transfected with siRNA targeting the A2A subtype showed a 76% reduction in A2A receptor RNA levels and a dramatically reduced antiproliferative response to adenosine receptor agonists Chloro-IB-MECA and HE-NECA (Figure 2B). Cells transfected with siRNA to A1, A2B, and A3 showed no diminished response to these agents despite the reduction of A1, A2B, and A3 receptors (Figure 2B).
Chemical and molecular genetic validation of the adenosine receptor subtype A2A, PDE3B and PDE7A as a novel drug targets for multiple myeloma. (A) MM.1S cells were incubated with 3.4μM trequinsin, 0.77μM Chloro-IB-MECA, or both drugs in the absence or presence of 89nM DPCPX (A1 antagonist), 78nM SCH 58261 (A2A antagonist), 89nM MRS 1754 (A2B antagonist), and 87nM MRS 1523 (A3 antagonist). Cells were seeded in wells of a 384-well plate, incubated with drugs for 72 hours, and effects on proliferation determined using ATP lite. (B) MM.1R cells were electroporated with 50nM control siRNA (siCON) or siRNA targeting the A1, A2A, A2B, or A3 adenosine receptor. Forty-eight hours later, cells were exposed to 510nM HE-NECA or 320nM Chloro-IB-MECA and incubated an additional 72 hours before the addition of ATP lite. At the time of drug addition, as determined by reverse transcription–PCR, target RNA knockdown was 88% for the adenosine A1 receptor, 76% for A2A, 37% for A2B, and 62% for the A3 adenosine receptor subtype. The results are from experiments performed in duplicate. MM.1R cells were electroporated with control siRNA (siCON) or siRNA targeting (C) PDE3B or (D) PDE7A. Cells not receiving PDE siRNA were electroporated with the control siRNA siCON. Forty-eight hours postelectroporation, cells were seeded in 384-well plates, treated with 250nM roflumilast (PDE4i), 2.3nM HE-NECA (A2A agonist), or the combination of both drugs and incubated an additional 72 hours followed by the addition of ATP lite. As determined by RT-PCR, at the time of drug addition, target RNA knockdown was 64% for PDE3B and 60% for PDE7A. All results are from experiments performed in duplicate.
Chemical and molecular genetic validation of the adenosine receptor subtype A2A, PDE3B and PDE7A as a novel drug targets for multiple myeloma. (A) MM.1S cells were incubated with 3.4μM trequinsin, 0.77μM Chloro-IB-MECA, or both drugs in the absence or presence of 89nM DPCPX (A1 antagonist), 78nM SCH 58261 (A2A antagonist), 89nM MRS 1754 (A2B antagonist), and 87nM MRS 1523 (A3 antagonist). Cells were seeded in wells of a 384-well plate, incubated with drugs for 72 hours, and effects on proliferation determined using ATP lite. (B) MM.1R cells were electroporated with 50nM control siRNA (siCON) or siRNA targeting the A1, A2A, A2B, or A3 adenosine receptor. Forty-eight hours later, cells were exposed to 510nM HE-NECA or 320nM Chloro-IB-MECA and incubated an additional 72 hours before the addition of ATP lite. At the time of drug addition, as determined by reverse transcription–PCR, target RNA knockdown was 88% for the adenosine A1 receptor, 76% for A2A, 37% for A2B, and 62% for the A3 adenosine receptor subtype. The results are from experiments performed in duplicate. MM.1R cells were electroporated with control siRNA (siCON) or siRNA targeting (C) PDE3B or (D) PDE7A. Cells not receiving PDE siRNA were electroporated with the control siRNA siCON. Forty-eight hours postelectroporation, cells were seeded in 384-well plates, treated with 250nM roflumilast (PDE4i), 2.3nM HE-NECA (A2A agonist), or the combination of both drugs and incubated an additional 72 hours followed by the addition of ATP lite. As determined by RT-PCR, at the time of drug addition, target RNA knockdown was 64% for PDE3B and 60% for PDE7A. All results are from experiments performed in duplicate.
The analysis of 20 PDE inhibitors indicates that inhibitors targeting one or more PDEs from the subgroup PDE2, 3, 4, and/or 7 combined with adenosine A2A receptor agonists result in the synergistic inhibition of proliferation in MM cell lines. Furthermore, the inhibition of multiple PDEs from this subgroup results in greater combination activity than when targeting just one PDE (Table 1 and supplemental Figure 2). To confirm the role of PDE2, 3, 4, and 7 in the antiproliferative effect observed in A2A agonist plus PDE inhibitor combinations, we examined the antiproliferative effect of A2A agonist and PDE inhibitor single agents and combinations in MM.1R cells after transfection of siRNA targeting specific PDE isoforms. Cells were electroporated with control siRNA (siCON) or siRNA targeting PDE3B or PDE7A (Figure 2C-D) or PDE2A, PDE4A, PDE4B, or PDE4D (supplemental Figure 3). Forty-eight hours posttransfection, cells were incubated with HE-NECA (adenosine receptor agonist), a PDE inhibitor, or both agents. As seen in Figure 2C, siRNA inhibition of PDE3B had little effect in potentiating inhibition of proliferation of cells treated with the PDE4 inhibitor roflumilast. In contrast, siRNA inhibition of PDE3B increased the anitproliferative activity of HE-NECA and the HE-NECA/roflumilast combination. Similar potentiating effects were observed using siRNA targeting PDE7A (Figure 2D), PDE2A, PDE4B, and PDE4D (supplemental Figure 3). siRNA to PDE4A did not increase single agent or combination activity (supplemental Figure 3). The results confirm that PDE2A, PDE3B, PDE4B, PDE4D, and PDE7A are the PDE targets important for synergistic antiproliferative activity when cells are exposed to an A2A agonist.
Selectivity for B-cell malignancies
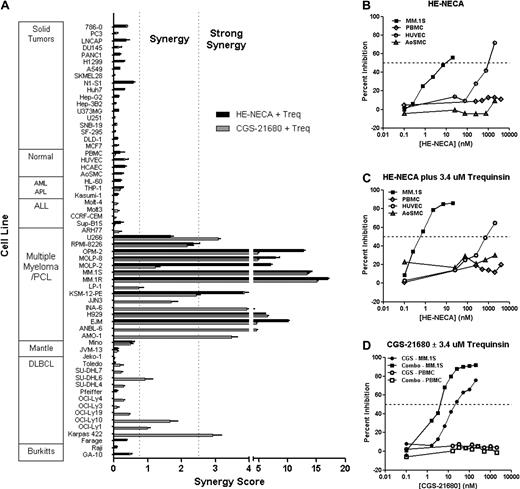
To determine the selectivity profile of A2A agonist/PDE inhibitor combinations, we examined the activity of A2A agonists HE-NECA and CGS-21680 in combination with trequinsin in a panel of 61 additional cell types including peripheral blood mononuclear cells (PBMCs), nontransformed cell lines, and transformed cells derived from solid tumors and hematologic malignancies (Figure 3A). A striking pattern of synergy was observed in cell lines representative of MM. Synergistic antiproliferative activity was also observed in cell lines representative of DLBCL but not observed for B-ALL, T-ALL, myeloid lineages, or solid tumor cell lines. Importantly, no synergy or efficacy was observed in the human primary nontransformed cell types such as PBMCs, human coronary artery endothelial cells (HCAECs), or aortic smooth muscle cells (AoSMCs), even at A2A agonist 3 to 4 orders of magnitude higher than the 50% inhibitory concentration (IC50) for MM activity (Figures 3B-D). This striking selectivity was observed for both single agent A2A agonists as well as for A2A agonists in combination with trequinsin. Primary human umbilical vein endothelial cells (HUVECs) showed modest inhibition of proliferation in response to high concentrations of HE-NECA with an IC50 2 orders of magnitude greater than that in MM.1S. Notably, the combination of HE-NECA and trequinsin showed substantially greater selectivity in HUVEC cells than single agent HE-NECA, with an IC50 shifted to 3 orders of magnitude greater in MM.1S cells. These results demonstrate that A2A agonists and A2A/PDE inhibitor combinations are highly selective for B-cell malignancies and that the synergy created by the combined agents confers additional selectivity.
Summary of A2A agonist/PDE inhibitor combination selectivity and breadth of activity. (A) Synergy scores were determined for 61 cell lines cells treated with HE-NECA and trequinsin and/or CGS-21680 and trequinsin combinations (see Figure 1 legend for details of how synergy scores were derived). GA-10, Raji, Farage, Pfeiffer, Toledo, Kasumi-1, HL-60, AoSMC, HCAEC, HUVEC, and all solid tumor cell lines were treated with HE-NECA and trequinsin. Jvm-13, Mino, EJM, H929, KSM-12-PE, MM.1R, MM.1S, Molp-2, Molp-8, OPM-2, RPMI-8226, U266, Sup-B15, and PBMCs were treated with HE-NECA/trequinsin and with CGS-21680/trequinsin. The rest of the cell lines received the combination of CGS-21680 and trequinsin. Synergistic activity was observed for the majority of the multiple myeloma cell lines examined. Strong combination activity was also observed in a subset of DLBCL cell lines tested. Synergy was not observed with either solid tumor or normal cells. (B) The single-agent dose response for HE-NECA was determined after 72-hour incubation in MM.1S, PBMC, HUVEC, and AoSMC. (C) The activity of increasing concentrations of HE-NECA was determined at a fixed concentration of 3.4μM trequinsin using MM.1S, PBMC, HUVEC, and AoSMC cells. (D) The activity of increasing concentrations of CGS-21680 was determined as a single agent or in combination with 3.4μM trequinsin using MM.1S and PBMC cells.
Summary of A2A agonist/PDE inhibitor combination selectivity and breadth of activity. (A) Synergy scores were determined for 61 cell lines cells treated with HE-NECA and trequinsin and/or CGS-21680 and trequinsin combinations (see Figure 1 legend for details of how synergy scores were derived). GA-10, Raji, Farage, Pfeiffer, Toledo, Kasumi-1, HL-60, AoSMC, HCAEC, HUVEC, and all solid tumor cell lines were treated with HE-NECA and trequinsin. Jvm-13, Mino, EJM, H929, KSM-12-PE, MM.1R, MM.1S, Molp-2, Molp-8, OPM-2, RPMI-8226, U266, Sup-B15, and PBMCs were treated with HE-NECA/trequinsin and with CGS-21680/trequinsin. The rest of the cell lines received the combination of CGS-21680 and trequinsin. Synergistic activity was observed for the majority of the multiple myeloma cell lines examined. Strong combination activity was also observed in a subset of DLBCL cell lines tested. Synergy was not observed with either solid tumor or normal cells. (B) The single-agent dose response for HE-NECA was determined after 72-hour incubation in MM.1S, PBMC, HUVEC, and AoSMC. (C) The activity of increasing concentrations of HE-NECA was determined at a fixed concentration of 3.4μM trequinsin using MM.1S, PBMC, HUVEC, and AoSMC cells. (D) The activity of increasing concentrations of CGS-21680 was determined as a single agent or in combination with 3.4μM trequinsin using MM.1S and PBMC cells.
Analysis of single agent and combination activity using patient tumor cells
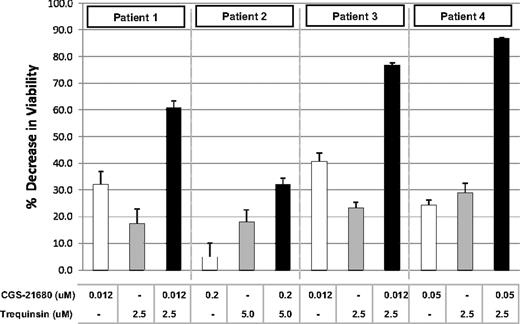
The selectivity and breadth of A2A agonist/PDE inhibitor synergy observed with MM cell lines prompted us to examine MM patient tumor cell single agent and combination drug sensitivity. CD138+ tumor cells isolated from MM patients were treated with single agents and combinations using a dose-ratio matrix format. Combination activity was observed with cells from all 4 patients (Figure 4 and supplemental Figure 4). Potent synergy was observed with patient samples 1, 3, and 4 at 0.012 to 0.05μM CGS-21680 and 2.5μM PDE inhibitor trequinsin. In contrast, combination activity was not seen when PBMCs were exposed to 5μM CGS-21680 and 3.4μM trequinsin (Figure 3D) or in non-B tumor cells or “normal cells” (HUVECs, AoSMCs, and HCAECs) exposed to 0.1μM CGS-21680 and 10μM trequinsin (Figure 3A). Weak combination effects were observed in tumor cells from patient 2 (Figure 4), which were not as sensitive to A2A agonist compared with cells from other patients. These results strongly support the results obtained with cell lines and the concept of a therapeutic window where B-cell malignancies are selectively eliminated by the combination.
A2A agonist and PDE inhibitor single-agent and combination activity in freshly isolated bone marrow plasma cells from patients with MM. Purified (CD138+) multiple myeloma cells from patients were treated with indicated concentrations of A2A agonist CGS-21680, PDE inhibitor trequinsin, or both agents. After 48 hours, cells were analyzed using the Cell Titer Glo assay and results plotted as a decrease in viability versus untreated control cells. The results for patients 1, 3, and 4 represent the mean and SD from 6 replicate measurements while with patient 2, N = 4.
A2A agonist and PDE inhibitor single-agent and combination activity in freshly isolated bone marrow plasma cells from patients with MM. Purified (CD138+) multiple myeloma cells from patients were treated with indicated concentrations of A2A agonist CGS-21680, PDE inhibitor trequinsin, or both agents. After 48 hours, cells were analyzed using the Cell Titer Glo assay and results plotted as a decrease in viability versus untreated control cells. The results for patients 1, 3, and 4 represent the mean and SD from 6 replicate measurements while with patient 2, N = 4.
A2A and PDE inhibitor combinations induce cAMP and cell death with compensatory up-regulation of PDE 4B
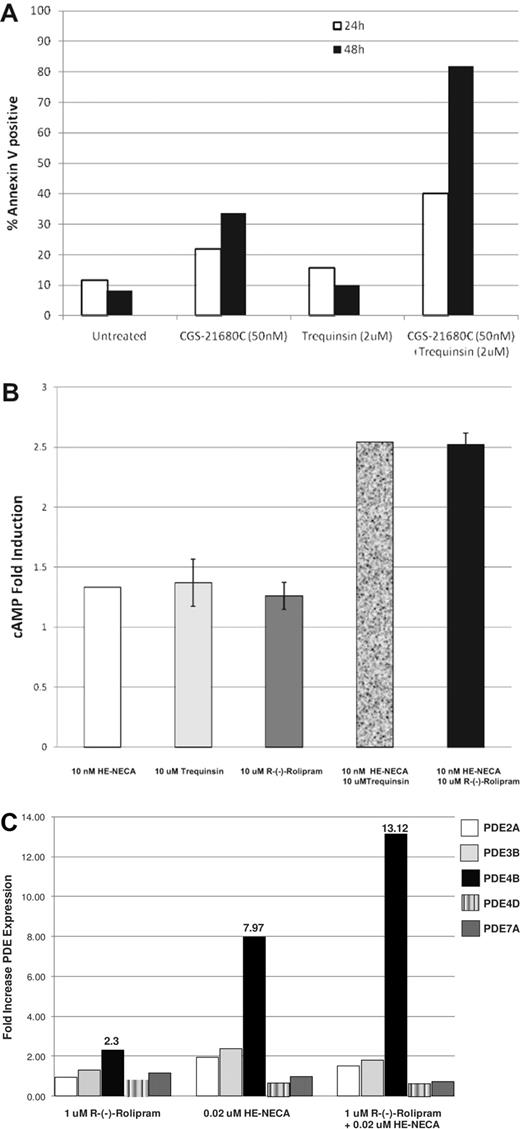
A2A agonists and select PDE inhibitors are highly synergistic in combination, potently inhibiting proliferation as determined using a surrogate assay (ATPlite) that measures both cytostatic and cytocidal activities. To better understand how these drugs affect cell growth, MM.1S cells were treated with the A2A agonist CGS-21680 and the PDE inhibitor trequinsin as single agents and in combination. At 24 and 48 hours postdrug treatment, cells undergoing apoptosis were identified using reagents that detect annexin V and quantified using FACS. As shown in Figure 5A, while each of the single agents can induce some cell death, apoptosis is rapid (within 24 hours) and potently induced by the A2A agonist and PDE inhibitor when in combination.
A2A agonists/PDE inhibitor combinations induce apoptosis, increase cellular cAMP, and elevate PDE subtype expression. (A) The combination of A2A agonist CGS-21680 and PDE inhibitor trequinsin triggers the rapid induction of apoptosis of multiple myeloma cells. MM.1S cells were incubated with 50nM CGS-21680, 2μM trequinsin, or the combination of both agents. At 24 and 48 hours, cells were harvested and analyzed by FACS for annexin V–positive cells. (B) MM.1S cells were incubated with 10nM HE-NECA, 10μM trequinsin, 10 μM R-(-)-rolipram, or the combination of 10nM HE-NECA and 10μM trequinsin or 10nM HE-NECA and 10μM R-(-)-rolipram for 1 hour at 37°C followed by preparation of cell lysates and measurement of cAMP levels. (C) MM.1R cells were incubated with 1μM R-(-)-rolipram, 20nM HE-NECA or the combination of 1μM R-(-)-rolipram and 20nM HE-NECA for 4 hours at 37°C and then the cells were lysed to isolate total RNA. The RNA was used for RT-PCR quantitation of PDE RNA levels using subtype specific primers as described in “siRNA transfections.”
A2A agonists/PDE inhibitor combinations induce apoptosis, increase cellular cAMP, and elevate PDE subtype expression. (A) The combination of A2A agonist CGS-21680 and PDE inhibitor trequinsin triggers the rapid induction of apoptosis of multiple myeloma cells. MM.1S cells were incubated with 50nM CGS-21680, 2μM trequinsin, or the combination of both agents. At 24 and 48 hours, cells were harvested and analyzed by FACS for annexin V–positive cells. (B) MM.1S cells were incubated with 10nM HE-NECA, 10μM trequinsin, 10 μM R-(-)-rolipram, or the combination of 10nM HE-NECA and 10μM trequinsin or 10nM HE-NECA and 10μM R-(-)-rolipram for 1 hour at 37°C followed by preparation of cell lysates and measurement of cAMP levels. (C) MM.1R cells were incubated with 1μM R-(-)-rolipram, 20nM HE-NECA or the combination of 1μM R-(-)-rolipram and 20nM HE-NECA for 4 hours at 37°C and then the cells were lysed to isolate total RNA. The RNA was used for RT-PCR quantitation of PDE RNA levels using subtype specific primers as described in “siRNA transfections.”
The adenosine A2A receptor is Gs-coupled, and receptor activation is known to increase cAMP levels.20 PDE2, PDE3, PDE4, and PDE7 are PDEs that hydrolyze cAMP.19,21 To determine whether cAMP levels are modulated by A2A/PDE inhibitor combinations, we measured cAMP levels after MM.1R cells were exposed to single agents and combinations for 1 hour. As seen in Figure 5B, while neither the A2A agonist HE-NECA or the PDE inhibitors trequinsin or R-(-)-rolipram had appreciable single-agent activity at the concentrations tested, cAMP induction was observed when drugs were used in combination. Similar results have been observed using the A2A agonist CGS-21680 (data not shown). To understand the consequences of increased cAMP levels in this system, we examined the levels of PDE2A, 3B, 4B, 4D, and 7A in response to R-(-)-rolipram, HE-NECA or the combination of these agents (Figure 5C). Although PDE inhibition by R-(-)-rolipram had little effect on PDE4B mRNA levels, treatment with HE-NECA alone or in combination with R-(-)-rolipram resulted in an 8- or 13-fold up-regulation of PDE4B mRNA levels, respectively. This effect was selective for PDE4B as no elevation in PDE2A, 3B, 4D, or 7A mRNA was observed. These results suggest that inhibition of the PDE4B isoform is particularly important for maximal antiproliferative activity in MM.
Discussion
Using a systematic combination screening approach, we have identified unexpected multitarget mechanisms and new drug combination leads selective for MM and other B-cell malignancies. We have discovered that adenosine receptor agonists and subtype-selective PDE inhibitors demonstrate synergistic antiproliferative activity when used in combination with GC. Furthermore, adenosine receptor agonists and select PDE inhibitors potently synergize with each other. Synergistic antiproliferative effects of A2A agonist/PDE inhibitor combinations are observed in the Dex-insensitive MM.1R cell line, suggesting that the mechanism of action of this combination is GC-independent. Combination synergy is highly selective for B-cell malignancies, including MM and DLBCL, and demonstrates a minimum 3 order of magnitude selectivity window in human primary cells compared with MM cell lines and patient tumor cells. Using a combination of chemical and molecular genetics, we have shown that the adenosine receptor subtype A2A and PDE isoforms PDE2A, PDE3B, PDE4B, PDE4D, and PDE7A are the primary targets conferring this activity. Treatment of MM cells with an A2A agonist/PDE inhibitor combination elevates cellular levels of cAMP, results in a compensatory elevation of PDE4B, and triggers the rapid induction of apoptosis. These effects require exposure of cells to both agents. Inhibition of PDE4 appears to be particularly important for combination activity.
Glucocorticoid-dependent and -independent synergies
GCs have been used for many years to treat MM but the mechanisms by which this class of drugs affect cell growth is poorly understood. GC receptor expression is necessary for activity as resistance correlates with an overall reduction in receptor mRNA levels.22 Previous work suggests that GC response element (GRE)–dependent transactivation and/or transrepression is important for the induction of apoptosis; however, the identity of the key target genes has not been elucidated.15,23 Several compounds have been identified that potentiate GC MM activity in vitro, including all-trans retinoic acid, rapamycin, and lenalidomide.
Using a screen for GC enhancers, we have discovered that both adenosine A2A receptor agonists and PDE inhibitors synergize with GCs to promote tumor cell death. We have also demonstrated that these agents synergize with each other in the absence of GC and can induce synergistic inhibition of proliferation and death in cells (ie, MM.1R) that lack the GC receptor,16 demonstrating complete independence from GC for this synergistic interaction (Figures 1, 3, and supplemental data). These results may have important implications for the translation of A2A/PDE inhibitor combinations to clinical disease. Because GCs are an important component of clinical regimens in MM and other B-cell malignancies, there is the potential for increased benefit by the addition of an A2A agonist, a PDE inhibitor or both to a GC-containing regimen. Importantly, we observe rigorous mathematical synergy in 3-way combinations containing A2A agonists, PDE inhibitors, and Dex at multiple concentrations and ratios (supplemental Figure 5A-B). Furthermore, we have observed combination activity when an A2A agonist (CGS-21680) is combined with Dex in an MM xenograft model (data not shown). Taken together, these data suggest that A2A agonist/PDE inhibitors, either as single agents or in combinations may be attractive as an adjunctive to clinical GC-containing regiments and may confer benefit in both GC-sensitive and -resistant populations.
Synergy and selectivity
A2A agonist/PDE inhibitor combinations potently and synergistically inhibit growth and induce the death of malignant B-cell lines such as MM, DLBCL, and primary tumor samples from MM patients. The synergies are highly selective and not observed in other cells (normal or transformed) over a wide concentration range (Figure 3). The selectivity observed suggests that A2A agonists/PDE inhibitor combinations can be deployed in the clinic without adverse indiscriminate killing of normal cells, resulting in less toxicity and better tolerability. The molecular determinants of selectivity have not yet been defined. However, using Western blot analysis, we have demonstrated that selectivity is not a consequence of restricted expression of the A2A receptor as the protein has been detected in cells that do not respond to A2A agonists (data not shown). The A2A receptor is a Gs-linked G coupled protein receptor (GCPR)20 and perhaps the receptor is uncoupled in unresponsive cells. Alternatively, selectivity may be conferred by differences in events that occur downstream of receptor signaling in sensitive and insensitive cells. In this model, resistant cells would be have distinct signaling networks and thus respond to the combination in different ways. A more thorough understanding of the determinants of sensitivity and resistance could ultimately lead to the selection of patients most likely to respond to A2A agonist/PDE inhibitor combinations. Importantly, the combination may enable greater selectivity than single agents alone as a result of synergistic interactions. This conclusion is supported by our analysis of 94 110 multidose combinations indicating that synergistic drug combinations are generally more specific to particular cellular contexts than single-agent activities.14
cAMP elevation and PDE inhibition in the induction of cell death in B-cell malignancies
The mechanisms by which A2A agonists and PDE2, 3, 4, and 7 inhibitors synergize to rapidly and potently induce multiple myeloma cell apoptosis are currently under investigation. Both classes of drugs can elevate intracellular levels of cAMP, which is cytotoxic to several tumor types of lymphocytic origin including ALL24 and chronic lymphocytic leukemia.25
The activities of the PDE inhibitors as single agents in MM are particularly noteworthy. The inhibitors studied here have limited single agent activity until high concentrations are used which results in a loss of target specificity and become nonselectively toxic (see supplemental Figure 2). The relative insensitivity of MM cells to PDE inhibitors suggest that MM cells may have low basal adenylate cyclase activity or that multiple PDEs keep cAMP levels low. Both scenarios are probable. We have shown here that PDE2A, PDE3B, PDE4A, PDE4B, PDE4D, and PDE7A (PDEs that hydrolyze cAMP) are expressed in MM.1S, and compounds that block cAMP hydrolysis when used as single agents do not appreciably elevate cAMP levels or inhibit proliferation (Figure 5B and data not shown). MM cells also appear to have a compensatory mechanism for maintaining low cAMP levels. Treatment of MM cells with the A2A agonist HE-NECA results in a 7-fold induction of PDE4B with only a modest 1.3-fold induction of cAMP and 50% inhibition of proliferation. In contrast, when agonists of the Gs-coupled adenosine A2A receptor are combined with inhibitors of PDEs that hydrolyze cAMP in MM cells, cAMP elevation is potentiated approximately 2.5-fold, PDE4B mRNA is induced 13-fold, and MM cell proliferation is inhibited more than 80%. As a result, MM synergy is demonstrated at PDE concentrations below those where any single-agent activity is observed. Taken together, these results suggest that neither A2A agonists nor PDE inhibitors alone are sufficient to induce therapeutically relevant cAMP-mediated cell death in MM and that only through the synergy of the combination can adequate levels of tumor cell death be achieved.
PDE2, 3, 4, and 7 are present in MM cells and all contribute to cAMP hydrolysis and synergy with A2A agonists. The best MM A2A agonist combination activity is observed with trequinsin, a PDE inhibitor with PDE3>PDE4>PDE2-selective activity (Table 1 and supplemental Figure 2). While inhibition of multiple PDEs gives maximal synergy, inhibition of PDE4 is a particularly important target, probably because PDE4B is up-regulated when cellular levels of cAMP increase (Figure 5C). Still, selective inhibitors for PDE2, 3, 4, and 7 are all effective in synergizing with A2A agonists (Table 1 and supplemental Figure 2). In contrast to MM, low concentrations of PDE inhibitors are active in inhibiting DLBCL cell line proliferation (supplemental Figure 6A-B roflumilast data). Basal adenylate cyclase activity may be higher in DLBCL and as a result, a PDE4 inhibitor such as roflumilast has significant single-agent activity at target-relevant concentrations. Our strong synergy results with A2A and PDE4 inhibitors (supplemental Figure 6) are consistent with gene expression and inhibitor studies which identify PDE4 as a drug target for DLBCL.26,27 We note that the PDE inhibitor profile required for synergistic inhibition of proliferation is different for MM versus DLBCL. In MM cell lines, inhibition of multiple PDEs, including PDE2, 3, 4, and 7 is important for maximal synergy while in DLBCL cell lines, selective PDE4-specific inhibitors (eg, roflumilast) are sufficient to demonstrate maximal synergy (supplemental Figure 6A-B and data not shown). The differential profiles of PDE inhibitors important for antiproliferative activity in different B-cell malignancies are only revealed through this differential synergy analysis. These results demonstrate the importance of systematically evaluating synergistic interactions in tumor cells. Furthermore, these results provide a concrete example of how synergistic network interactions vary with specific tumor subtypes, underscoring the selectivity that is conferred through synergistic network interactions.
Chemical matter selective for the target classes we describe are available with drug-like properties. An A2A agonist (regadenoson) was recently FDA-approved for cardiac stress imaging and several others are in development for various indications. The in vivo properties of numerous PDE inhibitors have been established in humans with several in phase 3 clinical trials or with FDA approval. The robust synergies we observe suggest the ability to use flexible dose and ratio combinations of A2A agonists and PDE inhibitors in conjunction with GCs for the treatment of MM. Both combinations that boost the maximal achievable effect and enable substantial dose sparing are possible. Such flexibility in clinical deployment has the potential to minimize adverse effects and increase the possibility of achieving clinical efficacy when treating B-cell malignancies such as MM and DLBCL. Taken together, this body of work supports the further investigation of A2A agonists and PDE inhibitors in the treatment of B-cell malignancies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank our colleagues at CombinatoRx for their comments throughout the course of this work, Peter Grossman and Jeb Ledell for help with informatics, and Margaret Shipp, Constantine S. Mitsiadies, Stephen Rosen, and Robert Orlowski for cell lines. We also thank Yanjun Wang for guidance when performing the cAMP measurements.
Authorship
Contribution: R.J.R. designed and performed the experiments, interpreted data, and wrote the manuscript; L.T.P., T.P.G., W.F.T., D.W.M, J.D., and J.P.L. performed the experiments; A.A.B. and P.G.R. discussed the results and provided helpful insights; and M.S.L. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: R.J.R., L.T.P., T.P.G., W.F.T., A.A.B., and M.S.L. performed this work while employees at CombinatoRx. P.G.R. is on the advisory committee for Millennium Pharmaceuticals and Celgene and receives funding from Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Richard Rickles, CombinatoRx Inc, 245 1st St, Cambridge, MA 02142; e-mail: rrickles@combinatorx.com.