To the editor:
Acute episodes of acquired autoimmune thrombotic thrombocytopenic purpura (TTP) are associated with severe ADAMTS13 deficiency, commonly defined as <10%.1 Patients may also have severe ADAMTS13 deficiency during remission after recovery, without thrombocytopenia, microangiopathic hemolytic anemia, or other clinical features of an acute episode.2,3 The occurrence of severe ADAMTS13 deficiency during remission may predict risk of relapse4,5 ; therefore, preemptive treatment with rituximab and other immunosuppressive modalities has been advocated.3,6,7
In 2004, the Oklahoma TTP Registry began annual remission evaluations. During each evaluation, a complete blood count was done, with prompt results, and samples were collected for subsequent measurement of ADAMTS13 activity by both fluorescence resonance energy transfer system (FRETS-VWF73) and immunoblotting (IB) methods in Bern, Switzerland. In this report, FRETS-VWF73 measurements are reported unless IB is specified. Results were not available for several months. This was consistent with our practice of not treating patients during remission if they were asymptomatic with no clinical features of TTP, including microangiopathic hemolytic anemia and thrombocytopenia, regardless of remission ADAMTS13 activity. Thus, we have observed the natural history of ADAMTS13 activity during remission and its association with relapse.
From 1995 through 2014, the Registry enrolled 357 patients with suspected TTP or hemolytic uremic syndrome.1,8 Eighty patients with ADAMTS13 activity <10% were diagnosed with their first episode of acquired TTP (cohort 1). Of these 80 patients, 67 survived their initial episode; however, 3 patients died before annual remission evaluations began. Of the remaining 64 patients, 57 (89%) have had remission evaluations and are described in 3 categories related to their remission ADAMTS13 activity.
Eleven additional patients were enrolled at the time of a relapse (cohort 2); their initial episode(s) had occurred before 1995 or outside the Registry region. Of these 11 patients, 1 died and 10 have had remission evaluations.
Seventeen (30%) of the 57 cohort 1 patients have had at least 1 remission ADAMTS13 activity <10% (median number of measurements per patient, 6). Four of these patients had relapsed before remission ADAMTS13 measurements began. ADAMTS13 activity was <10% in 44 (41%) of their 108 remission measurements; the range of all measurements was <10% to 100%. Eight (47%) of the 17 patients had only 1 measurement <10% (of 2-10 total remission measurements). In 7 of the 17 patients, ADAMTS13 activity spontaneously recovered to normal (60%-96%) during subsequent annual evaluations. Among all 44 measurements <10%, no functional inhibitor was demonstrable in 15 (34%); 19 (43%) had inhibitor titers of ≥2 BU/mL. The presence and strength of ADAMTS13 functional inhibitors were also inconsistent within individual patients.
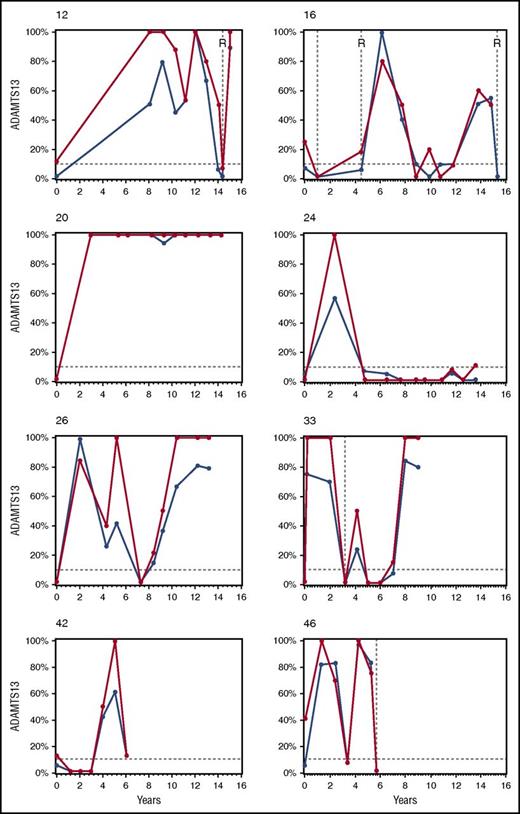
Ten (59%) of the 17 patients have relapsed after remission ADAMTS13 activity <10%. The Kaplan-Meier estimate of the median time to relapse after remission ADAMTS13 activity <10% for these 17 patients was 5.4 years (supplemental Figure 1, available on the Blood Web site). The time between the first observation of ADAMTS13 <10% and the first relapse ranged from 0.3 to 9.5 years. Two of these 10 patients had spontaneous recoveries of remission ADAMTS13 activities of 55% to 96% for 2 years before they relapsed (Figure 1; patients 16 and 46). Median follow-up for the 7 patients who have not relapsed after remission ADAMTS13 <10% was 5.8 years (range, 4.5-12.1 years).
ADAMTS13 activity during remission and relapse in 8 selected patients. These 8 patients were selected from the 57 patients in cohort 1 to illustrate the different patterns of ADAMTS13 activity during remission (2004-2015). At all points, ADAMTS13 activity was measured by both the FRETS-VWF73 (blue lines) and IB (red lines) methods. All ADAMTS13 activities for each patient, both during acute episodes and during relapse, are presented. The patients’ numbers refer to their number in supplemental Table 1. Each graph begins with the patients’ initial episode of TTP. Relapses are indicated by vertical broken lines. All relapses were confirmed by ADAMTS13 activity <10% with at least 1 of the 2 assays. Rituximab treatment of relapse episodes is indicated by R. None of these patients was treated with rituximab for their initial episode. The horizontal broken line indicates ADAMTS13 activity of 10%. The clinical features of each patient are described in the supplemental Legend for Text Figure 1. No treatment was given related to remission ADAMTS13 activity, and the frequency of remission ADAMTS13 measurements was not changed.
ADAMTS13 activity during remission and relapse in 8 selected patients. These 8 patients were selected from the 57 patients in cohort 1 to illustrate the different patterns of ADAMTS13 activity during remission (2004-2015). At all points, ADAMTS13 activity was measured by both the FRETS-VWF73 (blue lines) and IB (red lines) methods. All ADAMTS13 activities for each patient, both during acute episodes and during relapse, are presented. The patients’ numbers refer to their number in supplemental Table 1. Each graph begins with the patients’ initial episode of TTP. Relapses are indicated by vertical broken lines. All relapses were confirmed by ADAMTS13 activity <10% with at least 1 of the 2 assays. Rituximab treatment of relapse episodes is indicated by R. None of these patients was treated with rituximab for their initial episode. The horizontal broken line indicates ADAMTS13 activity of 10%. The clinical features of each patient are described in the supplemental Legend for Text Figure 1. No treatment was given related to remission ADAMTS13 activity, and the frequency of remission ADAMTS13 measurements was not changed.
In 24 (42%) of the 57 cohort 1 patients, remission ADAMTS13 activity was always normal (≥60%; median number of measurements per patient, 5). None of these 24 patients has relapsed since their initial remission ADAMTS13 measurement; 3 patients each had 1 relapse before remission ADAMTS13 activity measurements began.
In the remaining 16 (28%) patients, remission ADAMTS13 activity was not always normal but was never <10% (median number of measurements per patient, 4.5); the range of remission ADAMTS13 activity was 10% to 100%. Six of these patients had a total of 16 relapses before remission ADAMTS13 activity measurements began; 2 of these 6 patients and 3 additional patients relapsed after remission ADAMTS13 activity measurements. One patient was treated with maintenance rituximab for 1 year after his fourth relapse.
In cohort 2, 2 patients have had remission ADAMTS13 activities of <5% to 15% since 2006 without relapse. Another patient was treated with maintenance rituximab for 3 years after her fourth relapse.
Data for all cohort 1 and cohort 2 patients are described in supplemental Table 1, which presents all remission ADAMTS13 activity and functional inhibitor measurements, as well as occurrence of relapse and treatment with rituximab.
To determine the relationship between remission ADAMTS13 activity and relapse, we calculated the frequency of relapse per patient-years for cohort 1 patients (Table 1). For all 57 patients, there were 445 patient-years of observations. During these 445 patient-years, 18 patients had 27 relapses, equivalent to 6 relapses per 100 patient-years. During the 285 patient-years after a remission ADAMTS13 activity measurement, 9 patients had 10 relapses (Table 2). The interval between relapse and the preceding remission ADAMTS13 measurement was 1.6 to 50.7 weeks (median, 21 weeks). The preceding ADAMTS13 activities ranged from <5% (40 weeks preceding relapse) to 83% (20 weeks preceding relapse). The relative risk for relapse (hazard ratio)9 was significantly greater for patients with preceding remission ADAMTS13 activity <15% compared with ≥15% (hazard ratio, 4.01; P = .031) and also for patients with preceding remission ADAMTS13 activity <60% compared with ≥60% (hazard ratio, 6.95; P = .015). However, relapses occurred in all categories of preceding ADAMTS13 activity, including normal activity (≥60%). For comparisons at other remission ADAMTS13 activity levels, the hazard ratio for relapse was not significant.
Relapse incidence rates related to remission ADAMTS13 activity measurements within 1 year
| Patient-years with and without ADAMTS13 measurements . | Patient-years . | Relapses (patients), n . | Incidence of relapse per 100 patient-years (95% CI) . |
|---|---|---|---|
| All patient-years | 445.6 | 27 (18) | 6.06 (2.24-13.14) |
| Patient-years without ADAMTS13 measurement | 160.6 | 17 (13) | 10.59 (5.20-19.15) |
| Patient-years with ADAMTS13 measurement | 285.0 | 10 (9) | 3.51 (0.85-9.52) |
| Patient-years with and without ADAMTS13 measurements . | Patient-years . | Relapses (patients), n . | Incidence of relapse per 100 patient-years (95% CI) . |
|---|---|---|---|
| All patient-years | 445.6 | 27 (18) | 6.06 (2.24-13.14) |
| Patient-years without ADAMTS13 measurement | 160.6 | 17 (13) | 10.59 (5.20-19.15) |
| Patient-years with ADAMTS13 measurement | 285.0 | 10 (9) | 3.51 (0.85-9.52) |
Patient-years were calculated by assigning 12 months for each ADAMTS13 measurement during remission. For example, patient 11 (supplemental Table 1) contributed 6 patient-years. She contributed 1 patient-year to the ADAMTS13 <10% row, 5 patient-years to the ADAMTS13 activity ≥10% row, 3 patient-years to the ADAMTS13 <15% row, and 3 patient-years to the ADAMTS13 activity ≥15% row (see Table 2). She did not have measurements for 4 years; therefore, she also contributed 4 patient-years to the row “Patient-years without ADAMTS13 measurements.” Twenty-seven relapses occurred in the 57 cohort 1 patients (2004-2015). Ten relapses were preceded by an ADAMTS13 measurement within 12 months, and 17 were not.
Relapse incidence rates in 285 patient-years and hazard ratios after remission ADAMTS13 activity measurements within 1 year
| ADAMTS13 activity level, % . | Patient-years . | Relapses (patients), n . | Incidence of relapse per 100 patient-years (95% CI) . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|---|---|
| <10 | 40.1 | 3 (3) | 7.48 (3.12-15.07) | 2.11 (0.54-8.25) | .285 |
| ≥10 | 244.9 | 7 (6) | 2.86 (0.56-8.55) | ||
| <15 | 54.6 | 5 (5) | 9.16 (4.22-17.29) | 4.01 (1.14-14.11) | .031 |
| ≥15 | 230.4 | 5 (4) | 2.17 (0.30-7.49) | ||
| <20 | 61.4 | 5 (5) | 8.14 (3.55-15.95) | 3.51 (1.00-12.36) | .051 |
| ≥20 | 223.5 | 5 (4) | 2.24 (0.32-7.60) | ||
| <30 | 68.1 | 5 (5) | 7.34 (3.03-14.88) | 3.24 (0.92-11.42) | .067 |
| ≥30 | 216.9 | 5 (4) | 2.31 (0.34-7.70) | ||
| <50 | 87.9 | 6 (6) | 6.83 (2.71-14.19) | 3.38 (0.93-12.34) | .065 |
| ≥50 | 197.1 | 4 (4) | 2.03 (0.25-7.27) | ||
| <60 | 103.1 | 8 (8) | 7.76 (3.30-15.44) | 6.95 (1.45-33.28) | .015 |
| ≥60 | 181.9 | 2 (2) | 1.10 (0.04-5.74) |
| ADAMTS13 activity level, % . | Patient-years . | Relapses (patients), n . | Incidence of relapse per 100 patient-years (95% CI) . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|---|---|
| <10 | 40.1 | 3 (3) | 7.48 (3.12-15.07) | 2.11 (0.54-8.25) | .285 |
| ≥10 | 244.9 | 7 (6) | 2.86 (0.56-8.55) | ||
| <15 | 54.6 | 5 (5) | 9.16 (4.22-17.29) | 4.01 (1.14-14.11) | .031 |
| ≥15 | 230.4 | 5 (4) | 2.17 (0.30-7.49) | ||
| <20 | 61.4 | 5 (5) | 8.14 (3.55-15.95) | 3.51 (1.00-12.36) | .051 |
| ≥20 | 223.5 | 5 (4) | 2.24 (0.32-7.60) | ||
| <30 | 68.1 | 5 (5) | 7.34 (3.03-14.88) | 3.24 (0.92-11.42) | .067 |
| ≥30 | 216.9 | 5 (4) | 2.31 (0.34-7.70) | ||
| <50 | 87.9 | 6 (6) | 6.83 (2.71-14.19) | 3.38 (0.93-12.34) | .065 |
| ≥50 | 197.1 | 4 (4) | 2.03 (0.25-7.27) | ||
| <60 | 103.1 | 8 (8) | 7.76 (3.30-15.44) | 6.95 (1.45-33.28) | .015 |
| ≥60 | 181.9 | 2 (2) | 1.10 (0.04-5.74) |
Only the 10 relapses that were preceded by an ADAMTS13 measurement within 12 months were included in this analysis. These 10 relapses occurred in 9 patients. In 1 patient who had 2 relapses, 1 relapse occurred after ADAMTS13 activity of 34% and the other relapse occurred after ADAMTS13 activity of 64%; therefore, he is included in both rows for the <50%/≥50% analysis and the <60%/≥60% analysis, creating the appearance of 10 patients. Hazard ratios were calculated using a modified Cox regression analysis,9 a time-to-event analysis that allows TTP relapse as a recurring event related to the time-varying measurement of ADAMTS13 activity during remission. This model also accounts for the competing events of death unrelated to an acute TTP episode. Due to ADAMTS13 activity being treated as a time-varying covariate, the assumption of proportional hazards is not relevant because the modification of the Cox regression model uses the partial likelihood estimation method.9
Other morbidities that may occur after recovery from TTP (hypertension, chronic kidney disease, depression, minor cognitive impairment, and death)10,11 did not appear to be related to remission ADAMTS13 activity (supplemental Table 2).
Our experience documents that the occurrence of ADAMTS13 deficiency during remission is associated with relapse, but the clinical courses of individual patients are unpredictable. Figure 1 illustrates the variability of remission ADAMTS13 activity within individual patients. A remarkable observation is that remission ADAMTS13 activity can spontaneously change from normal to <10% and back to normal across several years, with no apparent clinical signs of TTP.
Our observations are consistent with previous observations that patients with acquired4,5 or hereditary12,13 TTP may have prolonged periods of severe ADAMTS13 deficiency without an acute TTP episode. These observations support the current concepts that severe ADAMTS13 deficiency may be necessary for the occurrence of acute episodes of TTP, but severe ADAMTS13 deficiency alone may not be sufficient to trigger the occurrence of an acute episode. Additional conditions associated with inflammation can contribute to acute episodes in patients with acquired or hereditary TTP.14-16
The limitations of our analysis are the small number of patients, the small number of relapses that occurred during our follow-up, and that we only measured remission ADAMTS13 activity annually. However, even this small number of patients followed across many years revealed clinically important observations. Remission ADAMTS13 activity can spontaneously and repeatedly vary between <10% and normal. ADAMTS13 deficiency during remission, even intermittently, was associated with relapse. However, whether a relapse will occur after remission ADAMTS13 deficiency, or when it may occur, was not predictable.
Further studies are required to understand whether monitoring ADAMTS13 activity during remission may improve management of patients with TTP. Currently, we continue our practice of only measuring remission ADAMTS13 activity for research, not for management decisions. We continue our practice of treating patients only when clinical features of TTP (microangiopathic hemolytic anemia and thrombocytopenia) are present. We recognize that management of patients during remission involves critical decisions that require careful consideration of the values and preferences of both patients and their physicians.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The project was supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (U54GM104938), the Hematology Research Fund of the University of Oklahoma Health Sciences Center, and a Swiss National Research Foundation grant (310030-160269).
Contribution: E.E.P. organized and analyzed the data, created the figures, and reviewed the manuscript; J.A.K.H. supervised and analyzed ADAMTS13 measurements and reviewed the manuscript; D.R.T. organized the Registry protocols, maintained the institutional review board approvals, supervised the remission evaluations, and reviewed the manuscript; S.K.V. organized the Registry protocols, supervised the data analysis and interpretation, and reviewed the manuscript; and J.N.G. managed the patients, organized the data, assisted with analysis and interpretation, and wrote the manuscript.
Conflict-of-interest disclosure: E.E.P., S.K.V., and J.N.G. are supported by a contract with Ablynx to provide data from the Oklahoma TTP Registry for background information about TTP related to the development of caplacizumab. J.A.K.H. is a consultant and serves on the advisory boards of Ablynx for the development of caplacizumab and Baxalta for the development of recombinant ADAMTS13. D.R.T. declares no competing financial interests.
Correspondence: James N. George, College of Public Health, Room CHB 237, The University of Oklahoma Health Sciences Center, P.O. Box 26901, Oklahoma City, OK 73126-0901; e-mail: james-george@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal