Abstract
Survival of patients with thrombotic thrombocytopenic purpura (TTP) improved dramatically with plasma exchange treatment, revealing risk for relapse. The Oklahoma TTP Registry is a population-based inception cohort of all 376 consecutive patients with an initial episode of clinically diagnosed TTP (defined as microangiopathic hemolytic anemia and thrombocytopenia with or without signs and symptoms of ischemic organ dysfunctions) for whom plasma exchange was requested, 1989 to 2008. Survival was not different between the first and second 10-year periods for all patients (68% and 69%, P = .83) and for patients with idiopathic TTP (83% and 77%, P = .33). ADAMTS13 activity was measured in 261 (93%) of 282 patients since 1995. Survival was not different between patients with ADAMTS13 activity < 10% (47 of 60, 78%) and patients with 10% or more (136 of 201, 68%, P = .11). Among patients with ADAMTS13 activity < 10%, an inhibitor titer of 2 or more Bethesda units/mL was associated with lower survival (P = .05). Relapse rate was greater among survivors with ADAMTS13 activity < 10% (16 of 47, 34%; estimated risk for relapse at 7.5 years, 41%) than among survivors with ADAMTS13 activity of 10% or more (5 of 136, 4%; P < .001). In 41 (93%) of 44 survivors, ADAMTS13 deficiency during remission was not clearly related to subsequent relapse.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/cme/blood; and (4) view/print certificate. For CME questions, see page 1662.
Disclosures
Johanna A. Kremer Hovinga, Deirdra R. Terrell, Bernhard Lämmle, and James N. George serve as consultants for Baxter Inc for development of recombinant ADAMTS13. Author Sara K. Vesely, the Associate Editor Mortimer Poncz, and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe patterns in ADAMTS13 activity among patients with a history of thrombotic thrombocytopenic purpura (TTP)
List characteristics associated with deficient ADAMTS13 activity
Describe mortality trends in patients with TTP
Describe risk factors for and prognosis of relapse of TTP
Introduction
The introduction of plasma exchange treatment (PEX) dramatically increased the survival of patients with thrombotic thrombocytopenic purpura (TTP). Before the plasma exchange era, only 10% of patients survived1 ; initial reports of PEX, 1981 to 1991, described survival rates of 70% to 79%.2-4 The availability of effective treatment decreased the stringency of criteria required for diagnosis of TTP. Before the plasma exchange era, the characteristic clinical features (anemia, thrombocytopenia, neurologic and renal abnormalities, fever) occurred in 88% to 98% of patients.1 For patients in the clinical trial that documented superiority of PEX compared with plasma infusion, only microangiopathic hemolytic anemia and thrombocytopenia without another apparent etiology were required for the diagnosis of TTP; the frequency of each of the other 3 abnormalities decreased to 24% to 63%.4 Availability of effective treatment and decreased diagnostic stringency increased the frequency of patients treated for TTP by 7-fold5 and may also have increased the heterogeneity of patients diagnosed with TTP.
The discovery of severe ADAMTS13 deficiency as part of the pathogenesis of TTP6,7 provided an opportunity for a more specific diagnosis. However, patient heterogeneity has persisted.8-11 Patients are described as having idiopathic TTP if they have no apparent other condition that may cause thrombotic microangiopathy; they commonly have severe ADAMTS13 deficiency and they have a higher survival rate.9,10 Patients are described as having secondary TTP if other conditions are identified that may cause thrombotic microangiopathy, such as hematopoietic stem cell transplantation (HSCT), pregnancy, drug association, other autoimmune diseases, HIV infection, and cancer; they rarely have severe ADAMTS13 deficiency and they have poor survival.9,10 The improved survival rates in recent reports of patients with severe ADAMTS13 deficiency, 82% to 100%,10,12-15 compared with the initial reports of patients treated with PEX,2-4 may reflect patient selection rather than more effective management.
Soon after the availability of PEX, relapse rates of 37%3 and 36%16 were reported. Recent studies have reported that severe ADAMTS13 deficiency at the time of the initial episode predicts a risk for relapse9,10,12,13,15,17 and have suggested that severe ADAMTS13 deficiency during remission may also predict risk for relapse.15,17,18
We report the experience of the Oklahoma TTP Registry, a 20-year population-based inception cohort of all 398 consecutive patients in our 58 county region for whom PEX was requested for a diagnosis of TTP or hemolytic uremic syndrome (HUS), to address questions concerning survival and relapse: (1) What clinical and laboratory features predict survival? (2) Have survival rates changed across the past 20 years? (3) What is the risk for relapse? (4) Who is at risk for relapse? (5) When do relapses occur?
Methods
Oklahoma TTP-HUS Registry
The Registry has enrolled all consecutive patients since January 1, 1989, with a diagnosis of TTP or HUS for whom the Oklahoma Blood Institute was requested to provide PEX.9,19 The Oklahoma Blood Institute is the sole provider of PEX for all hospitals in 58 of the 77 Oklahoma counties, a region with a population of 2 310 000.20 Because standard practice in this region is to treat all adults who are diagnosed with either TTP or HUS and all children who are diagnosed with TTP with PEX, the Registry is a population-based inception cohort of consecutive patients in whom a diagnosis of TTP or HUS is made and PEX is requested. Children with typical (diarrhea-associated) HUS are not typically treated with PEX; therefore, most of these children are not included in the Registry. All patients have consented to be enrolled. Data are collected prospectively.9 The Registry is approved by the institutional review boards of the University of Oklahoma Health Sciences Center and each participat-ing hospital.
Nomenclature
Because these syndromes in adults, with or without renal failure or neurologic abnormalities, are commonly known as TTP,21 because Registry patients are almost all adults, and because patient descriptions focus on clinical presentations and levels of ADAMTS13 activity without testing for abnormalities of complement regulation, we describe patients in this report as having TTP. We recognize that some of our patients may be appropriately described as HUS, rather than TTP, particularly if complement regulatory abnormalities had been recognized22 or if a Shiga-like toxin-associated infection had been identified.
ADAMTS13 activity and inhibitor measurements
ADAMTS13 activity was measured by both quantitative immunoblotting of degraded, plasma-derived von Willebrand factor substrate6,23 and a fluorogenic assay using FRETS-VWF73 substrate.24,25 Pefabloc SC (Boehringer), 1mM, was present in all assay buffers to irreversibly block serine proteases, including thrombin and plasmin.25 To examine reliability and validity, ADAMTS13 activity in serial dilutions of normal plasma with plasma from patients with congenital TTP was measured by both methods in a blinded fashion.26
Serum has been routinely collected immediately before the first PEX since November 13, 1995; therefore, serum has been used for ADAMTS13 assays. To determine whether ADAMTS13 activity was the same in serum and plasma, activity was measured by both immunoblotting and FRETS-VWF73 methods on serum and plasma simultaneously obtained from 13 patients. ADAMTS13 levels in serum and plasma correlated for both methods (r = 0.983, immunoblotting; r = 0.996, FRETS-VWF73); agreement was good (mean paired difference ± SD: immunoblotting, 3.6% ± 6.0%; FRETS-VWF73, 5.3% ± 6.5%; Appendix). ADAMTS13 activity was measured by both methods at the time of initial diagnosis. Patients are designated as having ADAMTS13 activity < 5% or < 10% when measurement by either method was < 5% or < 10%. ADAMTS13 activity during clinical remission was measured only by the FRETS-VWF73 method in patients who had ADAMTS13 levels < 10% at the time of their initial episode.
ADAMTS13 functional inhibitor activity was measured on samples with ADAMTS13 activity of 20% or less by determination of residual ADAMTS13 activity of normal human plasma after 1:1 (v:v) incubation for 2 hours at 37°C with heat-inactivated patient's serum by the FRETS-VWF73 method. Inhibitor titers are reported in Bethesda units (BU)/mL up to 2 BU/mL. Titers greater than 2 BU/mL are reported as > 2 BU/mL when residual ADAMTS13 activity was 11% to 25% and >> 2 BU/mL when residual ADAMTS13 activity was 10% or less. Assays were performed without knowledge of the patients' clinical data.
Patient clinical categories
Patients were assigned in a hierarchical, sequential order to one of 6 clinical categories related to associated conditions and potential etiologies that were apparent during their initial episode9 : (1) allogeneic HSCT, (2) pregnancy/postpartum, (3) drug association, (4) bloody diarrhea prodrome, (5) additional or alternative disorder, and (6) idiopathic, if criteria for the previous categories were not present. Patients with additional or alternative disorders were further divided into 6 subcategories (Table 1). These patients were heterogeneous; in some, the presenting clinical features were attributed to another (alternative) etiology after PEX was begun and then PEX was stopped; in other patients, additional disorders were present and PEX was continued because the diagnosis of TTP seemed valid. Assignments to clinical categories were made before results of ADAMTS13 activity were known.
Patient outcome definitions
Remission is defined as no PEX for 30 days; laboratory and clinical abnormalities had typically resolved at this time.9 Survival is defined as achievement of remission.9 Death from TTP is defined as occurring within 30 days of stopping PEX; deaths beyond 30 days were not attributed to TTP.9 Recurrence of thrombocytopenia and microangiopathic hemolytic anemia and reinitiation of daily PEX within 30 days of stopping PEX is defined as an exacerbation of a continuing episode.9 Relapse, considered to be the occurrence of a new episode of TTP, is defined as the recurrence of thrombocytopenia and microangiopathic hemolytic anemia after achievement of a remission.9
Statistical analysis
To determine the correlation between immunoblotting and FRETS-VWF73 methods, a Spearman correlation coefficient was calculated. To determine whether the probability of measuring ADAMTS13 < 10% by the FRETS-VWF73 method compared with the immunoblotting method was different, the McNemar test with exact inference was used. We used the nonparametric Wilcoxon Mann-Whitney test for continuous data and the χ2 test or Fisher exact test for categorical data to compare the demographics and clinical features between different groups of patients. A Kaplan-Meier curve was created to illustrate the time of occurrence of relapse or the time of last follow-up or death among surviving patients with ADAMTS13 levels < 10% or of 10% or more followed by a log-rank test to determine differences in survival curves between groups. To estimate relapse (failure) probability in the presence of death before relapse (a competing event), cumulative incidence methodology was used.27 Statistical analyses were performed using SAS, Version 9.1.3 (SAS Institute). An alpha of 0.05 was used.
Results
Patients
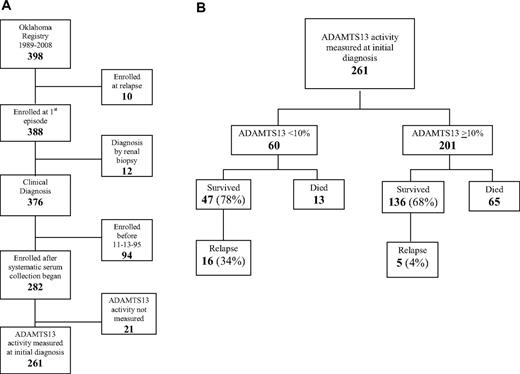
The Registry enrolled all 398 consecutive patients for whom PEX was requested for a diagnosis of TTP or HUS across 20 years, January 1, 1989, to December 31, 2008. Ten patients were excluded from this analysis because they had had a previous episode of TTP that occurred before 1989 or outside of the Registry region (Figure 1A). Twelve patients whose initial diagnosis was made by renal biopsy were also excluded; these patients often did not have clinical diagnostic criteria for TTP. Survival was analyzed in the remaining 376 patients with their first episode of clinically diagnosed TTP. Systematic collection of serum samples at the time of the initial diagnosis of TTP began on November 13, 1995; since that time, ADAMTS13 activity has been determined on 261 (93%) of 282 patients. Among the 21 patients without ADAMTS13 measurements, 9 died after PEX was requested but before it was begun and a sample collected; 2 of these 9 patients had autopsies; both autopsies confirmed the clinical diagnosis of TTP by demonstration of systemic microvascular thrombosis. ADAMTS13 measurement was not valid in 2 patients because of extreme hemoglobinemia28 caused by hemodialysis complications. Ten other patients did not have a sample for other reasons. Because ADAMTS13 deficiency at disease onset has been associated with risk for relapse, relapses were analyzed only in the 261 patients who had ADAMTS13 activity measured at the time of their initial diagnosis; 183 (70%) of these patients survived; follow-up is complete through October 2009 for 182 of the 183 survivors; the median duration of follow-up is 4.7 years (range, 0.05-13.4 years). For analysis of survival and relapse, patients were divided into groups with ADAMTS13 activity < 10% and 10% or more (Figure 1B).
Patient selection diagram. (A) Patient selection for analysis of survival and relapse. (B) Survival and relapse in patients with ADAMTS13 activity < 10% and ADAMTS13 activity 10% or more at the time of their initial diagnosis with TTP.
Patient selection diagram. (A) Patient selection for analysis of survival and relapse. (B) Survival and relapse in patients with ADAMTS13 activity < 10% and ADAMTS13 activity 10% or more at the time of their initial diagnosis with TTP.
ADAMTS13 measurements
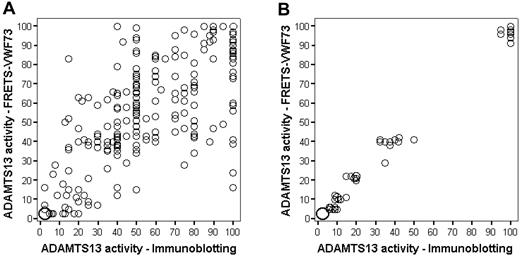
Figure 2A illustrates the results of measurements of ADAMTS13 activity in all 261 patients at the time of their initial episode by both immunoblotting and FRETS-VWF73 methods. Although variability of results is apparent, the agreement was strong (r = 0.80, P < .001) and the mean paired difference was small, 0.36% (SD = 20.61%). Figure 2B demonstrates that, when serial normal plasma dilutions into Upshaw-Schulman plasma were measured by both methods, there was strong correlation between the 2 methods (r = 0.98) and between each method and the expected value.26
ADAMTS13 activity measurements by both immunoblotting and the fluorogenic assay with FRETS-VWF73 substrate. The size of the circle corresponds to number of observations. For example, in the lower left corner of panel A, the large circle represents 33 observations. (A) Measurements on 261 patients at the time of diagnosis of their first episode of TTP. The immunoblotting technique at higher ADAMTS13 activity is semiquantitative with activity measured at increments of 5%. (B) Measurements of one normal human plasma sample serially diluted into congenital TTP (Upshaw-Schulman syndrome) plasma. Ten replicates of 6 dilutions (0%, 5%, 10%, 20%, 40%, and 100%) each were measured on 10 different days in a blinded fashion. These data from our laboratory have been previously published as part of a multicenter collaborative study.26
ADAMTS13 activity measurements by both immunoblotting and the fluorogenic assay with FRETS-VWF73 substrate. The size of the circle corresponds to number of observations. For example, in the lower left corner of panel A, the large circle represents 33 observations. (A) Measurements on 261 patients at the time of diagnosis of their first episode of TTP. The immunoblotting technique at higher ADAMTS13 activity is semiquantitative with activity measured at increments of 5%. (B) Measurements of one normal human plasma sample serially diluted into congenital TTP (Upshaw-Schulman syndrome) plasma. Ten replicates of 6 dilutions (0%, 5%, 10%, 20%, 40%, and 100%) each were measured on 10 different days in a blinded fashion. These data from our laboratory have been previously published as part of a multicenter collaborative study.26
For determination of severe ADAMTS13 deficiency, only 18 (7%) of the 261 patient samples showed discordant values with the 2 assay methods for < 10% versus 10% or more; 201 (77%) samples had ADAMTS13 activity of 10% or more by both assays; 42 samples had ADAMTS13 activity < 10% by both assays. Among the 18 discordant samples, 13 were < 10% by the FRETS-VWF73 method but not by immunoblotting, and 5 were < 10% by immunoblotting but not by the FRETS-VWF73 method (Figure 3A); the probability of having ADAMTS13 activity < 10% by the FRETS-VWF73 method compared with the immunoblotting method was not significantly different (P = .10). Among the 60 patients with ADAMTS13 activity < 10% by at least one assay method, 50 (83%) had demonstrable inhibitors. Six of the 10 patients without a demonstrable functional inhibitor by the FRETS-VWF73 assay had evidence for acquired ADAMTS13 deficiency: normal activity during remission or presence of an inhibitor during relapse.
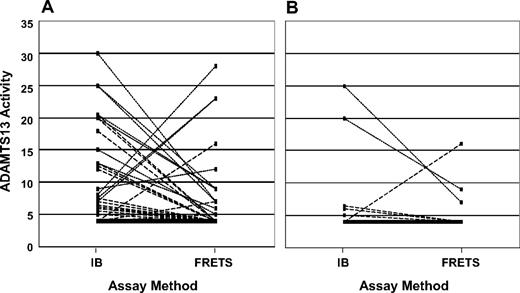
ADAMTS13 activity measurements by both immunoblotting and the fluorogenic assay with FRETS-VWF73 substrate. (A) Sixty patients who had ADAMTS13 activity < 10% by at least 1 of the 2 methods of measurement at the time of their initial diagnosis. The solid line represents 33 patients who had ADAMTS13 activity < 5% by both assays, arbitrarily designated as 4%. The broken lines represent each of the 16 patients who had ADAMTS13 activity < 5% by one assay but 5% or more by the other assay. The dotted lines represent each of the 11 patients who had ADAMTS13 activity of 5% to 9% by one assay but more than 10% by the other. (B) The 16 patients who had ADAMTS13 activity < 10% by at least 1 of the 2 methods of measurement at the time of their initial diagnosis and who have relapsed. The solid line represents 10 patients who had ADAMTS13 activity < 5% by both assays, arbitrarily designated as 4%. The broken lines represent each of the 4 patients who had ADAMTS13 activity < 5% by one assay but 5% or more by the other. The dotted lines represent each of the 2 patients who had ADAMTS13 activity of 5% to 9% by one assay but more than 10% by the other.
ADAMTS13 activity measurements by both immunoblotting and the fluorogenic assay with FRETS-VWF73 substrate. (A) Sixty patients who had ADAMTS13 activity < 10% by at least 1 of the 2 methods of measurement at the time of their initial diagnosis. The solid line represents 33 patients who had ADAMTS13 activity < 5% by both assays, arbitrarily designated as 4%. The broken lines represent each of the 16 patients who had ADAMTS13 activity < 5% by one assay but 5% or more by the other assay. The dotted lines represent each of the 11 patients who had ADAMTS13 activity of 5% to 9% by one assay but more than 10% by the other. (B) The 16 patients who had ADAMTS13 activity < 10% by at least 1 of the 2 methods of measurement at the time of their initial diagnosis and who have relapsed. The solid line represents 10 patients who had ADAMTS13 activity < 5% by both assays, arbitrarily designated as 4%. The broken lines represent each of the 4 patients who had ADAMTS13 activity < 5% by one assay but 5% or more by the other. The dotted lines represent each of the 2 patients who had ADAMTS13 activity of 5% to 9% by one assay but more than 10% by the other.
ADAMTS13 levels related to clinical categories
Table 1 presents the clinical categories of all 376 patients with an initial episode of clinically diagnosed TTP. Tests for HIV infection were performed on 365 (97%) patients; HIV infection was documented in 6 (1.6%) patients who were assigned to their appropriate clinical categories: 3 malignant hypertension, 1 systemic malignancy, and 2 idiopathic.29 All 6 patients with HIV infection had ADAMTS13 measurements; 1 patient with systemic malignancy had ADAMTS13 activity < 10%; 1 idiopathic patient had normal ADAMTS13 activity with his initial episode but activities < 10% with his fourth to sixth episodes.29,30 Table 1 also presents the clinical categories of all 261 patients who had ADAMTS13 activity measurements at the time of their initial diagnosis and also the subgroups of patients who had ADAMTS13 activity of 10% or more, < 10%, and < 5% (patients in the < 5% subgroup are also in the < 10% subgroup).
Among the 60 patients with ADAMTS13 activity < 10%, 46 (77%) were in the idiopathic category: 3 patients who presented postpartum, 2 who presented with bloody diarrhea, and 3 who had previously established diagnoses of additional autoimmune disorders (systemic lupus erythematosus, 2; Sjögren syndrome, 1) had clinical courses typical of TTP. In 6 (10%) patients, the clinical features that had initially suggested the diagnosis of TTP were subsequently attributed to an alternative disorder: acute graft-versus-host disease and aspergillus sepsis in 1 after HSCT, documented systemic infection in 4, and systemic malignancy in 1. Five of these 6 patients had ADAMTS13 activity 5% to 9% by one assay but more than 10% by the other; 1 had ADAMTS13 activity < 5% by both assays; 4 had demonstrable ADAMTS13 inhibitors. Two of the patients with systemic infection survived; 2 of the 4 who died had autopsies; there was no evidence for TTP on either autopsy.
The 49 patients with ADAMTS13 activity < 5% were examined separately to determine the clinical spectrum of patients with a more stringent definition of severe ADAMTS13 deficiency. This group excluded 1 patient after HSCT, 1 postpartum patient, 3 patients with systemic infection, 1 patient with systemic malignancy, and 5 idiopathic patients. In only 1 (2%) patient with ADAMTS13 activity < 5% (no demonstrable inhibitor) were the clinical features that had initially suggested TTP subsequently attributed to an alternative disorder (bacterial endocarditis); she survived. Seven other patients with ADAMTS13 activity < 5% were in clinical categories other than the idiopathic category (2 pregnant/postpartum, 2 bloody diarrhea, and 3 autoimmune disorder).
Presenting features and clinical outcomes related to ADAMTS13 levels
Table 2 compares the demographic, clinical, and laboratory features at presentation and the clinical outcomes of the 201 patients with ADAMTS13 activity of 10% or more to the 60 patients with ADAMTS13 activity < 10%. Patients with ADAMTS13 levels < 10% were younger and more obese. There were significant sex and race disparities with more women and blacks among patients with ADAMTS13 levels < 10%. Thrombocytopenia was more severe and renal failure was less severe among patients with ADAMTS13 levels < 10%, but there was no difference in the frequency of severe neurologic abnormalities. Six patients with ADAMTS13 < 10% had acute renal failure; 2 survived and both regained normal creatinine levels. A total of 109 patients with ADAMTS13 of 10% or more had acute renal failure; 80 survived; 54 (68%) regained normal creatinine values. Comparison of clinical outcomes demonstrated that there was no difference in the frequency of TTP-associated death; but among patients who survived, those with ADAMTS13 levels < 10% required more PEX to achieve remission and had a greater risk for relapse.
Survival related to clinical categories and years of Registry enrollment
Overall survival was 69% (258 of 376 patients), but survival was extremely variable across clinical categories (Table 1). Survival was 93% for patients diagnosed during pregnancy or postpartum, 80% for patients in the idiopathic category, but less than 30% for patients after HSCT and in patients with systemic infection, malignancy, or multiorgan failure. Survival was unchanged across the 20 years of patient accrual. For all patients, survival was 68% (115 of 169 patients) during the first 10 years, 1989 to 1998, and 69% (143 of 207 patients) during the second 10 years, 1999 to 2008 (P = .83). For idiopathic patients, survival was 83% (55 of 66 patients) during the first 10 years and 77% (63 of 82 patients) during the second 10 years (P = .33).
Survival related to ADAMTS13 activity levels
Survival was 68% for patients with ADAMTS13 activity of 10% or more and 78% for patients with ADAMTS13 activity < 10% (Figure 1B; Table 2). Among the 60 patients with ADAMTS13 activity < 10%, the 47 survivors are compared with the 13 patients who died (Table 3). Only serum creatinine (P < .01) and frequency of an ADAMTS13 inhibitor of 2 or more BU (P = .05) were significantly greater among the 13 nonsurvivors. Death was attributed to thrombotic complications of TTP in 6 patients and to hemorrhagic or infectious complications of PEX in 3 patients (Table 4). In the 4 remaining patients, death was attributed to alternative disorders rather than to TTP. Sixty-five patients with ADAMTS13 activity of 10% or more died, 2 from PEX (1 hemorrhage related to catheter insertion, 1 sepsis); 13 of the remaining 63 had autopsies, and none had systemic microvascular thrombosis consistent with TTP. Forty-five of the 63 patients had additional or alternative disorders or had had an allogeneic stem cell transplantation.
Relapse related to ADAMTS13 activity levels
Relapse among survivors was significantly more frequent among patients with ADAMTS13 activity < 10% than among patients with ADAMTS13 activity of 10% or more (P < .01, Figure 1B; Table 2). Among the 16 patients with ADAMTS13 activity < 10% who have relapsed, 3 (19%) had ADAMTS13 activity < 10% by only one method of measurement (Figure 3B). Four of the 16 patients had no demonstrable inhibitor at the time of their initial diagnosis, but 3 of these 4 patients had inhibitors at the time of a relapse. Relapse in the fourth patient was diagnosed only at death and confirmed by autopsy; ADAMTS13 activity was not measured (described in “Survival of patients with relapsed TTP”). One woman who relapsed was postpartum at the time of her initial episode; the other 15 patients were in the idiopathic category.
The 5 patients who relapsed and whose ADAMTS13 activity was 10% or more at the time of their initial diagnosis are described in Table 5. Patient 1 had quinine-induced TTP confirmed by demonstration of quinine-dependent platelet-reactive antibodies. In patients 2 and 3, the clinical features that had suggested the diagnosis of TTP may have been related to flares of systemic lupus erythematosus (SLE) with advanced lupus nephritis, malignant hypertension, and/or sepsis. The other 2 patients may have had in vivo abnormalities of ADAMTS13 function despite normal activity with in vitro measurements. In patient 4, ADAMTS13 deficiency evolved over the course of 6 TTP episodes; ADAMTS13 activity was < 5% with a demonstrable inhibitor in his fifth and sixth episodes.29,30 Patient 5 had discordant values of ADAMTS13 activity between immunoblotting and FRETS-VWF73 measurements and a demonstrable ADAMTS13 inhibitor at both of her episodes.
Relapse related to clinical features of the initial episode of TTP
Among the 47 surviving patients who had ADAMTS13 activity < 10%, the demographic, clinical, and laboratory features of the 16 patients who have relapsed were compared with the 31 patients who have not relapsed; only male sex was significantly associated with relapse (Table 3). Duration of follow-up was significantly longer for patients who have relapsed compared with those who have not.
Relapse related to duration of remission
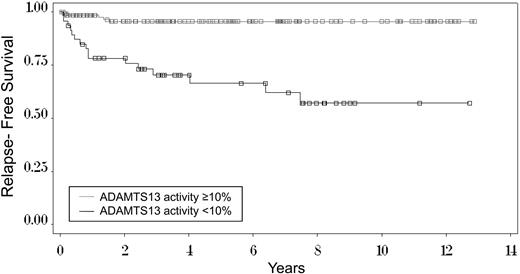
Figure 4 illustrates the time to occurrence of the first relapse among the 136 surviving patients with ADAMTS13 levels of 10% or more and the 47 surviving patients with ADAMTS13 levels < 10%. Among the patients with ADAMTS13 of 10% or more, relapses occurred within 2 years. Among the patients with ADAMTS13 levels < 10%, 10 (63%) initial relapses occurred within the first year; 14 (88%) within 4 years. The remaining 2 patients had their initial relapses at 6.4 and 7.5 years. The cumulative incidence rate, taking into account the competing risk of death before a relapse in 5 patients,27 estimated that the risk for relapse at 7.5 years is 41%. Seven (44%) of the 16 patients who have relapsed have had only 1 relapse; 9 patients have had 2 to 4 relapses.
Kaplan-Meier curve of time to first relapse among 47 surviving patients with ADAMTS13 activity < 10% and among 136 patients with ADAMTS13 activity of 10% or more at the time of their initial diagnosis with TTP. Among the 47 surviving patients with ADAMTS13 activity < 10%, the 1 patient who has been lost to follow-up had a relapse before she refused further follow-up; therefore, data are complete for this analysis. The median follow-up for all 47 patients is 7.5 years. Median follow-up for the 26 living patients who have not relapsed is 4.6 years. (□), the 31 censored patients who have not relapsed: for 26 surviving patients, the time of last follow-up; for 5 patients, the time of death (competing event) before a relapse (event of interest) had occurred; deaths occurred at 0.3, 1.2, 3.5, 7.8, and 8.6 years. The Kaplan-Meier estimate of relapse at 7.5 years, the time of the last relapse (43%), is greater than the estimate using cumulative incidence methodology27 to account for the competing events of death (41%). For the 136 patients with ADAMTS13 activity of 10% or more, median follow-up is 4.4 years. The Kaplan-Meier estimate of relapse at 1.5 years, the time of the last relapse (4%), is the same as the estimate using cumulative incidence methodology.27
Kaplan-Meier curve of time to first relapse among 47 surviving patients with ADAMTS13 activity < 10% and among 136 patients with ADAMTS13 activity of 10% or more at the time of their initial diagnosis with TTP. Among the 47 surviving patients with ADAMTS13 activity < 10%, the 1 patient who has been lost to follow-up had a relapse before she refused further follow-up; therefore, data are complete for this analysis. The median follow-up for all 47 patients is 7.5 years. Median follow-up for the 26 living patients who have not relapsed is 4.6 years. (□), the 31 censored patients who have not relapsed: for 26 surviving patients, the time of last follow-up; for 5 patients, the time of death (competing event) before a relapse (event of interest) had occurred; deaths occurred at 0.3, 1.2, 3.5, 7.8, and 8.6 years. The Kaplan-Meier estimate of relapse at 7.5 years, the time of the last relapse (43%), is greater than the estimate using cumulative incidence methodology27 to account for the competing events of death (41%). For the 136 patients with ADAMTS13 activity of 10% or more, median follow-up is 4.4 years. The Kaplan-Meier estimate of relapse at 1.5 years, the time of the last relapse (4%), is the same as the estimate using cumulative incidence methodology.27
Relapse related to ADAMTS13 activity during remission
ADAMTS13 activity was measured 1 to 4 times (2004, 2006, 2007-2008, and 2009) during clinical remission in 41 (93%) of the 44 patients whose ADAMTS13 activity was < 10% at the time of their initial episode and who survived until 2004 (Tables 6–7). Seven (17%) patients have had one or more relapses after a remission ADAMTS13 measurement; in 4 of these patients, the most recent preceding ADAMTS13 level was lower than normal 1 to 33 months preceding relapse (< 5%-14%). In the other 3 patients, ADAMTS13 levels 12 to 23 months preceding relapse were normal (64%-85%). Eight other patients have had ADAMTS13 levels of < 5% to 15% without a subsequent relapse for 15 to 64 months. The cause of death of patient 18 is unknown; she was 47 years old and died unexpectedly at home in her sleep; autopsy was not done; she was being treated for hypertension and a major psychiatric disorder.
Relapse related to rituximab treatment
Since December 2003, 13 patients with ADAMTS13 activity < 10% have been treated with rituximab; 7 patients were treated during their initial episode because of severe, unresponsive TTP or because of exacerbations when PEX was stopped; 1 died before achieving a remission. Five patients were treated during their second or third relapse. One patient was treated for an acquired factor VIII inhibitor that occurred 11 months after her only relapse (patient 28, Table 6). All 12 surviving patients have had ADAMTS13 activity measured during remission after rituximab treatment; 2 have had ADAMTS13 levels < 10%, and none has had a subsequent relapse (median follow-up after rituximab treatment, 42 months; range, 4-70 months). All patients received the standard regimen (375 mg/m2 per week for 4 weeks) at the time of their PEX for an acute episode, with the exception of the patient treated for an acquired factor VIII inhibitor. Among patients with ADAMTS13 activity < 10%, the decision to give rituximab was solely based on the clinical course. In addition, 4 patients with ADAMTS13 of 10% or more have been treated with rituximab: 3 were treated for a concurrent disorder (2 SLE, 1 bronchiolitis obliterans); 1 woman who was treated for an exacerbation of her initial episode had ADAMTS13 activity of 11%; she has not relapsed during the following 12 months.
Survival of patients with relapsed TTP
Two (12%) of 16 patients with ADAMTS13 activity < 10% at the time of their initial episode died during an episode of relapsed TTP. One patient died during her 63rd PEX for her first relapse; her platelet count had been normal for 5 days; autopsy documented a discrete ventricular septum myocardial infarction but no microvascular thrombosis. One man required urgent coronary artery bypass surgery 4 years after his initial episode of TTP; his platelet count was normal at the time of surgery; he died suddenly 7 days after surgery; TTP was diagnosed at death and confirmed by autopsy. The other 14 patients have survived 30 relapses of TTP. None of the 5 patients with ADAMTS13 levels of 10% or more at the time of their initial diagnosis died during relapsed episodes.
Discussion
Despite greater understanding of the pathogenesis of TTP and effective treatment with PEX, mortality from acute episodes and morbidity among survivors remain substantial. We have defined the clinical outcomes of survival and relapse in a population-based cohort of 376 consecutive patients with an initial episode of TTP enrolled and followed continuously over 20 years. Therefore, data from this cohort of unselected patients identified at the time of their first diagnosis by the intention to treat with PEX, unaffected by referral and reporting bias, should be generalizable to other populations.
Patients with a clinical diagnosis of TTP are heterogeneous and have different outcomes. In some patients, the clinical features that had initially supported the diagnosis of TTP were subsequently attributed to another disorder; these patients had poor survival. In all patients and also in patients defined as having idiopathic TTP, survival rates did not change across 20 years. The survival rate of our patients with idiopathic TTP, 80%, was similar to the survival rates of 70% to 79% reported in the initial descriptions of PEX 18 to 28 years ago.2-4
Patients defined as idiopathic were also heterogeneous. Although these patients were not recognized to have any of the conditions defining the other established clinical categories, some had preceding or concurrent conditions, such as pancreatitis,34 infections,35 or surgery,36,37 which may have triggered the onset of TTP, reflecting the current arbitrary definition of “idiopathic” TTP. Only 46 (47%) of the 98 patients defined as idiopathic had ADAMTS13 activity < 10%.
Even patients who presented with ADAMTS13 levels < 10% were heterogeneous, initially presenting in multiple clinical categories (HSCT, postpartum, bloody diarrhea prodrome, additional or alternative disorders, as well as idiopathic). This experience suggests that dichotomous descriptions of TTP as either idiopathic or secondary11,38 do not accurately represent the heterogeneity among patients who are diagnosed and treated for TTP.
Severely deficient ADAMTS13 activity has been considered to be specific for TTP,23 but the level of activity that defines severe deficiency and provides clinical value has not been established. We evaluated 2 methods of measurement of ADAMTS13 activity: quantitative immunoblotting and the FRETS-VWF73 method. Although the mean paired difference was small, there was substantial variability between the 2 methods. However, the 2 methods agreed on the determination of ADAMTS13 activity < 10% or 10% or more in 93% of the 261 patient samples. That the variability between the 2 assays reflects complexity of individual patient samples rather than analytical precision was supported by the close agreement of the 2 methods for measurement of ADAMTS13 activity in samples of serially diluted normal plasma. Examples of patient complexity with discordant results between the 2 assays are patients 4 and 5 in Table 5. Multiple factors may contribute to the variability of assay results: severely elevated bilirubin, hemoglobin, or other plasma components may cause quenching of fluorescence.28,39 Some genetic variants of ADAMTS13 may be sensitive to urea, resulting in lower ADAMTS13 activity measured by the immunoblotting assay.40,41 Circulating immune complexes of ADAMTS13 with low affinity autoantibodies may dissociate during in vitro assays, with different ADAMTS13 activities reported with different incubation conditions and times.30 One assay was not better than the other for identifying patients with ADAMTS13 activity < 10%, but the use of both assays increased the ability to identify patients with ADAMTS13 activity < 10% who were at increased risk for relapse.
For determination of clinical value, we evaluated 2 levels of ADAMTS13 deficiency: < 5% and < 10% activity. ADAMTS13 activity < 5% identified only 1 patient (of 49 patients) who had another disorder (bacterial endocarditis) that may have explained her presenting clinical features. However, an ADAMTS13 level < 5% was less sensitive to identify patients at risk for relapse; 2 (13%) of the 16 patients with initial ADAMTS13 levels < 10% who have relapsed had levels of 7% and 9%.
ADAMTS13 activity < 10% identified 16 (76%) of the 21 patients who were diagnosed with a relapse. The 5 patients with ADAMTS13 activity of 10% or more who were diagnosed with relapses of TTP had exceptional circumstances. One patient with quinine-induced TTP-HUS took quinine again. In 2 patients, there were multiple possible alternative etiologies for the acute episodes that had suggested the diagnosis of TTP: SLE with severe nephritis, malignant hypertension, and/or sepsis. The other 2 patients may have had a functional deficiency of ADAMTS13 not detected by either method of measurement; 1 of these patients had ADAMTS13 levels < 10% at the time of his fourth to sixth episodes.29,30 However, ADAMTS13 activity < 10% appeared to have less specificity for the diagnosis of TTP than levels < 5%. Six (10%) of the 60 patients with ADAMTS13 activity < 10% were considered to have possible alternative explanations for their clinical features.
Among the 60 patients with ADAMTS13 activity < 10%, the survival was 78%, less than in recent reports describing 82% to 100% survival in patients with severe ADAMTS13 deficiency.10,12-15 The survival rate of 78% may underestimate the survival of patients with TTP because of the inclusion of 6 patients who may have had alternative etiologies for their clinical features, 4 of whom died. However, this survival rate may also overestimate actual survival because it did not include patients with a clinical diagnosis of TTP who died before plasma exchange could be started and a sample for ADAMTS13 measurement obtained and who had pathologic confirmation of TTP at autopsy. Three of the 13 deaths in these 60 patients were attributed to complications of PEX,32,33 emphasizing that safer as well as more effective treatment is required. No demographic, clinical, or laboratory features were associated with death other than a higher serum creatinine and an ADAMTS13 inhibitor level of 2 or more BU/mL, as previously observed.42
Although recovery from acute episodes typically appears to be complete, observations of long-term outcomes have documented both persistent problems, such as minor cognitive abnormalities43 and impaired health-related quality of life,44 as well as risk for relapse. Among the 47 survivors who had ADAMTS13 activity < 10% at the time of their initial diagnosis, no demographic, clinical, or laboratory features were associated with relapse other than male sex. Serial observations of the 16 patients who relapsed documented the inconsistency of measurement of ADAMTS13 inhibitors. Four of the 16 patients had no demonstrable inhibitor activity at the time of their initial diagnosis; 3 of these 4 patients had inhibitors at the time of a relapse. ADAMTS13 deficiency has been commonly observed during clinical remission,14,15,17,18 with suggestions that it predicts risk for relapse.15,17,18 Among our patients, ADAMTS13 activity and inhibitor levels during clinical remission were often inconsistent across time, as previously observed,18 and were not clearly related to subsequent relapses. Prospective studies on survivors of TTP with systematic ADAMTS13 monitoring are needed to determine whether ADAMTS13 deficiency predicts relapse and then to also determine whether any treatment intervention may be appropriate.
Most initial relapses among patients with ADAMTS13 activity < 10% at the time of their initial episode occurred within the first year after remission and the rate of relapse decreased with time. Seven of 16 patients who relapsed had only one relapse. The observed relapse rate was 34%, remarkably similar to the 2 initial reports of relapse after the introduction of PEX (36%16 and 37%3 ). The estimate of the cumulative risk for relapse at 7.5 years among patients with ADAMTS13 levels < 10% was 41%. However, analysis of the relapse rate may be confounded by increasing use of rituximab. Among the 6 surviving patients treated with rituximab for their initial episode, none has had a relapse with a median follow-up of 42 months, similar to a previous report.45 However, rituximab is not yet standard care for patients with TTP; the role of rituximab is being investigated in a current clinical trial (www.clinicaltrials.gov; #NCT00799773).46,47
In conclusion, mortality among patients diagnosed with TTP has not changed across the 20 years since the introduction of PEX. Complications of PEX accounted for at least 5 deaths, emphasizing the need for safer as well as more effective treatments. The availability of ADAMTS13 measurements has allowed the identification of patients who have a high risk for relapse.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant 32003B_124892) and the Hematology Research Fund of the University of Oklahoma Health Sciences Center.
Authorship
Contribution: J.A.K.H., S.K.V., B.L., and J.N.G. designed research, performed research, analyzed data, and wrote the paper; and D.R.T. designed research, performed research, and wrote the paper.
Conflict-of-interest disclosure: J.A.K.H., D.R.T., B.L., and J.N.G. serve as consultants for Baxter Inc for development of recombinant ADAMTS13; these authors declare no conflict with the topic or data of this manuscript. S.K.V. declares no competing financial interests.
Correspondence: Sara K. Vesely, Department of Biostatistics and Epidemiology, College of Public Health, University of Oklahoma Health Sciences Center, Room CHB 309, PO Box 26901, Oklahoma City, OK 73126-0901; e-mail: sara-vesely@ouhsc.edu.
Appendix
As shown in the table, ADAMTS13 activity was determined in citrated plasma (1 volume of 0.106M trisodium citrate per 9 volumes of whole blood) and serum (obtained from native blood left to clot completely before centrifugation) from samples withdrawn at the same time point from 13 patients with clinically diagnosed TTP (designated patients A to M) by the quantitative immunoblotting assay and the FRETS-VWF73 assay using our established methods.
References
Author notes
*J.A.K.H. and S.K.V. contributed equally to this manuscript.