Abstract
Immune thrombocytopenia (ITP) is a bleeding disorder characterized by antibody-opsonized platelets being prematurely destroyed in the spleen, although some patients with ITP may have a cell-mediated form of thrombocytopenia. Although several animal models of ITP have been developed, few mimic primary chronic ITP nor have any shown cell-mediated platelet destruction. To create this type of model, splenocytes from CD61 knockout mice immunized against CD61+ platelets were transferred into severe combined immunodeficient (SCID) (CD61+) mouse recipients, and their platelet counts and phenotypes were observed. As few as 5 × 104 splenocytes induced a significant thrombocytopenia and bleeding mortality (80%) in recipients within 3 weeks after transfer. Depletion of lymphocyte subsets before transfer showed that the splenocyte's ability to induce thrombocytopenia and bleeding completely depended on CD4+ T helper cells and that both CD19+ B cell (antibody)– and CD8+ T cell (cell)–mediated effector mechanisms were responsible. Treatment of the SCID mouse recipients with intravenous γ-globulins raised platelet counts and completely prevented bleeding mortality induced by antibody-mediated effector mechanisms but did not affect cell-mediated disease. This novel model not only shows both antibody- and cell-mediated ITP and bleeding but also suggests that these 2 effector mechanisms have a differential response to therapy.
Introduction
Immune thrombocytopenia (ITP), one of the most common hematologic autoimmune bleeding disorders, is characterized by premature platelet clearance by Fcγ receptor (FcγR)–mediated phagocytosis in the reticuloendothelial system.1-8 ITP was distinguished by 2 forms, termed acute and chronic, but an international group of recognized experts has significantly revised the definitions of and recommendations for the clinical diagnosis of ITP.8 Primary ITP is now defined as an autoimmune disorder characterized by isolated thrombocytopenia (peripheral blood platelet count < 100 × 109/L) in the absence of other causes or disorders that may be associated with thrombocytopenia.8 The various phases of ITP are defined by the time since diagnosis; newly diagnosed ITP occurs within 3 months from diagnosis, whereas persistent ITP is between 3 and 12 months from diagnosis, and chronic ITP is now defined as thrombocytopenia lasting for more than 12 months.8 The classification of severe ITP is now reserved for those patients in whom there is the presence of bleeding symptoms at presentation or the occurrence of new bleeding symptoms requiring therapeutic intervention.8 First-line treatments for patients with chronic ITP include steroids and intravenous γ-globulins (IVIgs), and previous studies have shown that IVIg can also protect against passive ITP in mice.9-12
At least 70% of patients with chronic ITP have identifiable serum autoantibodies that are composed primarily of IgG1 and IgG3 isotypes and generally target platelet glycoproteins (GPs) IIb/IIIa and/or GPIbIX.3-5,13,14 However, some patients with ITP have no detectible antiplatelet autoantibodies yet are thrombocytopenic, and it has been shown that, in these patients, cell-mediated immune mechanisms such as CD8+ T-cell cytotoxicity may lead to the thrombocytopenia.15,16 At present, however, it is unknown whether cell-mediated platelet destruction contributes to the severity of disease or the difficulty of treatment in some patients with ITP. Thus, an animal model that mimics chronic ITP pathophysiology would be desirable.
There have been several animal models of ITP, including the secondary autoimmune model, in which ITP is secondary to either other diseases (eg, lupus nephritis17-20 ) or infections,21-23 and the passive transfer model, in which continuous injection of platelet-specific antibodies are required to maintain a steady state of thrombocytopenia.9-12 Although these models have been instrumental in understanding the pathophysiology of secondary ITP and the mechanisms of action of treatments such as IVIg, they are not ideal for chronic ITP pathophysiology where both T-cell and B-cell autoimmune attacks are focused on platelet-specific antigens. Recently, however, a platelet-specific immune model of neonatal alloimmune thrombocytopenia was developed with the use of immune CD61 knockout (KO) mice24 that were pregnant with wild-type (WT) CD61+ fetuses. Fetal death because of bleeding occurred, and in successful pregnancies the neonatal pups were thrombocytopenic and exhibited a bleeding diathesis.24 In this report, we have created a novel murine model of severe chronic ITP that shows both antibody-mediated and cell-mediated platelet and megakaryocyte destruction and show that, although antibody-mediated thrombocytopenia is responsive to IVIg treatment, cell-mediated disease is not. This model may be applicable for understanding the immune nature of severe ITP, and the lack of response of cell-mediated thrombocytopenia and bleeding to IVIg therapy may suggest that other therapeutic strategies may be needed to treat this form of ITP.
Methods
Mice
Female BALB/c (H-2d), C57BL/6 (H-2b) mice and CB.17 (H-2d) severe combined immunodeficient (SCID) mice, 8 to 12 weeks of age, were used as either platelet donors or spleen-cell transfer recipients and were obtained from Charles River Laboratories. BALB/c CD61 KO mice were bred in the laboratory of H.N. and originally obtained from Dr Richard O. Hynes (Massachusetts Institute of Technology). All animal studies were approved by the St Michael's Hospital Animal Care Committee (protocol no. 942).
Platelet preparation and immunization of CD61 KO mice
For platelet preparation, the indicated donor mice were anesthetized, and blood was drawn by cardiac puncture into phosphate-buffered saline (PBS) containing citrate/phosphate, dextrose, acetate buffer. The blood was pooled, diluted with PBS, and centrifuged at 150g for 15 minutes, and the platelet-rich plasma was collected. The platelet-rich plasma was washed 3 times by centrifuging at 450g for 18 minutes, and the washed platelets were resuspended in PBS and counted and their concentration adjusted to 109 cells/mL.
For immunization, BALB/c CD61 KO mice were transfused with 100 μL of 108 platelets from WT BALB/c mice weekly for 3 weeks. Sera from these immune KO mice have been previously shown to induce a profound thrombocytopenia when injected into naive mice.24,25 To produce third-party immune control animals, BALB/c mice were transfused as above with platelets from major histocompatibility complex allogeneic C57BL/6 mice. Immunization was monitored by measuring IgG antiplatelet antibody production in the sera by flow cytometry.
Splenocyte preparations and cell depletion
The antiplatelet immune CD61 KO mice were killed, and their spleens were removed and prepared into a splenocyte suspension by crushing and straining through a mesh filter in PBS. The suspension was washed 3 times by centrifugation at 400g for 15 minutes. The splenocytes were then adjusted in cell medium (PBS + 2% fetal bovine serum with 1mM ethylenediaminetetraacetic acid [EDTA]) to a concentration of 108 cells/mL; titrations were made and transferred to SCID mice to determine the optimal dose for inducing thrombocytopenia (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Engraftment of 105 immunized splenocytes caused 67% death in mice by day 15 after transfer, whereas engraftment of 104 splenocytes had little effect on the rate of mortality of the SCID mice (supplemental Figure 1). An intermediate dose of 5 × 104 cells was chosen for all experiments so that the engrafted SCID mice would exhibit symptoms of thrombocytopenia and survive at least 2 weeks after irradiation.
In the indicated cell-depletion studies, splenocytes (108 cells/mL cell medium) were first depleted of either CD4+, CD8+, and/or CD19+ lymphocytes before transfer by the procedures and reagents provided by the EasySep Magnetic cell sorting kit (StemCell Technologies; CD4+ kit catalog no. 18752, CD8+ kit catalog no. 18753, and CD19+ kit catalog no. 18754). Briefly, cells were coated with the kit-specific CD antibody reagent, the tube was inserted into a magnet, and the negatively selected nonbound cells were collected by decanting, counted, and resuspended with RPMI-5% heat-inactivated fetal bovine serum for engraftment. Depletion efficiencies were determined by flow cytometry (supplemental Figure 2) and were greater than 96% for the specific lymphocyte subpopulations removed.
Splenocyte transfer and platelet counts
SCID mice were bled before the experiments, and sera were screened for the presence of murine IgG by an enzyme-linked immunoabsorbent assay as previously described.26 Any mouse with a serum IgG concentration greater than 15 μg/mL was deemed “leaky” and killed.
On the day before splenocyte transfer, screened SCID mice were injected intraperitoneally with 50 μL of rabbit anti-asialo GM1 (Wako Pure Chemical Industries Ltd) to primarily remove natural killer cells, and on the day of transfer the SCID mice were subjected to 200 cGy total body γ irradiation to inhibit recipient innate immune responses and to enhance engraftment. Within 3 hours of irradiation, mice were bled from the saphenous vein for prebleed platelet counts and then injected intraperitoneally with 100 μL of the indicated splenocyte preparations (at 5 × 104 cells/mL final). Platelet counts and phenotypes were then recorded weekly. For platelet counts, blood was diluted 1/100 in PBS:citrate/phosphate, dextrose, acetate buffer, and the platelet count was measured by a Beckman Coulter Counter-LH750 hematology analyzer.
IVIg treatment
SCID mice were weighed and IVIg (10% wt/vol; Gamunex; Talecris Biotherapeutics) was administered intraperitoneally at a dose of 2 g/kg 1 day before splenocyte transfer and twice weekly thereafter to ensure that a relatively constant in vivo level was maintained. This dose was chosen because of its common use clinically in patients with ITP.1,2,6-8 Control human serum albumin (25% wt/vol; Baxter Healthcare Corporation) was diluted to a final concentration of 10% (wt/vol) with sterile PBS (pH 7.4) and administered intraperitoneally at a dose of 2 g/kg similar to IVIg. Although the albumin dose has a greater oncotic load than does IVIg, it did not adversely affect the mice.
Flow cytometry to detect serum platelet CD61-specific antibodies
To determine the levels of platelet-specific antibodies in the engrafted SCID mice, platelets were prepared from BALB/c mice, and 5 × 106 platelets were incubated with 4 μL of undiluted serum from the SCID mouse recipients for 30 minutes at room temperature. The platelet suspension was washed once with PBS, and 100 μL of a FITC-conjugated goat anti–mouse IgG (Invitrogen; 1/200 final) was added and incubated in the dark for 30 minutes at room temperature. The suspension was washed and then analyzed by flow cytometry.
Bone marrow histology
Femurs were dissected from killed mice, their ends were cut off, and the long bones were placed in fixative (B+ Fixative; BBC Biochemicals) for 12 hours. Decalcification was achieved with 10% nitric acid, followed by embedding in wax and staining with hematoxylin and eosin (H&E). To concentrate megakaryocytes in the bone marrows from selected mice, the long bones were prepared as above, and bone marrow cells were flushed from the lumen in PBS. The cells were collected and concentrated onto glass microscope slides with the use of a Shandon Cytospin 2 Centrifuge (Block Scientific). Slides were fixed and stained with H&E stain.
Statistical analysis
Data are expressed as mean plus or minus SEM and were analyzed with the Student t test. P values less than .05 were considered significant.
Results
Induction of severe ITP
When irradiated CD61+ SCID control mice received either no transfer or splenocytes from BALB/c mice immunized against a third-party platelet antigen (H-2b major histocompatibility complex class I from C57BL/6 platelets), an irradiation-induced thrombocytopenia occurred at day 7 but recovered in all mice by day 14 (Figure 1), and no mortality was observed. In contrast, CD61+ SCID mice engrafted with 5 × 104 splenocytes from CD61 KO mice immunized against CD61+ platelets exhibited a profound thrombocytopenia, which did not recover (Figure 2A), and there was an 80% bleeding mortality within 21 days after transfer (Figure 3A). Bleeding diathesis occurred in the intestines, lungs, abdomen, subcutaneous tissues, and brain (supplemental Figure 3), and veterinary necropsy confirmed that the morbid mice had hyperplasia of splenic red pulp associated with anemia, thrombocytopenia, and megakaryocytopenia. Histologic sectioning of the bone marrows between healthy and morbid mice indicated that the latter had virtually no identifiable megakaryocytes (Figure 4) and that those megakaryocytes that were found after cytospin concentration appeared to have irregular, enlarged nuclei, membrane blebbing, and cytoplasmic abnormalities consistent with apoptosis (Figure 4). Determination of serum antibody levels also showed that the transferred SCID mice had significant levels of IgG antiplatelet CD61-specific antibodies by the second week after transfer (Figure 5).
Control SCID mice recover from radiation-induced thrombocytopenia. Platelet counts in control irradiated SCID mice transferred with either nothing (□; n = 9) or 5 × 104 splenocytes from BALB/c mice immunized against third-party antigenic platelets from C57BL/6 mice (○; n = 15). Thrombocytopenia at day 7 occurred in all mice irradiated (asialo GM-1 treatment had no effect on platelet counts). The data are expressed as platelet counts (× 109/L) ± SEM over time (days). The solid horizontal line represents the normal mean platelet count ± SEM (hatched lines) from 149 healthy SCID mice. The star refers to statistical significance (P < .001) between the observed platelet count for all control mice and the normal mean platelet count.
Control SCID mice recover from radiation-induced thrombocytopenia. Platelet counts in control irradiated SCID mice transferred with either nothing (□; n = 9) or 5 × 104 splenocytes from BALB/c mice immunized against third-party antigenic platelets from C57BL/6 mice (○; n = 15). Thrombocytopenia at day 7 occurred in all mice irradiated (asialo GM-1 treatment had no effect on platelet counts). The data are expressed as platelet counts (× 109/L) ± SEM over time (days). The solid horizontal line represents the normal mean platelet count ± SEM (hatched lines) from 149 healthy SCID mice. The star refers to statistical significance (P < .001) between the observed platelet count for all control mice and the normal mean platelet count.
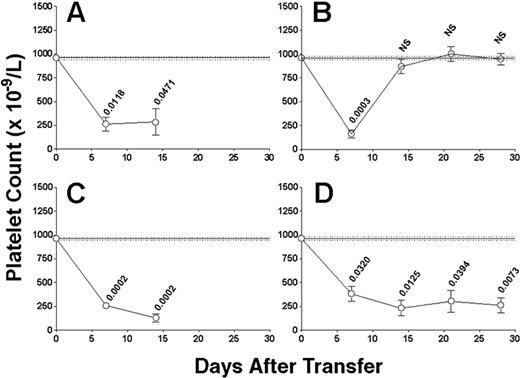
Evidence of both antibody- and cell-mediated thrombocytopenia. Platelet counts in irradiated SCID mice transferred with (A) 5 × 104 nondepleted splenocytes from CD61 KO mice immunized against WT BALB/c platelets (n = 15) or 5 × 104 splenocytes depleted of either (B) CD4+ T cells (n = 15), (C) CD8+ T cells (n = 18), or (D) CD19+ B cells (n = 14). Data are expressed as in Figure 1. (A,C) Platelet counts could not be determined past day 14 after transfer because of the poor health of the surviving mice. The numbers above the data points are the P values between the observed platelet counts for all mice and the normal mean platelet count. NS indicates not significant.
Evidence of both antibody- and cell-mediated thrombocytopenia. Platelet counts in irradiated SCID mice transferred with (A) 5 × 104 nondepleted splenocytes from CD61 KO mice immunized against WT BALB/c platelets (n = 15) or 5 × 104 splenocytes depleted of either (B) CD4+ T cells (n = 15), (C) CD8+ T cells (n = 18), or (D) CD19+ B cells (n = 14). Data are expressed as in Figure 1. (A,C) Platelet counts could not be determined past day 14 after transfer because of the poor health of the surviving mice. The numbers above the data points are the P values between the observed platelet counts for all mice and the normal mean platelet count. NS indicates not significant.
Bleeding mortality associated with cell-mediated thrombocytopenia is weaker than that of antibody-mediated thrombocytopenia. Kaplan-Meier survival plots of irradiated SCID mice transferred with (A) 5 × 104 nondepleted immune splenocytes from CD61 KO mice immunized against BALB/c platelets (n = 15) or 5 × 104 immune splenocytes depleted of either (B) CD4+ T cells (n = 15), (C) CD8+ T cells (n = 18), or (D) CD19+ B cells (n = 14). The data are expressed as the percentage of survival and calculated from the mouse groups in Figure 2.
Bleeding mortality associated with cell-mediated thrombocytopenia is weaker than that of antibody-mediated thrombocytopenia. Kaplan-Meier survival plots of irradiated SCID mice transferred with (A) 5 × 104 nondepleted immune splenocytes from CD61 KO mice immunized against BALB/c platelets (n = 15) or 5 × 104 immune splenocytes depleted of either (B) CD4+ T cells (n = 15), (C) CD8+ T cells (n = 18), or (D) CD19+ B cells (n = 14). The data are expressed as the percentage of survival and calculated from the mouse groups in Figure 2.
Megakaryocytes are depleted and abnormal in ITP SCID mice. Histologic section of (A) healthy bone marrow and (B) bone marrow from a morbid mouse engrafted with 5 × 104 nondepleted splenocytes (40× magnification; numerical aperture, plan apo; objective lens, 0.75). (A) The yellow arrows point to megakaryocytes, and (A-B) each scale bar represents 50 μm. Because of the paucity of megakaryocytes in SCID mouse ITP bone marrows, the marrows were prepared by a cytospin method to concentrate them on a slide and stained with H&E. (C) An example of a megakaryocyte from the bone marrow of a healthy SCID mouse. (D) H&E staining of a rare megakaryocyte found in the bone marrow of a SCID mouse with ITP (60× magnification; numerical aperture, plan apo; objective lens, 1.4). (C,D) Each scale bar represents 10 μm. All pictures were taken with Nikon Eclipse E800 upright microscope.
Megakaryocytes are depleted and abnormal in ITP SCID mice. Histologic section of (A) healthy bone marrow and (B) bone marrow from a morbid mouse engrafted with 5 × 104 nondepleted splenocytes (40× magnification; numerical aperture, plan apo; objective lens, 0.75). (A) The yellow arrows point to megakaryocytes, and (A-B) each scale bar represents 50 μm. Because of the paucity of megakaryocytes in SCID mouse ITP bone marrows, the marrows were prepared by a cytospin method to concentrate them on a slide and stained with H&E. (C) An example of a megakaryocyte from the bone marrow of a healthy SCID mouse. (D) H&E staining of a rare megakaryocyte found in the bone marrow of a SCID mouse with ITP (60× magnification; numerical aperture, plan apo; objective lens, 1.4). (C,D) Each scale bar represents 10 μm. All pictures were taken with Nikon Eclipse E800 upright microscope.
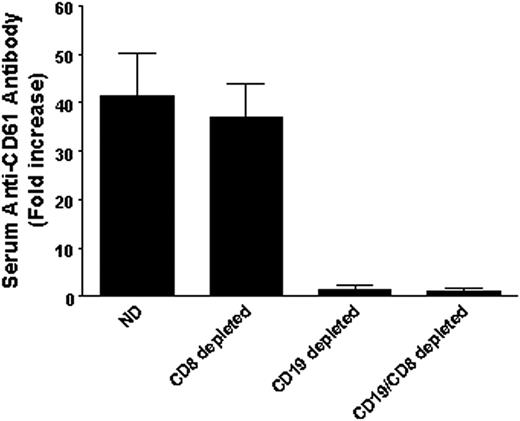
Antiplatelet CD61 antibodies in the sera of transferred SCID mice. Anti–CD61-specific antibody production in the sera of SCID mice transferred with either nondepleted (ND) splenocytes (n = 23), CD8+ T cell–depleted splenocytes (n = 16), CD19+ B cell–depleted splenocytes (n = 7), or CD19 and CD8 double-depleted splenocytes (n = 5). Platelets from either BALB/c mice or CD61 KO mice were incubated with sera from the indicated groups of SCID mice and analyzed by flow cytometry. The data are expressed as fold increase and were calculated by mean channel fluorescence (MCF) of test serum/MCF of naive serum. All the positive sera were not reactive against platelets from CD61 KO mice.
Antiplatelet CD61 antibodies in the sera of transferred SCID mice. Anti–CD61-specific antibody production in the sera of SCID mice transferred with either nondepleted (ND) splenocytes (n = 23), CD8+ T cell–depleted splenocytes (n = 16), CD19+ B cell–depleted splenocytes (n = 7), or CD19 and CD8 double-depleted splenocytes (n = 5). Platelets from either BALB/c mice or CD61 KO mice were incubated with sera from the indicated groups of SCID mice and analyzed by flow cytometry. The data are expressed as fold increase and were calculated by mean channel fluorescence (MCF) of test serum/MCF of naive serum. All the positive sera were not reactive against platelets from CD61 KO mice.
Induction of severe ITP depends on T helper cells and induced by both antibody- and cell-mediated effector cell responses
To determine the nature of the adaptive immune responses that may be responsible for the thrombocytopenia and bleeding, the splenocytes were first depleted of lymphocyte populations before transfer. In contrast with SCID mice transferred with nondepleted splenocytes (Figures 2A and 3A), depletion of CD4+ T cells completely abolished their ability to induce thrombocytopenia or bleeding mortality (Figures 2B and 3B, respectively) or to produce serum antiplatelet antibodies on transfer (Figure 5).
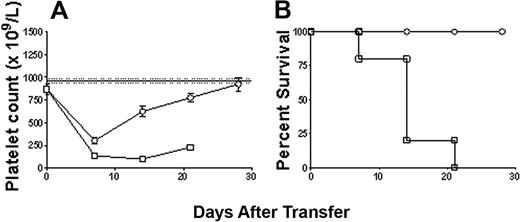
Further examination of the immune effector cell responses that may be responsible for the effects showed that depletion of CD8+ T cells did not affect the transferred splenocyte's ability to induce the development of antiplatelet antibodies (Figure 5), thrombocytopenia, or bleeding mortality (Figures 2C and 3C, respectively). However, as expected, depletion of CD19+ B cells significantly inhibited the transfer's ability to generate antiplatelet CD61 antibody production in the SCID mouse recipients (Figure 5); however, it still induced a significant thrombocytopenia (Figure 2D) and bleeding mortality (Figure 3D), suggesting the presence of a cell-mediated effector mechanism. Characterization of this cell-mediated thrombocytopenia and bleeding by performing double depletions of both CD19+ B cells and CD8+ T cells confirmed the presence of CD8+ T effector cells (Figure 6); like controls, the double-depletion group completely recovered from the irradiation-induced thrombocytopenia, and no antiplatelet antibody production (Figure 5) or bleeding mortality (Figure 6) was observed.
Cell-mediated thrombocytopenia and bleeding are specific to CD8+ T cell lymphocytes. (A) Platelet counts in irradiated SCID mice transferred with either 5 × 104 nondepleted immune spleen cells (n = 9; □) or 5 × 104 immunized spleen cells depleted of both CD8+ T cells and CD19+ B cells (n = 8; ○). (B) Kaplan-Meier survival plot of each group shown in panel A.
Cell-mediated thrombocytopenia and bleeding are specific to CD8+ T cell lymphocytes. (A) Platelet counts in irradiated SCID mice transferred with either 5 × 104 nondepleted immune spleen cells (n = 9; □) or 5 × 104 immunized spleen cells depleted of both CD8+ T cells and CD19+ B cells (n = 8; ○). (B) Kaplan-Meier survival plot of each group shown in panel A.
IVIg therapy has a differential effect on the 2 effector responses
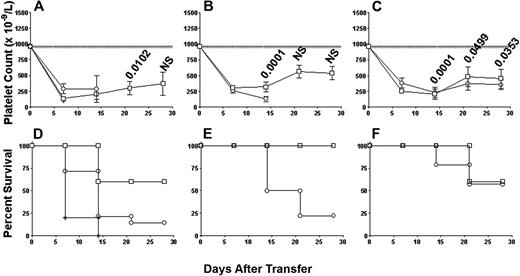
To determine the effect of a common first-line therapy clinically used in patients with ITP,1,2,6,8 2 g/kg IVIg was administered to the SCID mice the day before transfer and twice weekly. When nondepleted splenocytes were transferred into either nontreated or bovine serum albumin–treated SCID mice, significant thrombocytopenia and bleeding mortality occurred (Figures 7A and 7D, respectively). In contrast, treatment of the transferred SCID mice with 2 g/kg IVIg raised platelet counts (Figure 7A) and significantly reduced the bleeding mortality from 80% to 40% (Figure 7D). The IVIg efficacy could be completely separated if the SCID mice received splenocytes depleted of either CD8+ or CD19+ lymphocytes. IVIg treatment significantly prevented thrombocytopenia (Figure 7B) and completely reversed the bleeding mortality (Figure 7E) in the SCID mice transferred with antibody-mediated effectors (CD8+ T cell–depleted splenocytes; Figure 7C) but not in those mice transferred with cell-mediated effectors (CD19+ B cell–depleted splenocytes; Figure 7F).
IVIg treatment is effective against antibody-mediated ITP but not cell-mediated ITP. Platelet counts in irradiated SCID mice transferred with (A) 5 × 104 nondepleted immune splenocytes from CD61 KO mice immunized against BALB/c platelets (n = 15; ○) or 5 × 104 immune splenocytes depleted of either (B) CD8+ T cells (n = 18; ○) or (C) CD19+ B cells (n = 14; ○). Platelet counts in the SCID mice depletion groups treated with 2 g/kg IVIg (□) are also shown. Data are expressed as in Figure 1. The numbers above the data points are the P values calculated for the IVIg treatment groups as in Figure 1. NS indicates not significant. Kaplan-Meier survival plots for each group in panels A through C are shown in panels D through F. (D) The survival plot for control SCID mice that received human serum albumin (2 g/kg; *) is also shown.
IVIg treatment is effective against antibody-mediated ITP but not cell-mediated ITP. Platelet counts in irradiated SCID mice transferred with (A) 5 × 104 nondepleted immune splenocytes from CD61 KO mice immunized against BALB/c platelets (n = 15; ○) or 5 × 104 immune splenocytes depleted of either (B) CD8+ T cells (n = 18; ○) or (C) CD19+ B cells (n = 14; ○). Platelet counts in the SCID mice depletion groups treated with 2 g/kg IVIg (□) are also shown. Data are expressed as in Figure 1. The numbers above the data points are the P values calculated for the IVIg treatment groups as in Figure 1. NS indicates not significant. Kaplan-Meier survival plots for each group in panels A through C are shown in panels D through F. (D) The survival plot for control SCID mice that received human serum albumin (2 g/kg; *) is also shown.
Discussion
Severe chronic ITP is a diagnosis reserved for those patients with ITP who have bleeding symptoms either at presentation or with the occurrence of new bleeding symptoms requiring therapeutic intervention,8 and, over the past several years, information about the cellular immune pathophysiology of this disorder has been accumulating. For example, it is well established from studies in patients with chronic ITP that the disorder is associated with several T-cell abnormalities, including abnormally activated T helper cells with a T helper type 1 cytokine bias, a deficiency of T regulatory cells that lead to autoantibody production, and, in some patients, thrombocytopenia mediated by CD8+ T cells.27-43 To date, however, there are no known animal models that mimic these T-cell abnormalities or show the severe form of chronic ITP with active bleeding. We report here the development of such a murine model that shows both antibody (CD8 T cell–depleted)– and cell (CD19-depleted)–mediated effector responses that lead to thrombocytopenia and bleeding mortality. Of interest, the antibody-mediated bleeding mortality was significantly reduced by IVIg treatment, but the cell-mediated bleeding mortality was not. This model may offer several advantages in that it not only shows 2 major immunopathologic mechanisms of platelet destruction, but it will be amenable to further elucidating the T cell–associated immunopathogenesis of the disorder and for testing novel therapeutics for severe chronic ITP.
Both the B-cell and CD8+ T-cell effector mechanisms of thrombocytopenia and bleeding completely depended on the presence of CD4+ T helper cells (Figure 2B), suggesting that they are induced by an adaptive T helper cell immune response against platelet GPIIIa (CD61). These data appear to support previous observations in humans, showing that T helper cell abnormalities are responsible for the initiation and perpetuation of the immune effector mechanisms responsible for the human disease.27-43 In addition, it suggests that the CD4+ T cells can be examined with relation to how a relatively platelet-specific antigen (CD61) initiates their activation, which has relevance to understanding and developing platelet-derived peptide-based therapies for reducing immune responses against platelets. These types of therapies have been proposed for patients with chronic ITP and those patients with human platelet antigen–specific alloantibodies.44,45 Taken together, the complete dependence of thrombocytopenia and bleeding mortality on CD4+ T cells may, at least, mimic the pathophysiologic processes that lead to bleeding in those patients with severe ITP.
Of perhaps greater interest, this model may help to understand the immunopathophysiology of chronic ITP, particularly with respect to why some patients with ITP bleed and others with similar platelet counts do not. For example, immune thrombocytopenic mice display significant bleeding when they are subjected to various different types of inflammatory conditions, for example, contact dermatitis, stroke, or endotoxin-induced pulmonary inflammation.46 In addition, when some patients with ITP become infected, they can have precipitous drops in their platelet counts and increased bleeding symptoms. It is possible that the proinflammatory nature of the adaptive antiplatelet immune response in the SCID mice enhances the propensity to bleed. For example, when the transferred immune splenocytes first encounter CD61+ platelets in the SCID mice, they may promote proinflammatory cytokine cascades that, together with the ensuing ITP, enhances the bleeding diatheses. We are currently studying this.
Perhaps the most astonishing observation was that most SCID mice receiving immune KO splenocytes have a significant bleeding mortality which is not observed in most patients with chronic ITP. In addition, the level of thrombocytopenia in the recipient mice does not necessarily account for the increased bleeding because it is known that mice rendered thrombocytopenic by, for example, passive transfer of antiplatelet antibodies, do not bleed excessively.9-12,47 The excessive bleeding in our model suggests that additional immune factors are involved. For example, the IgG anti-CD61 antibody response in immune CD61 KO mice was extremely strong (titers > 1:12 000) and contains antibodies with multiple CD61 epitope specificities24,25 that not only induce FcR-mediated phagocytosis but also significantly inhibit platelet function.48 Thus, SCID mouse recipients that contain these antiplatelet antibodies are virtually devoid of platelet function, and this may be an important reason why they bleed excessively. Alternatively, the higher bleeding mortality observed in relation to the antibody-mediated platelet destruction may be also partially explained by the observations that CD61 can be expressed on endothelial cells during proinflammatory conditions.49 It is possible that a proinflammatory immune response developing in a SCID mouse recipient may activate endothelial cells, allowing the anti-CD61 antibodies to bind and perhaps disrupt or interfere with hemostatic mechanisms.49 Nonetheless, this model may at least mimic the bleeding observed in patients with severe ITP and may be useful in understanding why some patients with ITP go on to develop bleeding.
Compared with the severe antibody-mediated bleeding, the cell-mediated platelet destruction was associated with a lower bleeding mortality (Figure 3). The reasons for this are unclear but may be due to several reasons related to the nature of immune destruction mediated by autoantibodies compared with cell-mediated mechanism. Activated CD8+ T cells kill target cells directly by 2 different pathways; the main pathway is mediated by perforin and a series of serine proteases (granzymes), and the second minor pathway is by the Fas-Fas ligand interactions; ligands bind to the Fas receptors of target cells to induce apoptosis.50 H&E staining of the few megakaryocytes found by concentrating the bone marrows from morbid mice showed abnormalities indicative of apoptosis (Figure 4), which is, at least, consistent with cytotoxic T cell–mediated damage. In contrast, antibody-mediated immune destruction in ITP is primarily mediated by FcR-mediated phagocytosis within the spleen. Perhaps FcR-mediated phagocytosis is more efficient in platelet clearance and megakaryocyte destruction leading to bleeding compared with CD8+ T cell–mediated mechanisms. For example, platelet autoantibodies in patients with ITP have been shown to bind to and either destroy or inhibit megakaryocytes, which decreases platelet production.51 Histologic sections of the bone marrow of morbid mice showed almost a complete lack of megakaryocytes in comparison to healthy SCID mice (Figure 4). Perhaps cell-mediated platelet destruction together with an inability to generate new platelets may also predispose the SCID mouse to bleeding but to a lesser extent than the antibody-mediated form of ITP.
IVIg treatment of the SCID mice transferred with nondepleted splenocytes did raise the platelet counts, but it appeared not to be a complete response back to normal platelet numbers (Figure 7). In addition, IVIg treatment significantly reduced the bleeding mortality from 80% to 40% (Figure 7). These observations, particularly with regard to the IVIg therapeutic platelet response, do not appear to correlate to the significant platelet responses observed in most patients with ITP treated with IVIg. However, when the antibody-mediated disease was isolated from the cell-mediated effects by CD8+ T-cell depletions, IVIg treatment significantly elevated the platelet counts to normal levels and completely protected the mice from bleeding mortality. IVIg responses were not observed in those mice exhibiting only cell-mediated disease. It is possible that the combination of both forms of immune effector mechanisms in the nondepleted transfers is so robust that IVIg cannot counteract all the effects, and only when the 2 mechanisms are isolated, for example, by cell depletions, can the full IVIg response be seen. Alternatively, it has been shown in passive mouse models of ITP that antibodies with different platelet specificities may affect the efficiency of IVIG to alleviate thrombocytopenia.25 For example, IVIG was more effective in ameliorating thrombocytopenia caused by antiplatelet GPIIbIIIa antibody than anti-GPIbα antibody.25 It is thought that anti-GPIbα may cause thrombocytopenia through a different mechanism (Fc-independent pathway) from anti-GPIIbIIIa (Fc-dependent pathway).9,25 Perhaps related to the latter points, human IgG was used in the mouse model, which may have introduced extraneous xenogenic effects such as cross-reactivity with murine red blood cells). However, other studies have successfully used human IVIg in different animal models of ITP with no apparent xenogeneic effects,10-12,24,25 and, in our hands, human IVIg at least does not bind with murine red blood cells (J.W.S., unpublished data, December 1999). Thus, taken together, our data suggest that IVIg therapy is efficacious for antibody-mediated thrombocytopenia but not the CD8+ T cell–mediated form of the disease.
In summary, we have described a murine model of ITP that shows 2 major platelet/megakaryocyte destructive pathways, that is, antibody- and cell-mediated thrombocytopenia, and show that, although antibody-mediated thrombocytopenia is responsive to IVIg treatment, cell-mediated disease is not. This model will not only be important in understanding the immunopathologic processes in ITP but also will be useful in testing new therapeutic methods to treat the different modes of platelet destruction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Brian L. Sheridan for helping us with the bone marrow preparations.
This work is supported by the Canadian Blood Services (grant XT00054). L.C. is the recipient of a Canadian Blood Services Post-Doctoral Fellowship.
National Institutes of Health
Authorship
Contribution: L.C. designed research, performed and supervised all experiments, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript first draft; R.A., E.R.S., and M.K. performed experiments, collected and analyzed data; N.C. performed bone marrow experiments, collected data, and analyzed and interpreted data; M.L.W. and P.C. performed IVIg experiments, collected data, and analyzed and interpreted data; K.S. performed experiments, collected data, and analyzed and interpreted data; H.N. designed research, edited the manuscript, and contributed animals; A.H.L. and J.F. designed research, interpreted data, and edited the manuscript; M.B.G. provided the experimental design, analyzed bone marrow histology, interpreted data, and edited the manuscript; and J.W.S. provided financial resources, designed research, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Semple, St Michael's Hospital, 30 Bond St, Toronto, ON, Canada, M5B 1W8; e-mail: semplej@smh.toronto.on.ca.