Abstract
Recombinant adenovirus-engineered dendritic cells (Ad.DCs) are potent immunologic adjuvants of antiviral and anticancer vaccines. The effectiveness of Ad.DC-based vaccines may depend on the ability of Ad.DCs to crosstalk with natural killer (NK) cells and to activate, polarize, and bridge innate and adaptive immunity. We investigated, for the first time, whether and how human Ad.DCs activate NK cells, and compared the Ad.DC function with that of immature DCs and matured DCs (mDCs). We found that adenovirus transduction and lipopolysaccharide/interferon-γ-induced maturation increased expression of transmembrane tumor necrosis factor (TNF) and trans-presented (trans) interleukin-15 (IL-15) on DCs, leading to enhanced NK cell activation without enhancing DC susceptibility to NK cell-mediated killing. This crosstalk enhanced NK cell CD69 expression, interferon-γ secretion, proliferation, and antitumor activities, with Ad.DCs being significantly more effective than immature DCs, but less effective than mDCs. The Ad.DC and mDC crosstalk with NK cells was largely prevented by physical separation of DCs and NK cells, and neutralization of total TNF and IL-15, but not by selective sequestration of soluble TNF. These findings demonstrate that both Ad.DCs and mDCs can efficiently promote innate immune functions by activation of NK cells through the cooperative activities of tmTNF and trans-IL-15 mediated by cell-to-cell contact.
Introduction
Dendritic cells (DCs) and natural killer (NK) cells are essential components of the innate immune system that function at the interface of innate and adaptive immunity.1-3 DCs are potent antigen-presenting cells, best known for their unique ability to efficiently process and present antigenic epitopes and activate antigen-specific naive T cells.1 NK cells have the unique ability to rapidly recognize and directly eliminate microbial pathogens and transformed cells.4 Both NK cells and DCs produce proinflammatory and immunoregulatory cytokines and mediate inflammation as well as polarization and regulation of both innate and adaptive immune responses.2-4
DCs and NK cells interact, reciprocally regulate each other, and induce enhanced polarization of type 1 cytokine secretion.2,3,5,6 NK cells induce in DCs increases in expression of maturation markers and secretion of interleukin-12p70 (IL-12p70). Reciprocally, DCs induce in NK cells expression of activation markers, enhance interferon-γ (IFN-γ) secretion, perforin-mediated tumoricidal activity, and proliferation, and stimulate their abilities to control in vivo viral infections and tumor growth.7 The extent of DC/NK cell cross-stimulation is dependent on the level of DC maturation and the degree of NK-cell activation.2,3,5,8,9 This early cellular crosstalk is thought to be an essential regulatory immune mechanism that bridges innate and adaptive immune functions, and defines the quality and magnitude of antiviral and antitumor immune responses. The precise molecular pathways responsible for DC/NK cell crosstalk and their role in immune reactions are matters of controversy. Several lines of evidence suggest that DC/NK cell crosstalk is mediated by cell-to-cell contact, via plasma membrane-bound molecules, such as transmembrane tumor necrosis factor (tmTNF) and trans-IL-15.2,3,5,6,10-12 In contrast, several secreted cytokines produced by DCs, including IL-12, IL-2, IL-15, IL-18, and IFN-α, have also been implicated in DC/NK cell crosstalk.13-17 The contributions of these mediators in DC/NK cell interactions involving DCs matured with different factors and NK cells remain unknown.
In addition to reciprocal stimulation, a subset of activated NK cells are able to kill immature DCs (iDCs) by triggering NK activating receptor NKp30 and DC TNF-related apoptosis-inducing ligand death receptors.5,6,18,19 The elimination of DCs by NK cells is thought to be an important control switch that selectively removes DCs unsuitable for efficient antigen presentation and initiation of adaptive immune responses.
Ad.DCs are potent immunologic adjuvants that show promise as a vaccine in prevention and therapy of viral infections and cancers.20,21 They acquire an intermediate level of maturation based on the expression of cell-surface maturation markers, cytokine secretion, and antigen-presenting machinery, and increased ability to stimulate antigen-specific CD4+ and CD8+ T-cell responses.22-24 The effectiveness of Ad.DC-based vaccines may be dependent on the ability of Ad.DCs to crosstalk with NK cells and bridge innate and adaptive immunity.25 However, it has not yet been investigated whether and how Ad.DCs interact with NK cells.
We have previously observed that peripheral blood NK cells of melanoma patients immunized with autologous AdV.DCs encoding MART-1 melanoma-associated antigen express enhanced levels of activation markers.21 These findings indicate that human Ad.DCs may be able to activate NK cells. In the present study, we directly tested whether human Ad.DCs can activate NK cells and compared the potential and molecular mechanisms of Ad.DCs to stimulate NK cells with that of iDCs and mature DCs (mDCs). We found that Ad.DCs and mDCs effectively activated resting NK cells and induced increases in NK-cell activation marker expression, type 1 cytokine secretion, proliferation, and killing of tumor cell targets in vitro. In addition, Ad.DCs, but not iDCs, induced significant NK cell antitumor activity in vivo. Ad.DC- and mDC-mediated activation of NK cells required cell-to-cell contact and was mediated by cooperative activities of tmTNF and trans-IL-15. This is the first study to report that Ad.DCs effectively stimulate innate immune mechanisms via crosstalk with NK cells and juxtacrine signaling requiring cell-to-cell contact. The study also newly defines the importance of cooperative engagements of tmTNF and trans-IL-15 in both Ad.DC/NK cell and mDC/NK cell crosstalk, suggesting that this novel pathway might be a common molecular mechanism of DC-mediated activation of NK cells.
Methods
Antibodies and reagents
DCs and NK cell phenotypes were examined using fluorochrome-conjugated antibodies against the following cell-surface markers: CD80, CD83, CCR7, and CCR5 (BD Biosciences PharMingen), MICA/B (eBioscience), HLA-A, -B, and -C (BioLegend), HLA-DR and CD86 (Beckman Coulter), NKG2D and NKp30 (eBioscience), CD8, CD16, and CD3 (Beckman Coulter), and CD56 and CD69 (BD Biosciences PharMingen). Mouse anti–human (mαh) antibodies to extracellular domain of TNF (clone 6401) were used for fluorescence-activated cell sorter (FACS) analyses, and extracellular domains of TNFR1 (clone 16803) and TNFR2 (clone 22210) were used for FACS analyses and blocking experiments (R&D Systems). For confocal microscopy analysis, rat anti–human TNF antibody (clone MP9-20A4; AbD Serotec) was used. Nonselective neutralization of both tmTNF and solTNF was performed with the chimeric anti-TNF monoclonal antibody infliximab (Remicade; Schering-Plough) and the TNFR2-Fc construct etanercept (Enbrel; Amgen). To selectively inhibit solTNF, a dominant negative TNF that forms nonfunctional heterotrimers with nascent solTNF (XENP1595) (Xencor) was used.26-28 mαhIL-15 neutralizing antibody (R&D Systems; clone 34559), which does not block IL-15/IL-15Rα trans-presentation, was used for cell-surface FACS and confocal microscopy analyses and blocking experiments. R&D Systems mαhIL-2/15Rβ (clone 24212) and mαhIL-2/15Rγ (clone 31134) antibodies, and BioLegend mαhIL-15Rα antibody (clone JM7A4) were used for FACS analysis.
Cell lines
NK and LAK activities were tested against K562 erythroleukemia and DAUDI lymphoma cell lines (ATCC), respectively. The cell lines were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine (Invitrogen), at 37°C, in a humidified/5% CO2 atmosphere.
Recombinant AdV vector
AdV vector encoding Escherichia coli β-galactosidase gene (Ad.LacZ) was obtained from the University of Pittsburgh Vector Core Facility. Ad.LacZ is E1/E3-deleted and replication-defective. Multiplicity of infection (MOI) is calculated based on plaque-forming units (pfu) per milliliter.
Mice
Eight-week-old T-, B-, and NK-cell deficient NOD/SCID/IL2Rγ knockout (NS.IL2Rγ−/−) female mice were obtained from The Jackson Laboratory. Mice were housed at the University of Pittsburgh Cancer Institute's Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facility. Animal studies were performed in accordance with a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Isolation of PBMCs
Peripheral blood was obtained from 80 healthy donors with their written consent in accordance with the Declaration of Helsinki, under an Institutional Review Board–approved protocol (UPCI 04-001). Peripheral blood mononuclear cells (PBMCs) were separated from blood using Ficoll-Hypaque gradient centrifugation (Cellgro; Mediatech).
DC preparation
iDCs were generated from CD14+ human monocytes, isolated from PBMCs using magnetic cell sorting (Miltenyi Biotec), by 5 day culturing in 800 IU/mL granulocyte-macrophage colony-stimulating factor (Sargramostim; Amgen) and 500 IU/mL IL-4 (Schering Plough). iDCs were not treated, transduced with Ad.LacZ vector at the indicated MOI (Ad.DCs), or matured with IFN-γ (1000 IU/mL; PeproTech) and lipopolysaccharide (LPS, 250 ng/mL; Sigma-Aldrich) (mDCs). DCs were cultured an additional 24 hours before use.
NK cell isolation
NK cells were purified from PBMCs by negative magnetic cell sorting selection using a human NK cell isolation kit (Miltenyi Biotec), according to the manufacturer's protocol.
DC/NK cell coculture
Autologous DC and NK cells were resuspended in AIM-V medium supplemented with 5% human AB serum (Invitrogen) and cultured alone or together at 1:1 DC:NK cell ratio, for 24 hours, unless otherwise specified.
NK-cell proliferation assays
Purified NK cells were stained using Vybrant CFDA SE Cell Tracker Kit (CFSE; Invitrogen) per the manufacturer's instructions. Labeled NK cells were cultured alone (negative control), with IL-2 (6000 IU/mL; positive control) or with autologous DC at a 2:1 ratio. On day 6 of culture, cells were analyzed for CFSE dilution using the CyAn analyzer (Dako North America) and Summit Version 4.3 software.
Transwell experiments
The experiments were performed in 24-well plates with 6.5-mm-diameter, 0.4-μm-pore size polyester membrane inserts (Corning). DCs and NK cells were seeded in the plate individually or together, with DCs in the insert and NK cells in the well. After 24 hours of incubation, cell phenotype was analyzed by FACS, and supernatants were tested for secreted cytokines by multiplex assays.
ELISPOT
The frequencies of cytokine- or granzyme B–secreting NK cells were measured using human IFN-γ or granzyme B ELISPOT assays based on antibody pairs from Mabtech and MultiScreen HTS plates from Millipore, as previously described.29
Blockade of DC/NK cell interactions
These tests were performed using human IFN-γ ELISPOT assays. Antagonist antibodies or inhibiting agents were added to DC/NK cell or DC supernatant/NK-cell cocultures at a final concentration of 20 μg/mL. DC/NK cells and/or DC supernatant/NK cells were preincubated with blocking reagents for 30 minutes at room temperature and then cultured at 37°C for 24 hours in ELISPOT plates.
Cell staining with fluorochrome-conjugated antibodies and FACS analysis
One-step staining of cell-surface antigens was performed using fluorochrome-conjugated primary antibodies as previously described.22 Two-step staining of tmTNF and trans-IL-15 was performed as follows. Cells were washed and resuspended in FACS buffer. Cells were blocked at room temperature with 10% human AB serum for 15 minutes and labeled with primary antibodies (2 μg/mL) at 4°C for 1 hour. After washing, cells were stained with phycoerythrin (PE)–conjugated polyclonal goat anti–mouse immunoglobulin F(ab′)2 (Dako North America; 1:20 working dilution) at 4°C for 1 hour. Cells were washed and fixed with 2% weight/volume paraformaldehyde (Sigma-Aldrich) solution in phosphate-buffered saline (PBS). Three-step staining of TNFR was performed using unconjugated anti-TNFR antibodies, biotinylated goat anti–mouse antibody (Jackson ImmunoResearch) and PE-conjugated streptavidin (Jackson ImmunoResearch), as previously described.10,22 Intracellular staining of IL-15/15Rα was performed with PE-conjugated antibodies per BioLegend's protocol. TNFR expression analyses were performed using the CyAn flow cytometer (Beckman Coulter). tmTNF and trans-IL-15 expression analyses were performed by imaging flow cytometry using the ImageStream 100 IS100 camera, 36× magnification (Amnis). For these analyses, DC nuclei were counterstained with DRAQ5 (Enzo Life Sciences), and the data were analyzed with IDEAS software Version 3.0 (Amnis).
Confocal microscopy
Expression of tmTNF and trans-IL-15 was investigated on both nonadherent and adhered DCs. For nonadherent DCs, 2-step costaining of TNF and IL-15 was performed as described in “Cell staining with fluorochrome-conjugated antibodies and FACS analysis” using mαhIL-15 (R&D Systems) and rat anti–human TNF (Serotec) antibodies. Cy3-conjugated goat anti–mouse (Jackson ImmunoResearch) and Alexa Fluor 488–conjugated goat anti–rat (Invitrogen) were used as secondary antibodies. After the staining, DCs were resuspended in 25 μL of PBS, applied on poly-L-lysine-treated microscopic slides, and analyzed. For adherent DCs, DCs were seeded on ethanol-cleaned glass coverslips. After 24 hours of incubation at 37°C, nonadherent DCs were removed by washing and adherent-DCs were fixed in 2% paraformaldehyde. For this analysis, DCs were not permeabilized. They were stained with the aforementioned antibodies, as well as with Alexa Fluor 647 phalloidin (Invitrogen), to label F-actin, and with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich), to label DC nuclei; 0.4-μm-thick optical sections were generated using the Olympus FluoView 1000 scanning confocal microscope with a 60×/1.4 NA oil immersion lens (Olympus America). Final composites were constructed with IMARIS (Bitplane) software Version 7.0.
CTO-based flow cytometry cytotoxicity assay
The assay was performed as previously described.30 Briefly, DCs were labeled with cell tracker orange (CTO) and used as target cells (T). CTO+ DCs were coincubated with effector NK cells (E) at various ratios at 37°C for 3 hours. Cells were stained with 7-amino-actinomycin D (7-AAD) and cytotoxicity was quantified by flow cytometry. The percentage of cytotoxic activity was calculated using the following equation: % specific cell death =(% 7-AAD+CTO+DC + NK cells − % spontaneous 7-AAD+CTO+DC)/(100 − % spontaneous 7-AAD+CTO+DC) × 100%. Lytic units (LU)20/107 effector cells were determined using the formula 107/(TX20), where T is the number of target cells and X20 is the estimated effector/target ratio at which 20% of target cells are killed.31
Measurement of secreted and cell-associated cytokines
Cell-free supernatants of DC cultures and debris-free DC lysates were assessed for solTNF and tmTNF or secreted IL-15 and trans-IL-15, respectively, using the human TNF ELISA kit (BD Biosciences)10 and IL-15 Luminex assay (Biosource). Cell-free supernatants of DC/NK cell cocultures were assessed for IFN-γ using IFN-γ Luminex assay (Biosource). In some experiments, 6-hour brefeldin A (Sigma-Aldrich) blockade of cytokine secretion (10 μg/mL) was applied to demonstrate active secretion of cytokines by DCs.
In vivo measurement of antitumor effect of human Ad.DC/NK cells using a mouse-human tumor xenograft model
NS.IL2Rγ−/− mice were injected subcutaneously into the shaved right flank with 4 × 106 human K562 leukemia cells resuspended in 0.1 mL of PBS. After 24 hours, when small tumors were established and visible, mice were injected with 0.1 mL of PBS, or PBS suspensions of healthy human NK cells (1 × 106), Ad.DCs (0.4 × 106), mixture of iDCs (0.4 × 106), and NK cells (1 × 106) in tumors; Ad.DCs (0.4 × 106) subcutaneously in opposite flank and NK cells (1 × 106) in tumors, or mixture of Ad.DC (0.4 × 106) and NK cells (1 × 106) in tumors. Tumor size was measured with a caliper every second day in 3 orthogonal axes (a, b, and c), and the tumor volume was calculated based on the formula V = abc/2. Measurements were performed for 13 days, after which time mice were killed because some tumors became larger than 2000 mm3.
Statistical analysis
SEM was used to estimate the variations. One-sided paired t test analyses were used to estimate statistical significance of differences of cytokine secretion and ELISPOT data. Before analyses, data were log-transformed. For the mouse study, a 2-sided, 2-sample equal variance t test analysis was implemented. No correction for multiple comparisons was applied. P values less than or equal to .05 were considered to be statistically significant.
Results
Ad.DCs, like mDCs, are not susceptible to NK cell killing
Viral infections can directly damage cells or increase their susceptibility to killing by NK cells. For effective crosstalk with NK cells, AdV transduction should not damage or increase susceptibility of DC to NK cell-mediated killing. We have previously shown that E1-E3-deleted, replication-defective AdV is not cytopathic for human DCs in doses up to 1000 MOI.20,22,24 Using 500 to 1000 MOI of AdV, we consistently transduced DCs with 95% efficiency and without cytopathic effect. Here we show that AdV transduction resulted in Ad.DCs, which, similar to IFN-γ/LPS-mDC, were resistant to killing by resting NK cells. In accordance with previous findings, iDCs were killed at higher levels by NK cells than mDCs (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Therefore, AdV transduction of DCs, like cytokine-induced DC maturation, did not enhance DC susceptibility to NK cell killing but rather increased DC resistance to NK cell lysis. This effect may be attributed to AdV transduction-mediated DC maturation as shown by increased Ad.DC expression of the maturation markers CD80, CD86, CCR7, and CD83 (supplemental Figure 2A-D), confirming our previous observation.22,32
Ad.DCs and mDCs activate NK cells
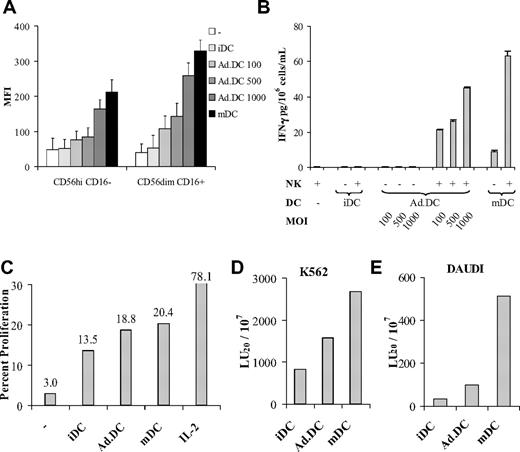
Previous studies have shown that mDCs induce high, whereas iDCs induce low, NK-cell activation.10 We examined the ability of Ad.DCs to activate resting NK cells compared with that of iDCs and IFN-γ/LPS-mDCs. In DC/NK cell cocultures, Ad.DCs induced, in an AdV dose-dependent manner, enhanced NK cell CD69 cell-surface expression (Figure 1A) and IFN-γ secretion (Figure 1B). In addition, Ad.DCs induced increases in NK cell proliferation (Figure 1C) and the ability to kill K562 (Figure 1D) and DAUDI (Figure 1E) tumor cell targets. The parameters of Ad.DC-induced NK cell activation were significantly greater than that of iDCs but lower than or similar to that of mDCs (Figure 1). Intriguingly, although NKG2D expression was not induced on NK cells, NKp30 was up-regulated to a similar extent by all 3 DC types, whereas granzyme B was up-regulated in NK cells stimulated with Ad.DCs and mDCs, but not by iDCs (supplemental Figure 3). These results demonstrate that Ad.DCs can crosstalk with and activate resting NK cells, mediating these functions more efficiently than iDCs, but less efficiently than mDCs. Reciprocally, NK cells enhanced Ad.DC expression of CD80 and CD86 (supplemental Figure 2A-B).22 The findings indicate that AdV and NK cells may additively induce DC maturation; thus, NK cell–matured Ad.DCs may become even more potent activators of NK and T cells.
Ad.DCs induce NK cell activation. NK cells and DCs were cultured alone or cocultured together at 1:1 ratio for 24 hours and subsequently examined. (A-B) iDCs, iDCs transduced with 3 different viral loads (100, 500, and 1000 MOI: Ad.DCs 100, Ad.DCs 500, Ad.DCs 1000, respectively) and LPS/IFN-γ-mDCs were assessed. (C-E) iDCs, mDCs, and Ad.DCs transduced with 500 MOI of AdV were examined. (A) CD69 expression on CD56dimCD16+ and CD56highD16− NKhigh cell subsets was measured by multicolor FACS analysis, and (B) secreted IFN-γ was tested in cell culture supernatants by multiplex cytokine assays. (C) NK cells from DC/NK cell cocultures were tested for proliferation by CFSE dilution assay using FACS analysis. (D-E) NK cells from DC/NK cell cocultures were tested for the ability to kill K562 and DAUDI tumor cell targets by CTO-based cytotoxicity assay, respectively. (A) Data are mean fluorescence intensities (MFI) for CD69. Bars represent the coefficient of variance for 5000 events acquired. (B) Data are mean ± SEM IFN-γ (pg/mL) of duplicate tests. (D-E) Data are LU20/107 NK cells based on the percentage of CTO+7-AAD+ cells. (A-B) Data are representative of 10 experiments. (C-E) Data are representative of 3 experiments.
Ad.DCs induce NK cell activation. NK cells and DCs were cultured alone or cocultured together at 1:1 ratio for 24 hours and subsequently examined. (A-B) iDCs, iDCs transduced with 3 different viral loads (100, 500, and 1000 MOI: Ad.DCs 100, Ad.DCs 500, Ad.DCs 1000, respectively) and LPS/IFN-γ-mDCs were assessed. (C-E) iDCs, mDCs, and Ad.DCs transduced with 500 MOI of AdV were examined. (A) CD69 expression on CD56dimCD16+ and CD56highD16− NKhigh cell subsets was measured by multicolor FACS analysis, and (B) secreted IFN-γ was tested in cell culture supernatants by multiplex cytokine assays. (C) NK cells from DC/NK cell cocultures were tested for proliferation by CFSE dilution assay using FACS analysis. (D-E) NK cells from DC/NK cell cocultures were tested for the ability to kill K562 and DAUDI tumor cell targets by CTO-based cytotoxicity assay, respectively. (A) Data are mean fluorescence intensities (MFI) for CD69. Bars represent the coefficient of variance for 5000 events acquired. (B) Data are mean ± SEM IFN-γ (pg/mL) of duplicate tests. (D-E) Data are LU20/107 NK cells based on the percentage of CTO+7-AAD+ cells. (A-B) Data are representative of 10 experiments. (C-E) Data are representative of 3 experiments.
Ad.DCs and mDCs mediate NK cell activation via plasma membrane-bound molecules
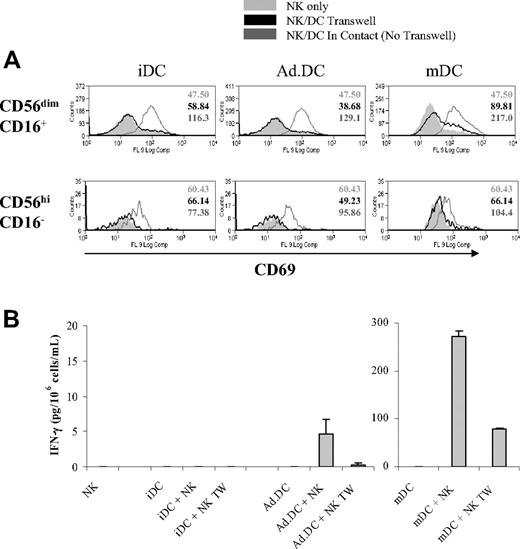
Although a large body of published evidence suggests that physical contact between DC and NK cells, presumably via membrane-bound mediators, is required for efficient DC/NK cell crosstalk, several secreted cytokines have also been implicated in the interaction, leaving unanswered the question whether secretory or nonsecretory mechanisms mediate DC/NK cell crosstalk, and whether the same mechanisms operate in interactions between NK cells and DC of different types or stages of activation/maturation.11,13,33-36 We investigated whether Ad.DCs and mDCs can activate NK cells when in cell-to-cell contact, via plasma membrane-bound mediators, and when physically separated, via soluble factors. In these experiments, DC and NK cells were either coincubated in the same wells, allowing for their cell-to-cell contact and involvement of transmembrane mediators, or were physically separated by a transwell membrane, allowing the involvement of soluble mediators. We found that iDCs, Ad.DCs, and mDCs induced in NK cells low, intermediate, and high expression levels of CD69, respectively, when DC and NK cells were cocultured in the same well (Figure 2A). The same culture conditions enabled Ad.DCs, and particularly mDCs, to induce IFN-γ secretion by NK cells (Figure 2B). Remarkably, both NK cell responses were completely abrogated when Ad.DCs and/or iDCs were separated from NK cells by transwell membrane (Figure 2), and partially (though significantly) when mDCs and NK cells were physically separated by the transwell membrane (Figure 2). These data indicate that Ad.DCs and iDCs mediate NK-cell activation exclusively, and mDCs principally, via plasma membrane-bound molecules.
Ad.DCs activate NK cells by cell-to-cell contact. DCs and NK cells were cultured in the same well or physically separated by a transwell membrane (TW). Ad.DCs were transduced using an MOI of 500. After 24 hours, (A) cell-surface expression of CD69 activation marker was measured by multicolor FACS analysis on CD56dimCD16+ and CD56highCD16− NK cell subsets, and (B) cell culture supernatants were tested for secreted IFN-γ by multiplex cytokine assays. (A) Values are MFI for CD69 expressed on NK cells. (B) Values are mean ± SEM IFN-γ (pg/mL) of duplicate tests. The results shown in all panels are representative of 4 experiments.
Ad.DCs activate NK cells by cell-to-cell contact. DCs and NK cells were cultured in the same well or physically separated by a transwell membrane (TW). Ad.DCs were transduced using an MOI of 500. After 24 hours, (A) cell-surface expression of CD69 activation marker was measured by multicolor FACS analysis on CD56dimCD16+ and CD56highCD16− NK cell subsets, and (B) cell culture supernatants were tested for secreted IFN-γ by multiplex cytokine assays. (A) Values are MFI for CD69 expressed on NK cells. (B) Values are mean ± SEM IFN-γ (pg/mL) of duplicate tests. The results shown in all panels are representative of 4 experiments.
Ad.DCs interact with NK cells and stimulate their antitumor activity in vivo
We next determined whether and how Ad.DCs could activate NK cells and promote their antitumor activity in vivo, in agreement with in vitro findings. Human tumor xenografts were consistently produced in NS.IL2Rγ−/− mice by subcutaneous injections of 4 × 106 K562 leukemia cells. Day 1 after tumor injections, small subcutaneous tumors were established, and Ad.DCs and NK cells were either injected jointly (Ad.DC/NK) into tumors, to enable their cell-to-cell contact in situ, or NK cells were injected into tumors and Ad.DCs into contralateral flanks of mice, to prevent their physical contact (Ad.DCcl-NKt). Control mice had tumors injected with vehicle (PBS), NK cells (NK), Ad.DCs, or mixed iDC and NK (iDC/NK). On days 6, 8, 10, and 13 after treatments, we found significantly and notably reduced tumor growth in AdDC/NK-treated mice relative to all control groups combined (44%, 64%, 58%, and 43%; P = .016, .030, .023, and .201, respectively), notable decreases relative to vehicle- and NK-treated mice (22%-74%), as well as significant (days 6-10) and notable (day 13) decreases relative to Ad.DC-, iDC/NK- or Ad.DCcl-NKt-treated mice (32%-67%, P = .044 to > .002; 21%-58%, respectively; Figure 3; supplemental Figures 4-6). In contrast, no slowed tumor growth was observed after NK, Ad.DC, iDC/NK, or Ad.DCcl-NKt treatments, indicating that resting NK cells, Ad.DCs, iDCs, or iDC-stimulated NK cells did not mediate antitumor activity against established solid tumors. In addition, no statistically significant differences in tumor growth between the control mice and Ad.DCcl-NKt-treated mice were observed, suggesting that physically separated Ad.DCs and NK cells did not interact in vivo. These findings show, for the first time, that crosstalk between Ad.DCs and NK cells can occur in vivo and cause significant NK-cell activation and strong antitumor activity capable of destroying malignant cells in solid tissues. In contrast, although resting NK cells readily kill K562 leukemia cells in single-cell suspensions, they do not exhibit antitumor activity against K562 solid tumors. The findings further indicate that Ad.DC-mediated activation of NK cells may have significant anticancer therapeutic potential. The data also indicate that cell-to-cell contact and, presumably, transmembrane molecules, mediate Ad.DC/NK cell crosstalk in vivo.
Ad.DCs stimulate in vivo significant NK-cell antitumor activity via cell-to-cell contact. NS.IL2Rγ−/− mice (4 or 5 mice per group) were injected subcutaneously with 4 × 106 K562 leukemia cells. On day 1 after tumor cell injection, mice were randomized into 6 groups. They were injected with: (1) PBS into tumor (Vehicle), (2) NK cells alone into tumor (NK), (3) Ad.DCs alone into tumor, (4) mixture of iDCs and NK (iDC/NK) into tumor, (5) Ad.DCs into contralateral, tumor-free flank, and NK cells into tumor (Ad.DCcl-NKt), and (6) mixture of Ad.DCs and NK (Ad.DC/NK) into tumor. Tumor growth was followed by caliper measurements every second day. Data are means of tumor volumes (mm3). Individual mouse tumor volumes are presented in supplemental Figure 4. Percentage inhibition of tumor growth of Ad.DC/NK-treated versus control mice is presented in supplemental Figure 5. *Statistical significance of differences of tumor volumes for Ad.DC/NK-treated versus control mice (also detailed in supplemental Figure 6).
Ad.DCs stimulate in vivo significant NK-cell antitumor activity via cell-to-cell contact. NS.IL2Rγ−/− mice (4 or 5 mice per group) were injected subcutaneously with 4 × 106 K562 leukemia cells. On day 1 after tumor cell injection, mice were randomized into 6 groups. They were injected with: (1) PBS into tumor (Vehicle), (2) NK cells alone into tumor (NK), (3) Ad.DCs alone into tumor, (4) mixture of iDCs and NK (iDC/NK) into tumor, (5) Ad.DCs into contralateral, tumor-free flank, and NK cells into tumor (Ad.DCcl-NKt), and (6) mixture of Ad.DCs and NK (Ad.DC/NK) into tumor. Tumor growth was followed by caliper measurements every second day. Data are means of tumor volumes (mm3). Individual mouse tumor volumes are presented in supplemental Figure 4. Percentage inhibition of tumor growth of Ad.DC/NK-treated versus control mice is presented in supplemental Figure 5. *Statistical significance of differences of tumor volumes for Ad.DC/NK-treated versus control mice (also detailed in supplemental Figure 6).
Ad.DCs and mDCs express enhanced levels of tmTNF and trans-IL-15 on the cell surface
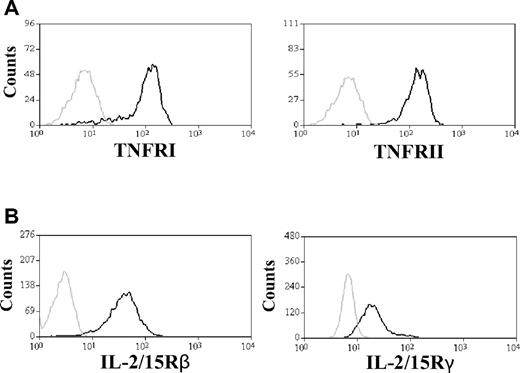
tmTNF and trans-IL-15 are expressed on DC plasma membrane and mediate NK cell activation in mice.10,11 We investigated whether these plasma membrane-bound molecules are also expressed on human Ad.DCs and mDCs and are mediators of cell contact-dependent interaction with, and activation of, NK cells in humans. Using imaging FACS and confocal microscopy analyses, we found that, 24 hours after AdV transduction or IFN-γ/LPS-induced maturation, Ad.DCs expressed increased levels of tmTNF and trans-IL-15 relative to iDCs, and mDCs expressed higher levels of both molecules than Ad.DCs (Figure 4A-B; supplemental Figure 7). Both analyses showed a particular distribution pattern of these molecules on DC plasma membrane. A more detailed analysis by confocal microscopy of nonadherent DCs revealed a punctate, microdomain-like appearance and balanced distribution of individual (red and green spots) and joint (yellow spots) TNF and IL-15 foci on the DC surface (Figure 4B). Intriguingly, adherent DCs showed mainly joint TNF and IL-15 expression in aggregated microdomains localized in small restricted areas on proximal parts of dendrites, adjacent to sites of cell-to-cell interaction (supplemental Figure 7). Both nonadherent and adherent Ad.DCs expressed intermediate levels of TNF and IL-15 relative to that of iDCs and mDCs. The increased tmTNF and trans-IL-15 expression levels on Ad.DCs were additionally confirmed by measurements of TNF (Figure 4C) and IL-15 (Figure 4D) concentrations in DC lysates. The increase in trans-IL-15 expression coincided with up-regulation of IL-15Rα on DCs (supplemental Figure 8). In addition, significant amounts of soluble TNF (solTNF) and IL-15 (solIL-15) were released by both Ad.DCs and mDCs during the initial 24-hour incubation, and the secretion was mostly exhausted after 24 hours of DC treatment (Figure 4E-F; supplemental Figure 9). Release of both cytokines was completely blocked by brefeldin A (supplemental Figure 9), demonstrating that it resulted from cell secretory activity. Importantly, the Ad.DCs and mDCs were used in coculture experiments 24 hours after treatments, when Ad.DCs stopped and mDCs significantly decreased secretion of solTNF and solIL-15 (Figure 4E-F; supplemental Figure 9). Reciprocally, we found high expression levels of both TNF receptors (Figure 5A) and IL-2/15Rβ and IL-2/15Rγ on resting NK cells (Figure 5B). Therefore, Ad.DCs and mDCs express plasma membrane-bound tmTNF and trans-IL-15, whereas resting NK cells express the corresponding receptors. These corresponding plasma membrane-bound molecules may be engaged by cell-to-cell contact, triggering NK cell activation by Ad.DCs and mDCs.
Ad.DCs and mDCs express tmTNF and trans-IL-15. (A) tmTNF and trans-IL-15 expression by iDCs, Ad.DCs (500 MOI), and mDCs was analyzed by imaging flow cytometry. Images show examples of individual cells, and the MFI values listed are averaged for the population of 10 000 cells. (B) Topography of tmTNF and trans-IL-15 expression on iDCs, Ad.DCs, and mDCs was examined with confocal microscopy. (Top panels) Optical cross-sections. (Bottom panels) Reconstructed (3-dimensional) images of 10 0.4-μm-thick optical sections. (A-B) Results are representative of 3 experiments. Lysates of 24 hours (C-D) and supernatants of 24 hours and 48 hours (E-F) cultured iDCs, Ad.DCs, and mDCs were tested for cell-associated (plasma membrane-bound) and soluble TNF and IL-15 by ELISA and cytokine multiplex assays, respectively. (B,D) TNF data are mean ± SEM (pg/mL) of 7 independent experiments. (C,E) IL-15 data are mean ± SEM (pg/mL) of 3 independent experiments. *P < .05.
Ad.DCs and mDCs express tmTNF and trans-IL-15. (A) tmTNF and trans-IL-15 expression by iDCs, Ad.DCs (500 MOI), and mDCs was analyzed by imaging flow cytometry. Images show examples of individual cells, and the MFI values listed are averaged for the population of 10 000 cells. (B) Topography of tmTNF and trans-IL-15 expression on iDCs, Ad.DCs, and mDCs was examined with confocal microscopy. (Top panels) Optical cross-sections. (Bottom panels) Reconstructed (3-dimensional) images of 10 0.4-μm-thick optical sections. (A-B) Results are representative of 3 experiments. Lysates of 24 hours (C-D) and supernatants of 24 hours and 48 hours (E-F) cultured iDCs, Ad.DCs, and mDCs were tested for cell-associated (plasma membrane-bound) and soluble TNF and IL-15 by ELISA and cytokine multiplex assays, respectively. (B,D) TNF data are mean ± SEM (pg/mL) of 7 independent experiments. (C,E) IL-15 data are mean ± SEM (pg/mL) of 3 independent experiments. *P < .05.
NK cells express necessary receptors for interaction with TNF and IL-15. (A) Expression of TNFR1 and TNFR2 on the plasma membrane of NK cells was analyzed by FACS. The gray line represents NK-cell staining with isotype control antibody; and the black line, anti-TNFR1 and anti-TNFR2 antibody staining. Data are representative of 5 experiments. (B) Expression of IL-15Rβ and IL-15Rγ on cell surface of NK cells was tested by FACS analysis. The gray line represents NK-cell staining with isotype control antibody; and the black line, staining with anti–IL-2/IL-15Rβ and anti–IL-2/IL-15Rγ antibodies. Data are representative of 3 experiments performed.
NK cells express necessary receptors for interaction with TNF and IL-15. (A) Expression of TNFR1 and TNFR2 on the plasma membrane of NK cells was analyzed by FACS. The gray line represents NK-cell staining with isotype control antibody; and the black line, anti-TNFR1 and anti-TNFR2 antibody staining. Data are representative of 5 experiments. (B) Expression of IL-15Rβ and IL-15Rγ on cell surface of NK cells was tested by FACS analysis. The gray line represents NK-cell staining with isotype control antibody; and the black line, staining with anti–IL-2/IL-15Rβ and anti–IL-2/IL-15Rγ antibodies. Data are representative of 3 experiments performed.
tmTNF and trans-IL-15 are major mediators of Ad.DC- and mDC-induced NK cell activation
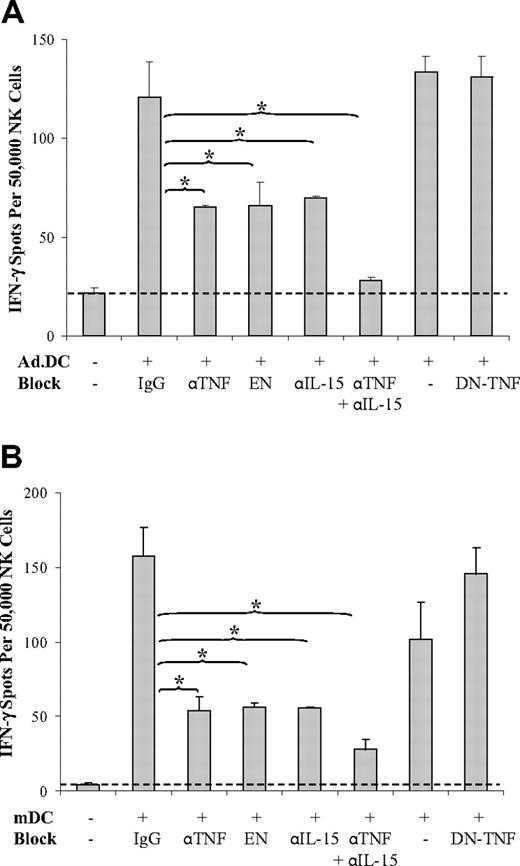
To determine whether plasma membrane-bound or secreted TNF and IL-15 produced by Ad.DCs and mDCs mediate activation of NK cells in DC/NK cell cocultures, we examined the effects of total TNF, solTNF alone, and total IL-15 neutralization on IFN-γ secretion by NK cells stimulated by DCs in cell-to-cell contact or by DC supernatants. We found that individual neutralization of total TNF or IL-15 by the corresponding neutralizing antibodies resulted in inhibition of NK cell activation by 56% and 51%, respectively, for Ad.DCs, and 67% and 66%, respectively, for mDCs. Their combined neutralization led to 94% and 85% inhibition of NK cell activation by Ad.DCs and mDCs, respectively, as measured by IFN-γ secretion (Figure 6). In addition, neutralization of total TNF by TNFR-Fc (Figure 6) and blockades of TNFR1 or TNFR2 by antibodies (supplemental Figure 10A-B) also produced significant inhibition of Ad.DC- and mDC-induced enhancement of NK cell IFN-γ secretion. In sharp contrast, selective sequestration of solTNF by XENP1595 DN-TNF did not affect Ad.DC- and mDC-mediated NK cell activation (Figure 6). These findings, together with the absence of solTNF and solIL-15 secretion by Ad.DCs, and low secretion of the same cytokines by mDCs during their coculture, demonstrate that tmTNF and trans-IL-15 are major mediators of DC/NK cell crosstalk that induce NK cell activation and increased secretion of the central Th1 cytokine IFN-γ. However, combined neutralization of TNF and IL-15 did not inhibit 15% (Figure 6B) and physical separation of the interacting cells did not inhibit 27% (Figure 2B) of mDC-induced NK cell activation. In addition, supernatants of mDC cultures induced significant activation of NK cells that could not be inhibited by either TNF or IL-15 neutralization (supplemental Figure 11). These findings indicate that additional undefined soluble factors produced by mDCs might have minor role in activation of NK cells.
Ad.DCs and mDCs activate NK cells via tmTNF and trans-IL-15. Ad.DCs (A) and mDCs (B) were cocultured with NK cells for 24 hours in the presence of 20 μg/mL of human and mouse nonreactive IgG, anti-TNF antibody (αTNF), TNFR2-Fc construct (EN), anti–IL-15 antibody (αIL-15), both αTNF and αIL-15 (αTNF + αIL-15), or DN-TNF. NK-cell activation was tested by IFN-γ ELISPOT. Data are representative of 3 experiments and are mean ± SEM of triplicates. *P < .05.
Ad.DCs and mDCs activate NK cells via tmTNF and trans-IL-15. Ad.DCs (A) and mDCs (B) were cocultured with NK cells for 24 hours in the presence of 20 μg/mL of human and mouse nonreactive IgG, anti-TNF antibody (αTNF), TNFR2-Fc construct (EN), anti–IL-15 antibody (αIL-15), both αTNF and αIL-15 (αTNF + αIL-15), or DN-TNF. NK-cell activation was tested by IFN-γ ELISPOT. Data are representative of 3 experiments and are mean ± SEM of triplicates. *P < .05.
Discussion
DCs transduced with tumor-associated antigen-encoding AdV efficiently stimulate tumor-associated antigen-specific adaptive CD8+ and CD4+ T-cell responses in vitro and in cancer patients21,24,32 and represent a promising and safe immune adjuvant of anticancer vaccines.20,21 mDCs possess an enhanced ability to present antigens and stimulate type 1 T-cell responses and are also promising as an immunotherapeutic adjuvant.37,38 Here we show that Ad.DCs and mDCs stimulate essential innate immune mechanisms by activating NK cells and amplifying their IFN-γ secretion and type 1 immune functions. Ad.DCs and mDCs directly induce increases in NK-cell CD69 expression, IFN-γ secretion, proliferation, and killing of tumor cell targets by cell-to-cell contact, via tmTNF and trans-IL-15. Importantly, our study also newly demonstrates that Ad.DCs can interact with NK cells in vivo via cell-to-cell contact and likely transmembrane mediators, and remarkably activate NK-cell antitumor activity that is capable of directly inhibiting growth of established tumors. Our current findings that Ad.DCs and mDCs, but not iDCs, stimulate increased expression of the cytotoxic molecule granzyme B, coupled with the observation that NKp30 is up-regulated on NK cells stimulated with DCs, suggest that Ad.DCs and mDC-mediated increases in NK-cell antitumor activity are mediated via up-regulation of NK-cell cytotoxic molecules and activating receptors. These DC-induced NK-cell functions may regulate the quality and extent of anticancer innate and adaptive immune responses25 and have remarkable biologic significance and anticancer therapeutic potential.
TNF and IL-15 mediate DC maturation and NK-cell activation, 2 important biologic responses that are reciprocally induced in DC/NK-cell crosstalk.11,39-47 Although both DCs and NK cells produce tmTNF and solTNF, only DCs produce solIL-15 and plasma membrane-expressed (IL-15Rα-bound) trans-IL-15.11,12,35,36,48 Here we show that AdV transduction and IFN-γ/LPS stimulation induce in DCs elevated plasma membrane expression of tmTNF and trans-IL-15. Up-regulation of trans-IL-15 coincided with the up-regulation of expression of high-affinity IL-15Rα on DCs, which is able to form stable complexes with IL-15 and mediate trans-presentation of IL-15.11,12,46 On the other hand, fresh NK cells express both TNF receptors and IL-2/15Rβγ complex needed for TNF- and IL-15–mediated signaling, respectively. In contrast, during coculture with NK cells, Ad.DCs do not secrete detectable levels of solTNF or solIL-15, whereas mDCs produce minute amounts of these 2 cytokines. In addition, sequestration of solTNF without blocking tmTNF does not prevent Ad.DC- and mDC-mediated activation of NK cells. Consequently, physical contact and engagement of Ad.DC and mDC tmTNF and trans-IL-15, but not solTNF and solIL-15, with the corresponding NK cell receptors lead to Ad.DC- and mDC-mediated NK-cell activation. Previous studies have suggested that IL-15 trans-presented by IL-15Rα expressed on mDCs and IL-15Rβ expressed on resting NK cells constitute an activating domain of NK cell immune synapse that mediates NK cell survival.49 Our current results further substantiate these findings and indicate that not only IL-15Rα-trans-IL-15/IL-15Rβ, but also tmTNF/TNFR, may constitute Ad.DC/NK cell and mDC/NK cell activating domains of NK cell immune synapse. Confocal microscopy findings that IL-15 and tmTNF are coexpressed on plasma membranes of Ad.DCs and mDCs in microdomains that become concentrated at sites of cell-to-cell contacts illustratively support this possibility.
It has been convincingly shown in mice that LPS-matured DCs rapidly express increased levels of tmTNF and use this plasma membrane-bound cytokine to interact with and activate NK cells in cell-to-cell contact.10 In contrast, it has been demonstrated that DCs matured by polyriboinosinic acid/polyribocytidylic acid express on their plasma membrane increased levels of IL-15Rα-bound trans-IL-15 and use this membrane-bound cytokine to crosstalk with and activate NK cells.11 These studies have left unanswered the question whether DCs matured by different methods use similar molecules to mediate DC/NK cell crosstalk and NK cell activation, and whether similar molecular mechanisms are used by murine and human DC and NK cells.10,11 Here we show that human AdV-transduced and IFN-γ/LPS-matured DCs crosstalk with resting NK cells by cell-to-cell contact, mediated exclusively (Ad.DCs) or principally (mDCs) via simultaneous engagement of tmTNF and trans-IL-15. Therefore, our present study demonstrates, for the first time, that cooperative activity of these 2 plasma membrane-bound cytokines represents a central and possibly general mechanism of DC/NK cell crosstalk, leading to polarized activation of NK cells.
Treatment of NK cells with anti-TNF neutralizing antibodies and TNF deficiency of NK cells have been shown to decrease the ability of NK cells to induce CD86 up-regulation in human DCs3,5 and CD40 expression and IL-12p70 secretion in mice,10 indicating that NK cell TNF could contribute to DC maturation induced in DC/NK cell crosstalk. We find that AdV induces up-regulation of multiple DC maturation markers, and CD80 and CD86 expression is further enhanced on Ad.DCs after coculture with resting NK cells. Together, these findings suggest that tmTNF could be a major mediator of Ad.DC/NK cell crosstalk in cell-to-cell contact, leading to not only Ad.DC-mediated activation of NK cells, but also to enhanced maturation of Ad.DC induced by NK cells. The former possibility has been well evidenced in the previous mouse10 and the present human studies, whereas the latter awaits formal demonstration.
Our present findings that AdV-transduction leads to significant increases in DC expression of maturation markers and tmTNF and trans-IL-15 confirm and extend our previous observation that AdV induces DC maturation.22,32 The exact mechanism of how AdV induces DC maturation and enhancement of tmTNF and trans-IL-15 expression and consequently increases the DC ability to activate NK cells is unknown. Previous studies have indicated that AdV induction of DC maturation and up-regulation of antigen-presenting machinery are independent of AdV replication and trans-gene function but dependent on viral structures.20,22 AdV tropism for DCs is mediated by a heparin-binding receptor that recognizes the shaft segment of Ad5 fiber. Triggering of this receptor can induce maturation of DCs.50 Therefore, it is possible that modulations of DC phenotype that impact NK-cell activation might be induced by AdV structural proteins.22
In conclusion, we defined for the first time in humans a novel juxtacrine signaling mechanism of DC/NK cell crosstalk, which is nonsecretory and mediated by cell-to-cell contact via simultaneous engagement of DC tmTNF and trans-IL-15 with the respective NK-cell receptors. This cell signaling leads to the activation, polarization, and enhancement of NK-cell tumoricidal and type 1 immune functions. As DCs also potently stimulate polyclonal antitumor T-cell responses, they may represent a unique and highly potent vaccine platform, which simultaneously induces activation, polarization, and bridging of innate and adaptive immune mechanisms and consequent mounting of enhanced immune functions that efficiently fight cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the University of Pittsburgh Cancer Institute (UPCI) Flow Cytometry Facility, the University of Pittsburgh Vector Core Facility (Ad.LacZ) and Center for Biologic Imaging, and the UPCI Immunologic Monitoring Laboratory (Luminex).
This work was supported by the University of Pittsburgh Cancer Institute and the Henry L. Hillman Foundation (L.H.B., N.L.V.) and by the National Institutes of Health (1P50CA121973 and RO1 CA104524, L.H.B.; and RO1 DE17150, N.L.V.).
National Institutes of Health
Authorship
Contribution: L.V., N.L.V., and L.H.B. were accountable for the conception and design of research and writing the manuscript; L.V. and N.L.V. performed all in vitro experiments and data analysis; D.E.S. provided DN-TNF for blocking experiments and contributed to writing the paper; and S.A. and S.C.W. performed confocal microscopy analysis of DCs.
Conflict-of-interest disclosure: D.E.S. is a Xencore employee. The remaining authors declare no competing financial interests.
Correspondence: Lisa H. Butterfield, Department of Medicine, Surgery and Immunology, University of Pittsburgh, 1.27d Hillman Cancer Center, 5117 Centre Ave, Pittsburgh, PA 15213; e-mail: butterfieldl@upmc.edu; or Nikola L. Vujanovic, Department of Pathology and Immunology, University of Pittsburgh, G.17d Hillman Cancer Center, 5117 Centre Ave, Pittsburgh, PA 15213; e-mail: vujanovicnl@upmc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal