Abstract
Excessive production of reactive oxygen species (ROS) is a feature of human malignancy and is often triggered by activation of oncogenes such as activated Ras. ROS act as second messengers and can influence a variety of cellular process including growth factor responses and cell survival. We have examined the contribution of ROS production to the effects of N-RasG12D and H-RasG12V on normal human CD34+ progenitor cells. Activated Ras strongly up-regulated the production of both superoxide and hydrogen peroxide through the stimulation of NADPH oxidase (NOX) activity, without affecting the expression of endogenous antioxidants or the production of mitochondrially derived ROS. Activated Ras also promoted both the survival and the growth factor–independent proliferation of CD34+ cells. Using oxidase inhibitors and antioxidants, we found that excessive ROS production by these cells did not contribute to their enhanced survival; rather, ROS promoted their growth factor–independent proliferation. Although Ras-induced ROS production specifically activated the p38MAPK oxidative stress response, this failed to induce expression of the cell-cycle inhibitor, p16INK4A; instead, ROS promoted the expression of D cyclins. These data are the first to show that excessive ROS production in the context of oncogene activation can promote proliferative responses in normal human hematopoietic progenitor cells.
Introduction
Reactive oxygen species (ROS) are a heterogeneous group of inorganic molecules and free radicals, with a wide spectrum of life span and reactivity. In physiologic systems, ROS formation begins with the univalent reduction of diatomic oxygen to produce superoxide radicals. There are 2 main sources of cellular superoxide; the first is the mitochondrial electron transport chain, where incomplete reduction of oxygen to water can result in superoxide formation. The other major source of superoxide is professional oxidases (exemplified by the NADPH oxidase [NOX] protein family),1 which are expressed and functional throughout hematopoietic development.2 These proteins form part of a membrane-bound complex that transfers single electrons from cytosolic nicotinamide adenine dinucleotide phosphate via flavin adenine dinucleotide to extracellular oxygen, producing superoxide. Superoxide is a short-lived radical but can dismutate forming hydrogen peroxide (H2O2), a relatively long-lived species that lies at the hub of a variety of potential chemical reactions and is the main molecule from which all other physiologic ROS molecules are derived. ROS levels are regulated by a complex network of antioxidant molecules and enzymes that detoxify ROS.3 For example, superoxide generation is balanced by the actions of superoxide dismutases (SODs), which convert superoxide to H2O2. Subsequently, H2O2 is destroyed during oxidation reactions involving glutathione or members of the peroxiredoxin family.
Although ROS production can have adverse consequences causing lipid peroxidation and DNA damage, it is also clear that ROS act as cell-signaling molecules. In recent years, it has become clear that H2O2 reacts with a variety of proteins sensitive to thiol oxidation.4 In particular, H2O2 can inhibit phosphatases via oxidation of cysteine at the active site and may consolidate growth factor signaling by preventing futile cycles of phosphorylation and dephosphorylation of proteins and phosphoinositides.5 The fact that H2O2 is freely membrane-permeable also has the consequence that its production affects not only the cells generating it but also neighboring cells. This applies particularly to transmembrane-bound NOX oxidases that release ROS into the extracellular environment. Rapid increases in ROS are observed after growth factor stimulation and inhibition of ROS production can lead to abolition of signaling.5-7 Other reports indicate that ROS may also directly promote G1-to-S progression, perhaps by direct modification of cell-cycle regulatory proteins.6,8
Excessive production of ROS (comprising mainly superoxide and H2O2) is a common feature of malignant disease including leukemia.9,10 Common leukemia-associated abnormalities such as activated Ras,9,11 activated FMS-like tyrosine kinase 3,12 and BCR-ABL13 are associated particularly with overproduction of ROS. One consequence of this is an increase in DNA damage,14 which may thereby contribute to leukemogenesis; however, its role in cell signaling suggests that overproduction of ROS may have more direct consequences. On the one hand, ROS overproduction may act as a tumor suppressor15 by promoting a stress response that leads to cell-cycle arrest,16 senescence, or apoptosis9 ; conversely, it is clear that cancer cells can evolve to evade this stress response,9 and several models indicate that sustained ROS overproduction is essential to maintain the transformed phenotype.11,13,17,18 Little is known of the consequences of ROS overproduction in leukemia.
Mutational activation of Ras is observed in 15% to 25% of acute myeloid leukemias.19 Using a model system based on normal human CD34+ hematopoietic progenitor cells, we have therefore examined the impact of this oncogene in terms of its capacity to promote ROS production in these cells and also the contribution that ROS make to the phenotype exhibited by activated Ras–expressing progenitor cells. Here we show that constitutively active Ras strongly promotes superoxide and H2O2 production in human CD34+ cells through activation of NOX oxidases. Ras also strongly promoted both survival and growth factor–independent proliferation of CD34+ cells in a culture density–dependent manner. We found that ROS overproduction did not contribute to the prosurvival phenotype, but did contribute to growth factor–independent proliferation by augmenting the expression of cyclins D1 and D3.
Methods
Generation of human CD34+ and murine Sca-1+ hematopoietic progenitor cells expressing activated Ras
Murine bone marrow was extracted from wild-type and Nox2−/−C57BL/6 littermates (kindly provided by Professor A. Shah, King's College Hospital) as described elsewhere.20 Human neonatal cord blood was obtained from healthy full-term pregnancies at the University Hospital Wales. These specimens were obtained with informed consent and with approval from the South East Wales Research Ethics Committee in accordance with the 1964 Declaration of Helsinki. Murine Sca-1+ and human CD34+ hematopoietic progenitor cells were positively selected using the MiniMACS Sca-1+ or CD34+ cell isolation kits, respectively, according to the manufacturer's instructions (Miltenyi Biotec). Ectopic expression of H-RasG12V or N-RasG12D and reporter genes (green fluorescent protein [GFP] or DsRed) was achieved using the PINCO retroviral vector. Retroviral transduction was carried out over 2 subsequent days as previously described.21 Transduced human CD34+ and murine Sca-1+ hematopoietic progenitor cells were cultured in supplemented Iscove modified Dulbecco medium (IMDM), as previously described,22 containing 20% fetal calf serum, supplemented with either 5 ng/mL human interleukin-3, human granulocyte colony-stimulating factor (CSF), human granulocyte-macrophage CSF, and 20 ng/mL human stem cell factor (for CD34+ cells), or with the same concentrations of murine interleukin-3, murine granulocyte-macrophage CSF, murine stem cell factor, and human granulocyte CSF (for Sca-1+ cells), unless otherwise stated.
Detection of superoxide using the chemiluminescent probe Diogenes
Superoxide measurement was carried out using the chemiluminescent probe Diogenes (National Diagnostics). Transduced human CD34+ cells or murine Sca-1+ cells were washed in phosphate-buffered saline (PBS) and resuspended in standard buffer (KH2PO4 4.6mM, Na2HPO4 8.0mM, NaCl 13mM, KCl 0.44mM, glucose 5.5mM, bovine serum albumin [low lipopolysaccharide] 0.1%, MgCl2 0.5mM, CaCl2 0.45mM dissolved in lipopolysaccharide–low water, pH 7.3) at 1 × 106 cells/mL. Cell suspensions (150 μL) were assayed in triplicate in FluoroNunc Maxisorp 96-well plates (Thermo-Fisher Scientific). Diogenes (50 μL) was added immediately before recording chemiluminescence (Dynex MLX Luminometer; Jencons). Some experiments were carried out in the presence of either 5 μg/mL superoxide dismutase (SOD), 100 μg/mL catalase, 50μM diphenyleneiodonium (DPI), 1mM apocynin, or 5μM rotenone (Sigma). Human peripheral blood monocytes stimulated with opsonised zymosan23 were used for comparative purposes.
Detection of superoxide using electron paramagnetic resonance spectroscopy
To confirm the presence of the superoxide radical, the spin-trap probe 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DEPMPO; Axxora) was used. Transduced cells were washed in PBS and resuspended at 1 × 107 cells/mL in standard buffer. DEPMPO (60mM) was added immediately before measurement. Electron paramagnetic resonance (EPR) spectra were detected in 10 scans of 60 seconds at 37°C using a Varian 104 EPR spectrometer (Varian Inc) at X-band (9.5 GHz). Typical recording conditions were field center, 3378 Gauss; gain, 2 × 105; modulation amplitude, 2.0; time constant, 0.25; microwave power, 10 mW; and field range, 160 Gauss. Addition of 5 μg/mL SOD served as a negative control. Background was established using DEPMPO probe alone.
Determination of mitochondrial superoxide production using MitoSOX
Mitochondrial superoxide was measured using MitoSOX (Invitrogen). Transduced CD34+ cells (days 5 to 7) were washed in warm PBS and then incubated with 5μM MitoSOX for 15 minutes at 37°C and washed in FACSFlow (BD Biosciences) before flow cytometric analysis (“Flow cytometric analysis”). For some experiments, cells were incubated for 15 minutes at 37°C before MitoSOX treatment with either 50μM rotenone (Sigma) or 5μM iron (III) 5, 10, 15, 20-tetrakis-4-carboxyphenyl porphyrin (FeTCPP; Frontier Scientific Inc) as a positive or negative control, respectively.
Detection of hydrogen peroxide using Amplex Red
Amplex Red (Invitrogen), a H2O2-sensitive fluorescent probe, was prepared according to the manufacturer's instructions. Transduced CD34+ cells (days 4 to 5; 2 × 105) were resuspended at 1 × 106 cells/mL in Krebs-Ringer phosphate buffer containing 5.50mM glucose, pH 7.35, for 4 hours at 37°C. After centrifugation (180g for 5 minutes), the conditioned medium (supernatant) was harvested and 50 μL was assayed (in triplicate) in 96-well fluorescent assay plates (Thermo Fisher Scientific) containing 50 μL/well Amplex Red solution. Fluorescence was recorded using a FluoStar Optima instrument (excitation, 540 nm; emission, 590 nm; BMG Labtech). The concentration of H2O2 was determined using a standard curve.
Western blotting
Western blotting was performed using the NuPage Bis-Tris (bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane–tris(hydroxymethyl)aminomethane) gel system (Invitrogen) using whole-cell lysates prepared as previously described.24 In some cases, transduced CD34+ cells (day 5) were incubated at 37°C in supplemented IMDM without growth factors for 16 hours before lysate preparation. For cell fractionation experiments, plasma membrane and cytosol fractions were prepared by 2 freeze-thaw cycles in the presence of homogenization buffer (sucrose 0.25M, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–KOH 10mM, magnesium acetate 1mM, ethylenediaminetetraacetic acid 0.5mM, ethyleneglycoltetraacetic acid 0.5mM, β-mercaptoethanol 10mM, 20 μg/mL DNase, pH 7.2). Homogenate (100 μL) was centrifuged at 16 000g for 1 hour at 4°C. Cytosolic proteins (supernatant) were aspirated and the membrane pellet was solubilized in homogenization buffer containing 1% Triton X-100. Nuclear and cytosol fractions were prepared using a cell fractionation kit (PerkinElmer) according to the manufacturer's instructions. Protein concentration was determined using the Bradford method, or by the DC Protein Assay Kit (Bio-Rad) according to the manufacturer's instructions. Subcellular fractions or whole-cell lysates (2-5 × 104 cell equivalents/lane) were loaded onto a 4% to 12% Bis-Tris gel and electroblotting was performed as previously described.24 Electroblotted membranes were probed overnight at 4°C using antibodies recognizing p67phox (clone H-300), p47phox (A-7), p40phox (D-8; all from Santa Cruz Biotechnology), pan Rac, cyclin D3, cyclin-dependent kinase 4 (CDK4), CDK6, anti-p16INK4A, p15INK4B, p21Cip1, p27Kip1, total p38MAPK, phospho-p38MAPK (Thr180/182), total Akt, phospho-Akt (Ser473), SOD1, anti–phospho-Rb (Ser780), anti–phospho-Rb (Ser795), anti–phospho-Rb (Ser807/811), anti-pan Rb, pan Ras (all from Cell Signaling Technology), catalase (Abcam), pan RasV12 (DWP; Calbiochem), and peroxiredoxin I (Lab Frontier). Anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 6C5; Santa Cruz Biotechnology), anti–β-actin (mAbcam 8226; Abcam) or anti–α-tubulin (DMA1; Calbiochem) was used to confirm equal loading. Purity of plasma membrane and nuclear or cytosolic fractions was verified using anti-CD45 (69; BD Biosciences), anti–Histone H1 (AE4; AbD Serotec), and anti-GAPDH. Target proteins were visualized using the ECL Advance Detection Kit (GE Healthcare) according to the manufacturer's instructions. Chemiluminescence was recorded using a LAS-3000 image analyser (Fujifilm UK Ltd), and analyzed using AIDA Image Analyser Version 4.19 (Fujifilm UK Ltd).
Cell survival, proliferation, and cell-cycle analysis assays
After gene transduction (day 3) and 2 washes in Hanks balanced salt solution (Invitrogen), 0.5 to 4 × 105 transduced CD34+ cells/mL were cultured in 96-well U-bottom microplates (Thermo Fisher Scientific) at 37°C for 48 hours in either IMDM without supplements, serum, or growth factors (for survival assay), or supplemented IMDM without growth factors (for proliferation assay). In some experiments, cells were incubated with either highly purified catalase (no. C100), DPI, or N-acetylcysteine (NAC; Sigma). After incubation, cells were washed in PBS, 1% bovine serum albumin and stained with an annexin V–cyanin 5 conjugate (BioVision) according to the manufacturer's instructions in the presence of 1 μg/mL 7-amino-actinomycin D (7-AAD) to discriminate membrane-permeable cells. Viable cells were counted by flow cytometry as described below using 5 × 103 allophycocyanin microbeads (BD Biosciences) to control for cell recovery. For cell-cycle analysis, 0.5 to 1 × 105 human CD34+ cells (day 5) were quiesced for 16 hours as in “Western blotting.” before fixation and determination of DNA content as previously described.25 The threshold for inclusion of apoptotic bodies was set at 25% of the fluorescence peak representing cells in G0. Proportion of cells in G0 and S+G2/M was determined using Cylchred Version 1.0 (T. Hoy, Cardiff University). All data were acquired by flow cytometry as described in “Flow cytometric analysis.”
Flow cytometric analysis
Transduced CD34+ cells were stained for cytometric analysis with unconjugated mouse anti-NOX2 antibody (7D5; Caltag Medsystems) or anti–CD34− phycoerythrin (PE) antibody (BD Biosciences). Unconjugated anti-NOX2 antibody was detected using secondary rat anti–mouse immunoglobulin G1-PE (BD). Background fluorescence was established by isotype-matched controls; autofluorescence of mock-infected cultures defined the threshold for GFP or DsRed positivity. Data were acquired using a BD FACSCalibur and analyzed using FCS Express Version 3 (De Novo Software).
Statistical analysis
Significance of difference was determined using one-way analysis of variance (ANOVA) with Tukey honestly significant differences test, Mann-Whitney test, or Student t test using Minitab Version 13.0 (Minitab Ltd).
Results
Expression of N-RasG12D or H-RasG12V in human CD34+ hematopoietic progenitor cells promotes ROS production
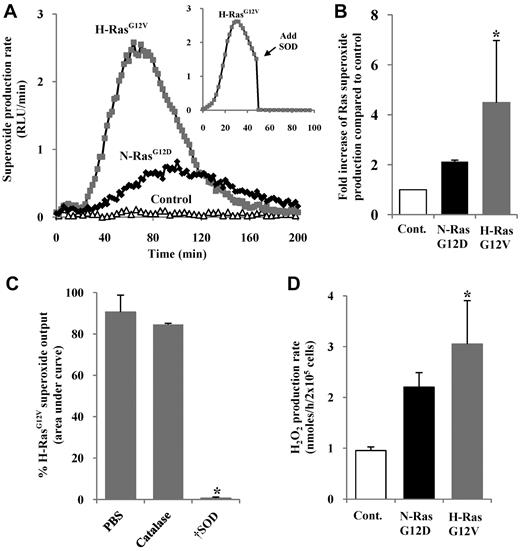
To establish whether expression of activated Ras promoted ROS production in normal hematopoietic progenitor cells, we transduced human CD34+ hematopoietic cells using the retroviral vector PINCO, encoding N-RasG12D or H-RasG12V and a reporter gene (GFP), or reporter gene alone. The resultant cell cultures are referred to hereafter as CD34+ N-RasG12D, CD34+ H-RasG12V, or control, respectively, and typically 50% to 80% of the cells were GFP+ after transduction (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Superoxide was assayed using the superoxide-sensitive chemiluminescent probe, Diogenes. A low level of constitutive superoxide production was observed in normal cells, and was strongly augmented by activated Ras (Figure 1A). As shown in Figure 1B, superoxide induction was typically more efficient with H-RasG12V (∼ 4-fold increase compared with controls) than with N-RasG12D (∼ 2-fold increase), though maximal superoxide induction was only a tenth of that generated by activated phagocytes (supplemental Figure 2B). SOD quenched chemiluminescence, whereas treatment with catalase (which decomposes H2O2 but not superoxide) did not, demonstrating the specificity of the probe and confirming superoxide as the major source of ROS in this assay (Figure 1C).
Measurement of ROS production by human hematopoietic progenitor cells expressing N-RasG12D or H-RasG12V. (A) Representative chemiluminescence trace of superoxide production measured using Diogenes. In some replicates (inset), superoxide production was quenched by addition of SOD at 50 minutes. (B) Summary of fold increase of total light emission due to expression of activated Ras over controls during early hematopoietic development. (C) Using Diogenes, superoxide production in H-RasG12V–transduced cells was measured, with either 5 μg/mL SOD or 100 μg/mL catalase present from the start of the assay (n = 3). Bar chart represents total superoxide produced (area under chemiluminescence trace). (D) Using Amplex Red, H2O2 production rate was determined over 4 hours. Data represent mean + 1 SD (n ≥ 3). Statistical significance was calculated by ANOVA using Tukey honestly significant differences, Mann-Whitney test, or Student paired t test. †P < .001; *P < .05.
Measurement of ROS production by human hematopoietic progenitor cells expressing N-RasG12D or H-RasG12V. (A) Representative chemiluminescence trace of superoxide production measured using Diogenes. In some replicates (inset), superoxide production was quenched by addition of SOD at 50 minutes. (B) Summary of fold increase of total light emission due to expression of activated Ras over controls during early hematopoietic development. (C) Using Diogenes, superoxide production in H-RasG12V–transduced cells was measured, with either 5 μg/mL SOD or 100 μg/mL catalase present from the start of the assay (n = 3). Bar chart represents total superoxide produced (area under chemiluminescence trace). (D) Using Amplex Red, H2O2 production rate was determined over 4 hours. Data represent mean + 1 SD (n ≥ 3). Statistical significance was calculated by ANOVA using Tukey honestly significant differences, Mann-Whitney test, or Student paired t test. †P < .001; *P < .05.
Because H2O2 rather than superoxide has been implicated as a second messenger, we measured levels of H2O2 in medium conditioned by N-RasG12D– and H-RasG12V–expressing cells. Hydrogen peroxide (which can be generated from superoxide via a dismutation reaction26 ) was assayed using the H2O2-sensitive fluorescent probe, Amplex Red. CD34+ H-RasG12V cells showed significantly higher rates of H2O2 production compared with controls (P < .05), whereas CD34+ N-RasG12D cells showed a similar trend, which did not reach significance (Figure 1D).
Aberrant Ras signaling affects transcription of a variety of genes27 and could potentially perturb the expression of endogenous antioxidant molecules resulting in an apparent increase in ROS production. However, no differences in the levels of common antioxidants were observed compared with control cultures (supplemental Figure 2D). Taken together, these data demonstrate that activated Ras stimulates significant overproduction of superoxide as well as the more stable and membrane-permeable putative second messenger, H2O2, in hematopoietic progenitor cells.
Ras-induced ROS are mediated via NOX family oxidase activity
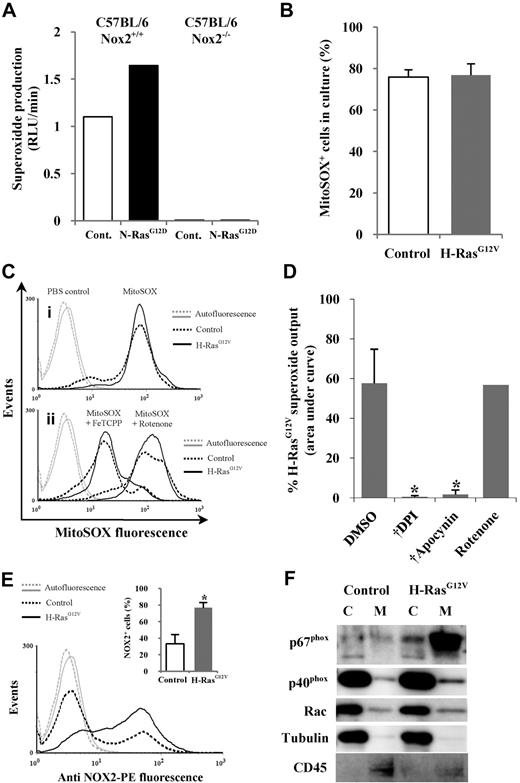
ROS can be generated by several mechanisms, the major sources being oxidase activity (eg, NOX family oxidases) or the mitochondrial electron transport chain.28 The NOX family oxidase Phox is a transmembrane multimeric complex consisting of the NOX2 (aka, gp91phox) catalytic subunit and several regulatory subunits. When N-RasG12D was expressed in Nox2 wild-type Sca-1+ murine hematopoietic cells, a 1.5-fold increase in superoxide production was observed compared with controls, whereas superoxide production was absent in Sca-1+ cells from Nox2−/− mice, suggesting that in murine hematopoietic cells, Nox2 is the major source of ROS in the context of activated Ras (Figure 2A). To establish whether this was also the case in human cells, we first used the mitochondria-specific superoxide-sensitive probe MitoSOX to determine whether activated Ras promoted ROS production from this source. No significant differences in mitochondrial ROS were observed in CD34+ H-RasG12V cells (which produced the highest levels of ROS) compared with controls (Figure 2B). The sensitivity of MitoSOX for mitochondrial superoxide was confirmed using the antioxidant FeTCPP or the mitochondrial poison rotenone, which reduce or increase mitochondrial superoxide, respectively (Figure 2C). These data indicated that excess superoxide production detected by Diogenes did not arise from the mitochondria. To determine whether NOX oxidases were the source of increased superoxide production, we used the NOX oxidase inhibitors DPI and apocynin. Superoxide production (measured using Diogenes) was completely blocked by these inhibitors in both CD34+ H-RasG12V cells and control cells, whereas the mitochondrial poison rotenone had no effect (Figure 2D). These data, together with the data from Nox2−/− mice (Figure 2A), indicate that the major source of increased superoxide production in human CD34+ cells expressing activated Ras is the NOX family oxidase, Phox.
Mutant Ras promotes ROS production via activation of NOX family oxidases. (A) Superoxide production by N-RasG12D–transduced murine Sca-1+ cells from either wild-type (Nox2+/+) or Nox2-deficient (Nox2−/−) mice was measured using Diogenes. Bar chart represents total superoxide produced during assay. (B) Mitochondrial superoxide was measured using MitoSOX. Figure depicts percentage of control or H-RasG12V cells positive for MitoSOX fluorescence after labeling. A PBS control was used to define positive threshold. (C) Representative histograms depict MitoSOX fluorescence in control and H-RasG12V cells labeled with MitoSOX (i) alone or (ii) in the presence of 5μM FeTCPP or 50μM rotenone. (D) Using Diogenes, superoxide production by CD34+ H-RasG12V cells was measured in the presence of 50μM DPI, 50μM apocynin, or 5μM rotenone (n = 3). Bar chart represents total superoxide produced. (E) Transduced CD34+ cells were incubated with anti–NOX2-PE and analyzed by flow cytometry. Typical histogram showing anti–NOX2-PE expression; bar chart (inset) indicates percentage of fluorescent cells after incubation with anti–NOX2-PE. Isotype control antibody was used to define positive threshold (n = 5). (F) Cytosol/membrane fractionated lysates were analyzed by Western blot using antibodies recognizing p67phox, p40phox, or Rac. Tubulin acted as a loading control and cytosolic marker. CD45 was used a membrane marker. C indicates cytosol; and M, membrane. Data represent mean + 1 SD. Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. †P < .001; *P < .05.
Mutant Ras promotes ROS production via activation of NOX family oxidases. (A) Superoxide production by N-RasG12D–transduced murine Sca-1+ cells from either wild-type (Nox2+/+) or Nox2-deficient (Nox2−/−) mice was measured using Diogenes. Bar chart represents total superoxide produced during assay. (B) Mitochondrial superoxide was measured using MitoSOX. Figure depicts percentage of control or H-RasG12V cells positive for MitoSOX fluorescence after labeling. A PBS control was used to define positive threshold. (C) Representative histograms depict MitoSOX fluorescence in control and H-RasG12V cells labeled with MitoSOX (i) alone or (ii) in the presence of 5μM FeTCPP or 50μM rotenone. (D) Using Diogenes, superoxide production by CD34+ H-RasG12V cells was measured in the presence of 50μM DPI, 50μM apocynin, or 5μM rotenone (n = 3). Bar chart represents total superoxide produced. (E) Transduced CD34+ cells were incubated with anti–NOX2-PE and analyzed by flow cytometry. Typical histogram showing anti–NOX2-PE expression; bar chart (inset) indicates percentage of fluorescent cells after incubation with anti–NOX2-PE. Isotype control antibody was used to define positive threshold (n = 5). (F) Cytosol/membrane fractionated lysates were analyzed by Western blot using antibodies recognizing p67phox, p40phox, or Rac. Tubulin acted as a loading control and cytosolic marker. CD45 was used a membrane marker. C indicates cytosol; and M, membrane. Data represent mean + 1 SD. Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. †P < .001; *P < .05.
During Phox activation, Phox regulatory subunits translocate from the cytosol to form a complex with NOX2 at the plasma membrane.26 Therefore, the ability of activated Ras to alter the expression and localization of Phox components was investigated. Flow cytometric analysis showed that activated Ras significantly increased in the expression of NOX2 compared with controls (P < .05; Figure 2E). In addition, Figure 2F shows that activated Ras also promoted the membrane translocation/expression of the Phox activators p67phox and p40phox. Taken together, these data support the observations that activated Ras promotes ROS production by increasing Phox activity in human CD34+ cells.
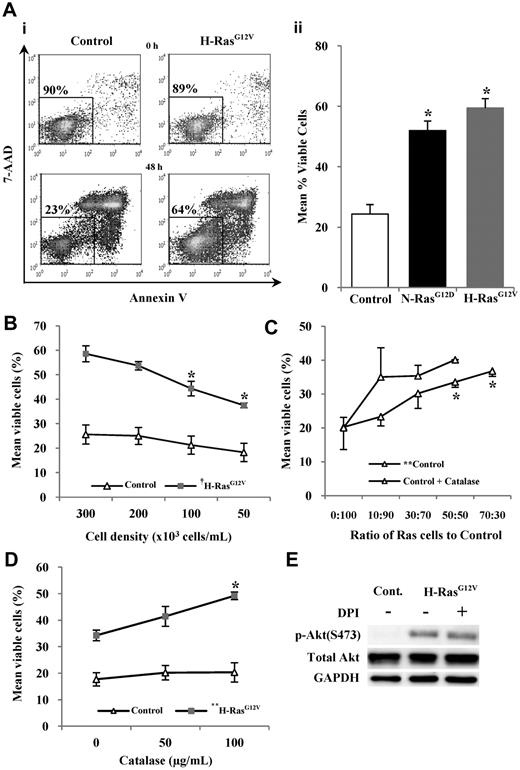
Activated Ras promotes survival in human CD34+ cells but Ras-induced ROS antagonize survival
Constitutively active Ras is associated with increased cell survival.29 Consistent with this, CD34+ N-RasG12D and CD34+ H-RasG12V cells were resistant to apoptosis when incubated in serum-free, growth factor–free medium for 48 hours compared with controls (P < .05; Figure 3A). Interestingly, the strength of the survival phenotype correlated with the level of ROS production. Excessive H2O2 exposure leads to oxidative stress and cell death, however low levels of H2O2 can promote cell survival.30 Therefore the role of H2O2 in the survival of CD34+ cells expressing H-RasG12V was investigated (because this oncogene induced the strongest survival phenotype). CD34+ H-RasG12V cells showed a significant decrease in cell survival with decreasing cell culture density (P < .001; Figure 3B). Furthermore, CD34+ control cells showed a significant increase in cell survival when cocultured with increasing numbers of CD34+ H-RasG12V cells (P < .01; Figure 3C). Given that H2O2 generated by CD34+ H-RasG12V cells is extracellular and membrane permeable, these data suggest that H2O2 may be acting as a paracrine prosurvival factor. To establish whether H2O2 was responsible for this effect, additional coculture experiments were carried out in the presence of catalase. The presence of catalase did not suppress control cell survival, but instead augmented it further, indicating that the prosurvival influence was not mediated by H2O2, which in fact antagonized the survival of control cells (Figure 3C). Indeed, catalase treatment also further promoted the survival of CD34+ H-RasG12V cells themselves, indicating that H2O2 production also antagonized the survival of these cells (Figure 3D). A similar trend was seen upon treatment with the antioxidant NAC or the NOX inhibitor DPI (data not shown). As expected, catalase treatment had a modest effect on control cells cultured alone because these cells produced very low levels of H2O2 (Figure 3D). Taken together, these data suggest that CD34+ H-RasG12V cells secrete factors that promote survival, but ROS production appears to antagonize the prosurvival effects of these factors.
Survival of human CD34+ hematopoietic cells expressing mutant Ras. CD34+ cells expressing mutant Ras or GFP control were incubated in serum-free, growth factor–free medium for 48 hours and stained with annexin V and 7-AAD. Annexin V− and 7-AAD− cells were defined as viable. (Ai) Typical flow cytometric plots obtained at start of incubation and after 48-hour incubation with percentage of viable cells indicated. (Aii) Bar chart representing survival averaged over 3 experiments. (B) Viability of CD34+ cells expressing H-RasG12V or GFP control while decreasing cell-culture density. (C) Normal (control) CD34+ cells were cocultured with an increasing proportion of CD34+ H-RasG12V cells coexpressing DsRed, enabling analysis of CD34+ control cell viability in mixed culture by flow cytometry. Some experiments were carried out in the presence of catalase. (D) CD34+ cells expressing mutant Ras or GFP were cultured at a cell density of 5 × 104 cells/mL in the presence of purified catalase. (E) Whole-cell lysates were analyzed by Western blot, using antibodies recognizing phospho-Akt (S473) or total Akt protein. GAPDH was used as a loading control. Data represent mean ± 1 SD (n = 3). Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. †P < .001; *P < .05; **P < .01.
Survival of human CD34+ hematopoietic cells expressing mutant Ras. CD34+ cells expressing mutant Ras or GFP control were incubated in serum-free, growth factor–free medium for 48 hours and stained with annexin V and 7-AAD. Annexin V− and 7-AAD− cells were defined as viable. (Ai) Typical flow cytometric plots obtained at start of incubation and after 48-hour incubation with percentage of viable cells indicated. (Aii) Bar chart representing survival averaged over 3 experiments. (B) Viability of CD34+ cells expressing H-RasG12V or GFP control while decreasing cell-culture density. (C) Normal (control) CD34+ cells were cocultured with an increasing proportion of CD34+ H-RasG12V cells coexpressing DsRed, enabling analysis of CD34+ control cell viability in mixed culture by flow cytometry. Some experiments were carried out in the presence of catalase. (D) CD34+ cells expressing mutant Ras or GFP were cultured at a cell density of 5 × 104 cells/mL in the presence of purified catalase. (E) Whole-cell lysates were analyzed by Western blot, using antibodies recognizing phospho-Akt (S473) or total Akt protein. GAPDH was used as a loading control. Data represent mean ± 1 SD (n = 3). Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. †P < .001; *P < .05; **P < .01.
In further demonstration of this, we examined the phosphorylation of the prosurvival protein, Akt. H-RasG12V increased the phosphorylation of Akt, which probably contributed to the increased survival of these cells (Figure 3E). However, incubation with the NOX inhibitor DPI (Figure 3E) or catalase (data not shown) did not reduce the phosphorylation of Akt, supporting our observations that ROS were not contributing to the enhanced survival of CD34+ H-RasG12V cells. Increased expression of the antiapoptotic protein Survivin has also been reported as an explanation for Ras-induced survival31 ; however, our study found no evidence of increased Survivin expression as a consequence of activated Ras expression (data not shown).
ROS production augments activated RAS-induced growth factor–independent proliferation
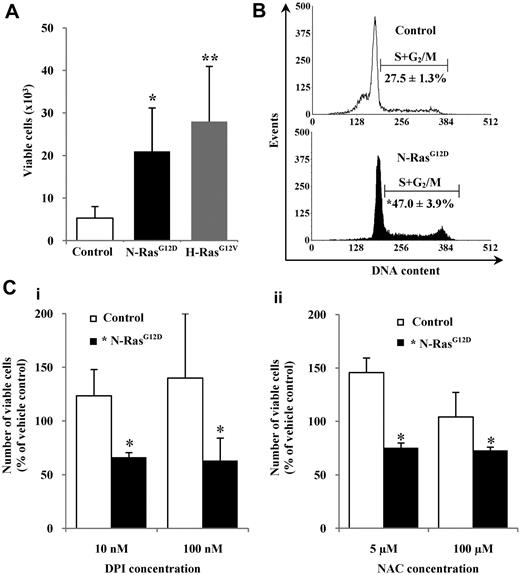
We observed that control cells were better able to survive in serum-replete medium but were unable to proliferate without the addition of growth factors, whereas CD34+ cells expressing N-RasG12D or H-RasG12V were able to proliferate under these conditions and showed a significant approximately 4-fold increase in cell numbers after 48 hours compared with controls (P < .05; Figure 4A). Cell-cycle analysis confirmed that CD34+ N-RasG12D cells exhibited a significant approximately 2-fold increase in the proportion of cells in cycle (S+G2/M) compared with controls (P < .05; Figure 4B). To determine whether ROS production played a role in the growth factor–independent proliferation, CD34+ N-RasG12D cells were incubated in serum-replete, growth factor–free medium for 48 hours in the presence of the antioxidants DPI and NAC. Both DPI and NAC suppressed proliferation of these cells, whereas control cells showed increased proliferation (Figure 4C). Similar effects were observed with CD34+ H-RasG12V cells (supplemental Figure 3). We also examined whether ROS production could influence progenitor frequency. In accordance with previously published work,32 we found no significant difference in progenitor frequency either in terms of CD34 positivity, morphology, or colony-forming ability. Treatment with DPI had no differential effect in each case, indicating that under these conditions excessive production of ROS (stimulated by Ras) does not significantly influence progenitor frequency (supplemental Figure 4). Taken together, these data suggest that Ras signaling strongly drives factor-independent growth, and indicate that Ras-induced ROS production plays a role in augmenting this proliferative signal.
Proliferation of hematopoietic cells expressing mutant Ras in the presence of antioxidants. (A) CD34+ cells (1 × 104) expressing mutant Ras or GFP control were incubated in serum-replete, growth factor–free medium for 48 hours, and viable cells (7-AAD−, annexin V−) were counted by flow cytometry. (B) Representative histograms showing cell-cycle distribution of CD34+ cells expressing N-RasG12D or GFP control after 16 hours in serum-replete, growth factor–free medium. The percentage of cells in S+G2/M is indicated. (C) CD34+ cells expressing N-RasG12D or GFP control were incubated as in panel A in the presence of (i) DPI or (ii) NAC. Data represent number of viable cells remaining as a percentage of that recovered from cells treated with vehicle control, set at 100%. Data are mean ± 1 SD; n ≥ 3. Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. **P < .01; *P < .05.
Proliferation of hematopoietic cells expressing mutant Ras in the presence of antioxidants. (A) CD34+ cells (1 × 104) expressing mutant Ras or GFP control were incubated in serum-replete, growth factor–free medium for 48 hours, and viable cells (7-AAD−, annexin V−) were counted by flow cytometry. (B) Representative histograms showing cell-cycle distribution of CD34+ cells expressing N-RasG12D or GFP control after 16 hours in serum-replete, growth factor–free medium. The percentage of cells in S+G2/M is indicated. (C) CD34+ cells expressing N-RasG12D or GFP control were incubated as in panel A in the presence of (i) DPI or (ii) NAC. Data represent number of viable cells remaining as a percentage of that recovered from cells treated with vehicle control, set at 100%. Data are mean ± 1 SD; n ≥ 3. Statistical significance was calculated by ANOVA using Tukey honestly significant differences or Mann-Whitney test. **P < .01; *P < .05.
ROS production modulates key cell-cycle regulatory proteins
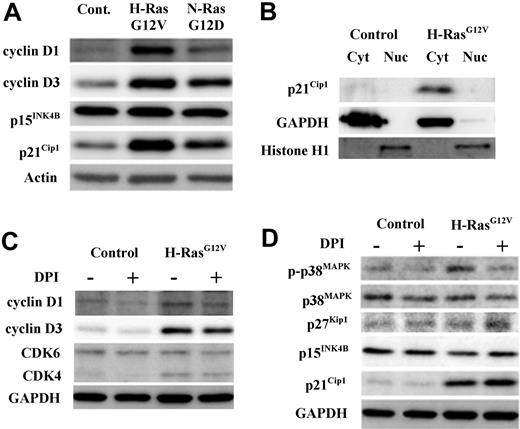
Consistent with their proproliferative role, N-RasG12D and H-RasG12V strongly promoted the expression of D-type cyclins D1 and D3; in each case the influence of H-RasG12V was greater than N-RasG12D. These oncogenes had no effect on the expression of the cell-cycle inhibitor p15INK4B, but did up-regulate the cell-cycle inhibitor p21Cip1, which seemed contrary to their proproliferative effect (Figure 5A). Previously, it has been reported that hematopoietic cells express high levels of extranuclear p21Cip1,33 therefore we examined the subcellular location of p21Cip1 and found that p21Cip1 expression was exclusively cytosolic (Figure 5B). This suggests that induction of p21Cip1 in these cells is concerned with its antiapoptotic cytoplasmic function rather than its nuclear cell-cycle inhibitory role.34
Determination of cell-cycle regulatory protein status by Western blot in CD34+ hematopoietic progenitors transduced with mutant Ras. CD34+ control, CD34+ N-RasG12D, or CD34+ H-RasG12V cells were incubated for 16 hours in serum-replete, growth factor–free medium in the presence or absence of 1μM DPI. Expression and phosphorylation state of key cell-cycle regulatory proteins in whole-cell lysates were determined by Western blot. In all cases, GAPDH was used as a loading control. (A) The endogenous expression of cell-cycle promoters cyclin D3 and cyclin D1 and cell-cycle inhibitors p15INK4B and p21Cip1 was determined. (B) Cytosol/nuclear fractionated lysates were probed with antibody recognizing the cell-cycle inhibitor p21Cip1. (C) Whole-cell lysates derived from both CD34+ control and CD34+ H-RasG12V cells (treated with either DPI or vehicle control) were probed with antibodies recognizing the cell-cycle promoters CDK6, CDK4, cyclin D1, and cyclin D3. (D) Whole-cell lysates as in panel C were probed with antibodies recognizing the following cell-cycle inhibitory proteins; p21Cip1 (which inhibits CDK/cyclin complexes); p15INK4B (which sequesters CDK4/6); p27Kip1 (which has a similar role to p21Cip1) and p38MAPK, both total and phosphorylated (T180/T182).
Determination of cell-cycle regulatory protein status by Western blot in CD34+ hematopoietic progenitors transduced with mutant Ras. CD34+ control, CD34+ N-RasG12D, or CD34+ H-RasG12V cells were incubated for 16 hours in serum-replete, growth factor–free medium in the presence or absence of 1μM DPI. Expression and phosphorylation state of key cell-cycle regulatory proteins in whole-cell lysates were determined by Western blot. In all cases, GAPDH was used as a loading control. (A) The endogenous expression of cell-cycle promoters cyclin D3 and cyclin D1 and cell-cycle inhibitors p15INK4B and p21Cip1 was determined. (B) Cytosol/nuclear fractionated lysates were probed with antibody recognizing the cell-cycle inhibitor p21Cip1. (C) Whole-cell lysates derived from both CD34+ control and CD34+ H-RasG12V cells (treated with either DPI or vehicle control) were probed with antibodies recognizing the cell-cycle promoters CDK6, CDK4, cyclin D1, and cyclin D3. (D) Whole-cell lysates as in panel C were probed with antibodies recognizing the following cell-cycle inhibitory proteins; p21Cip1 (which inhibits CDK/cyclin complexes); p15INK4B (which sequesters CDK4/6); p27Kip1 (which has a similar role to p21Cip1) and p38MAPK, both total and phosphorylated (T180/T182).
To assess whether ROS production contributed to the altered expression of cell-cycle regulatory proteins, CD34+ H-RasG12V cells were treated with DPI before Western blot analysis (Figure 5C-D). NOX inhibition resulted in a consistent reduction in cyclin D1 and cyclin D3 expression, indicating that ROS production cooperated with activated Ras in the induction of these D-type cyclins. Activated Ras also up-regulated cyclin-dependent kinase 4 (CDK4), a binding partner of D-type cyclins, however, DPI had no effect on the expression of this protein or CDK6 (Figure 5C). Next, we examined the effect of NOX inhibition on the cell-cycle inhibitor proteins p38MAPK, p16INK4A, p15INK4B, p27Kip1, and p21Cip1 (Figure 5D). ROS normally elicit a stress response mediated via phosphorylation of p38MAPK, which leads to the induction of the cell-cycle inhibitor, p16INK4A.9,15 We therefore examined whether this oxidative stress response was operating in CD34+ H-RasG12V cells. We found that H-RasG12V did promote p38MAPK phosphorylation, but not in the presence of DPI, demonstrating the specificity of ROS in the phosphorylation of p38MAPK (Figure 5D). However, we were unable to detect expression of p16INK4A in these cells (not shown), indicating either that the p38MAPK stress-response pathway was uncoupled in these cells or that it is not normally linked to p16INK4A induction in this context. As expected, DPI had no effect on p15INK4B or p27Kip1, because activated Ras did not alter the expression of these proteins (Figure 5D). However, DPI augmented p21Cip1 levels, indicating that ROS normally act to suppress p21Cip1 expression and (in this context) its associated antiapoptotic function (Figure 5D).
Finally, we examined the expression of the cell-cycle inhibitor, RB. H-RasG12V expression induced RB expression (3-fold), however, a similar increase was observed in the level of phosphorylated RB (consistent with the increase in cyclin D/CDK4 expression in these cells15 ), suggesting most of the RB was inactive (supplemental Figure 3C). Antioxidant treatment caused a consistent decrease in RB expression, however concomitant changes in RB phosphorylation level make it difficult to draw any inference from this observation.
In summary, these data indicate that ROS production by CD34+ N-RasG12D and CD34+ H-RasG12V cells contributes to cyclin D induction, which is consistent with these oncogenes' proproliferative influence.
Discussion
In agreement with previous research,2 we found that normal human CD34+ hematopoietic progenitors constitutively produce low levels of both superoxide and its derivative, H2O2. Expression of N-RasG12D and H-RasG12V strongly augmented constitutive production of superoxide and H2O2 (though the extent of production could also have been influenced by the concomitant overexpression of Ras in these cells). Activated Ras has been shown to promote ROS production in a variety of human cell types including transformed embryonic lung cells,35 fibroblasts,36 neuroblastoma cells,37 as well as in hematopoietic cells in vivo, indicating the potential for ROS to act as a proproliferative stimulus in an N-Ras leukemic context.12,14 Although untested in this study, activated K-Ras is not usually associated with increased ROS production, possibly due to isoform-specific cellular localization and signaling preferences.38 Cellular ROS production is balanced by expression of endogenous antioxidants. For example, Ras activity can decrease the activity of peroxiredoxins, leading to an increase in intracellular ROS.39 In this study, however, Ras expression did not affect levels of the major antioxidant enzymes, peroxiredoxin I catalase, and SOD1, suggesting that altered antioxidant expression was unlikely to account for the increased ROS detected.
ROS can be produced via oxidase activity (eg, NOX family) or via the mitochondrial electron transport chain.26 In this study, the use of a series of probes and inhibitors demonstrated that the source of excess ROS production was derived from NOX oxidases. This is consistent with other reports of Ras-induced ROS production in a variety of cell types.14,37,40,41 Specifically, we observed NOX2 overexpression and increased membrane translocation of NOX2 regulatory subunits and N-RasG12D was unable to induce ROS in mice deficient in Nox2. Taken together, these data suggest NOX2 is the major source of Ras-induced ROS in primitive hematopoietic cells. NOX1 may also contribute, because it is also expressed in human CD34+ progenitors2 and contributes to ROS production driven by activated Ras in rat fibroblasts.17
Activated Ras can promote survival of hematopoietic cell lines,31 but this is the first report that Ras can promote the survival of primary hematopoietic progenitors when deprived of growth factors and serum. Consistent with this, H-RasG12V strongly promoted the phosphorylation of Akt. Observations that survival of CD34+ cells expressing activated Ras was dependent on culture density and that these cells could promote the survival of control cells in paracrine fashion led us to examine whether ROS may be acting as a prosurvival factor (particularly as H2O2 has recently been shown to promote cell survival in neuronal cells30 ). Depletion of exogenous H2O2 (using catalase) merely promoted the survival of both CD34+ H-RasG12V and control cells, demonstrating that ROS production inhibited the survival of these cells. Correspondingly, neither catalase nor NOX inhibition suppressed the phosphorylation of Akt in CD34+ H-RasG12V cells. These data indicate that Ras provokes the secretion of prosurvival factors in CD34+ cells, but that their effects are antagonized by ROS. We have not investigated the nature of these prosurvival factors, however, a gene array analysis of CD34+ N-RasG12D cells has revealed that Ras strongly promotes the expression of several cytokine genes with this potential.42
In addition to promoting survival, we were surprised to observe that CD34+ N-RasG12D and CD34+ H-RasG12V cells were able to proliferate in serum-replete cultures without growth factors. Previous studies of the effect of activated Ras on CD34+ cells, including our own, have all made proliferative comparisons in the presence of growth factors,32,43 and under these conditions no proliferative advantage is observed; though N-RasG12D has previously been shown to promote a growth advantage when CD34+ cells were cultured with the MS5 stromal line.44 This increased proliferation is likely driven by the same secreted factors that promoted cell survival. However, unlike the cell survival experiments, we found that ROS production appeared to augment the proliferation of CD34+ N-RasG12D and CD34+ H-RasG12V cells, whereas ROS were growth inhibitory for control cells. These data suggest that activated Ras may not only promote ROS production in hematopoietic progenitors, but can simultaneously alter their response to it. Increased ROSs have previously been associated with increased cell proliferation in several models. For example, mitogenic signaling in Ras-transformed mouse fibroblasts was suppressed with NAC treatment,11 whereas overexpression of NOX1 alone (aka, Mox1) in mouse fibroblasts resulted in increased ROS production and proliferation.45
We also examined the changes in cell-cycle control proteins that may have mediated the proliferative changes elicited by activated Ras and ROS in these cells. Activated Ras induced strong up-regulation of cyclins D1 and D3, which has been reported previously,46 and also up-regulated their partner kinase, CDK4. In this study, ROS were shown to contribute to cyclin D expression, which is consistent with previous observations made in mouse embryo fibroblasts where ROS production was also associated with up-regulation of cyclin D expression and cell-cycle progression.47 Surprisingly, both isoforms of Ras also promoted expression of p21Cip1; though this appeared to have a purely cytosolic location making it unlikely that increased p21Cip1 was influencing cell-cycle progression. Finally, CD34+ H-RasG12V cells exhibited substantial phosphorylation of p38MAPK, which was solely a consequence of ROS production by these cells. In other contexts,9 this provokes cell-cycle arrest through induction of p16INK4A, but we were unable to demonstrate induction of this protein in these cells.
In conclusion, these data show that activated Ras strongly promotes the expression of ROS in early human progenitor cells, through a NOX-dependent process. A range of ROS was detected including H2O2, which has the capacity to affect a wide range of cell signaling processes. This species is membrane permeable, meaning its production can also influence neighboring cells. Our data therefore suggest that production of ROS may confer a competitive advantage on premalignant/malignant cells by promoting the proliferation of these cells, while at the same time inhibiting the proliferative capacity of normal cells in the surrounding microenvironment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Medical Research Council and Cardiff University. L.P. is funded by Leukemia Research UK.
Authorship
Contribution: P.S.H. cowrote the paper, designed and executed experiments, and analyzed data; L.P., A.J.T., and P.E.J. provided technical assistance; A.K.B. provided resources and clinical insight; and R.L.D. and A.T. designed experiments, provided project direction, and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alex Tonks, Department of Haematology, 7th Fl, School of Medicine, Cardiff University, Heath Park, Cardiff, CF14 4XN, United Kingdom; e-mail: tonksa@cf.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal