Abstract
Patients with multiple myeloma (MM) have an increased risk of venous thrombosis. Interestingly, excess risk of venous thromboembolism has been observed among patients with monoclonal gammopathy of undetermined significance (MGUS). Using population-based data from Sweden, we assessed the risks of venous and arterial thrombosis in 18 627 MM and 5326 MGUS patients diagnosed from 1958 to 2006, compared with 70 991 and 20 161 matched controls, respectively. At 1, 5, and 10 years after MM diagnosis, there was an increased risk of venous thrombosis: hazard ratios (95% confidence intervals) were 7.5 (6.4-8.9), 4.6 (4.1-5.1), and 4.1 (3.8-4.5), respectively. The corresponding results for arterial thrombosis were 1.9 (1.8-2.1), 1.5 (1.4-1.6), and 1.5 (1.4-1.5). At 1, 5, and 10 years after MGUS diagnosis, hazard ratios were 3.4 (2.5-4.6), 2.1 (1.7-2.5), and 2.1 (1.8-2.4) for venous thrombosis. The corresponding risks for arterial thrombosis were 1.7 (1.5-1.9), 1.3 (1.2-1.4), and 1.3 (1.3-1.4). IgG/IgA (but not IgM) MGUS patients had increased risks for venous and arterial thrombosis. Risks for thrombosis did not vary by M-protein concentration (> 10.0 g/L or < 10.0 g/L) at diagnosis. MGUS patients with (vs without) thrombosis had no excess risk of MM or Waldenström macroglobulinemia. Our findings are of relevance for future studies and for improvement of thrombosis prophylaxis strategies.
Introduction
Patients with multiple myeloma (MM) have an increased risk of venous thrombosis.1 During the past decade, the introduction of the oral immunomodulatory drugs (IMiDs) thalidomide and lenalidomide has improved clinical outcome of patients diagnosed with MM.2-7 However, an important complication related to IMiD therapy is the well-known risk of venous thromboembolism.8-17 Most studies have observed the most pronounced risk during the first months after MM diagnosis and when combining IMiDs with high-dose corticosteroids or chemotherapy.8-17 The underlying mechanisms for the excess of thromboembolism are largely unknown.
The risk of venous thromboembolism has also been explored among patients diagnosed with the precursor condition monoclonal gammopathy of undetermined significance (MGUS), with some,18,19 but not all,20 studies observing an increased risk. In a large study, based on more than 4 million veterans in the United States, we identified 2374 MGUS cases, of whom 31 developed deep vein thrombosis (DVT; crude incidence, 3.1 per 1000 person-years).21 Compared with non-MGUS cases, this translated to a statistically significant 3-fold increased risk of DVT among MGUS patients.
Although data are limited, there have been a few case reports indicating that the use of IMiDs in MM patients might also be associated with an increased risk of developing arterial thrombosis.22-27 To our knowledge, no large study has been conducted to evaluate the risk of arterial thrombosis among MGUS and MM patients.
Using high-quality population-based data from Sweden, we assessed the risks of venous and arterial thrombosis in 18 627 MM patients, 5326 MGUS patients, and more than 90 000 population-based matched controls. In MGUS patients, we further explored the risk of arterial and venous thromboembolism in relation to MGUS isotype and M-protein concentration at diagnosis. Our results should aid future studies designed to elucidate underlying mechanisms for thrombosis in plasma cell disorders and hopefully contribute to improvement in effective thromboembolic prophylaxis strategies.
Methods
Central registries, patients, and controls
The details of the study population have been described previously.28 In brief, Sweden provides universal medical care for the entire population, currently approximately 9 million people. In contrast to many other countries, patients with lymphoproliferative malignancies in Sweden are usually diagnosed, treated, and followed clinically by physicians at a few hospital-based hematology or oncology centers. MGUS patients are identified by a broad range of medical specialties. In Sweden, a clinician who detects a patient with an M-protein will typically consult with a hematologist at a hospital-based center and refer the patient for further workup to rule out a lymphoproliferative malignancy.
Since 1958, all physicians and pathologists/cytologists in Sweden are obliged by law to report each case of cancer they diagnose or treat to the centralized nationwide Swedish Cancer Registry, which has a very high completeness and diagnostic accuracy.29,30 Using the Swedish Cancer Registry, we identified all MM patients diagnosed between 1958 and 2006. We also used a nationwide MGUS cohort established from a national hospital network, including MGUS patients diagnosed in Sweden between 1958 and 2006.28 When available, information on MGUS immunoglobulin (Ig) isotype and concentration of the M-protein at diagnosis was collected. To minimize the influence of a potentially undetected lymphoproliferative malignancy, MGUS patients who within 6 months of diagnosis developed a lymphoproliferative malignancy were excluded from the analysis.
For each MM and MGUS patient, 4 population-based controls (matched by sex, year of birth, and county of residence) were chosen randomly from the Swedish Population database. All controls had to be alive and free of any preceding hematologic malignancy at the time of MM or MGUS diagnosis for the corresponding case.
The centralized Swedish Patient Registry captures information on individual patient-based discharge diagnoses and discharge listings from inpatient (since 1964) and outpatient care (since 2000), with a very high coverage.31 Information on occurrence and date of arterial (coronary artery disease and cerebrovascular disease) and venous (DVT and pulmonary embolism [PE]) thromboembolism was obtained using the seventh, eighth, ninth, and 10th revisions of the International Classification of Diseases. All conditions were analyzed both individually and grouped into the categories coronary artery disease, cerebrovascular disease, and venous thromboembolism. Through linkage with the Cause of Death Register and the Register of Total Population, we collected information on vital status until December 31, 2006. From the Swedish Medical Products Agency, we gathered information on the number of patients who were prescribed thalidomide and lenalidomide, during the study period.
Approval was obtained from the Karolinska Institutional Review Board for this study. Informed consent was waived because we had no contact with study subjects. An exemption from institutional review board review was obtained from the National Institutes of Health Office of Human Subjects Research because we used existing data without personal identifiers.
Statistical analysis
Cox proportional hazard models (PROC PHREG, SAS, Version 9.1; SAS Institute) were used to compare 1-, 5-, and 10-year risks for thrombosis in MM and MGUS patients compared with controls. The proportional hazards assumption for variables used in the models was assessed by visual inspection of the hazard function. Follow-up time for an MM or MGUS case started at the later of either his or her diagnosis of MM or MGUS or January 1, 1987, and for a control at the later of time of diagnosis of the matched case or January 1, 1987. The delayed entry was accommodated by the entry time option in PROC PHREG. Follow-up ended at the time of diagnosis of a specific cardiovascular event or at time of censoring. Censoring events were death, emigration, and the end of the data acquisition period (December 31, 2006). For the analysis of MGUS cases and controls, persons were additionally censored at the time of diagnosis of MM or Waldenström macroglobulinemia. Persons were not censored if they developed a cardiovascular condition other than the one being studied because they were still at risk of developing the specific thrombosis subsequently. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated overall and separately for men and women. Adjustment variables included in the models were sex, age at diagnosis in 6 groups (reference group, youngest age) or the logarithm of age at diagnosis, and year of diagnosis in quartiles (reference group, earliest decade). In addition to sex, models were stratified by year of diagnosis before or after 2000 for MM and additionally for MGUS isotype and M-protein concentration at diagnosis in MGUS patients, to assess interactions and formal interaction P values based on Wald tests were calculated. In a sensitivity analysis, we fitted the proportional hazard models using age as the underlying time metric, additionally adjusting for date of diagnosis.
Hazard curves for MGUS or MM patients and matched controls were estimated using the kernel-based method implemented in the R-package muhaz.32
Results
A total of 18 627 MM patients with 70 991 matched controls, and 5326 MGUS patients with 20 161 controls were included in the study. Demographic and clinical characteristics of MM and MGUS patients and controls are shown in Table 1. The median age at diagnosis of MM as well as of MGUS was 71 years with almost equal sex distribution. A total of 1756 patients received thalidomide in Sweden 2000 to 2005 and fewer than 100 before the year 2000. Lenalidomide was prescribed to 103 patients 2003 to 2005.
Arterial and venous thrombosis in MM patients
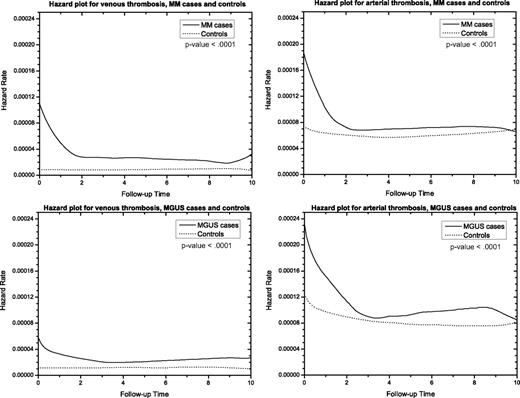
Compared with controls, MM patients had an HR of 7.5 (95% CI, 6.4-8.9), 4.6 (95% CI, 4.1-5.1), and 4.1 (95% CI, 3.8-4.5) for venous thrombosis based on 1, 5, and 10 years of follow-up, respectively, after MM diagnosis (Figure 1). The same pattern was observed when DVT and PE were analyzed separately, and the risks were highest during the first year of follow-up after diagnosis (Table 2). Risks for arterial thrombosis were significantly increased at 1, 5, and 10 years of follow-up, with HR 1.9 (95% CI, 1.8-2.1), HR 1.5 (95% CI, 1.4-1.6), and HR 1.5 (95% CI, 1.4-1.5), respectively (Figure 1). The same pattern was seen in the hazard plots in Figure 2. When analyzed separately, the risks for coronary artery disease and cerebrovascular events were also increased at 1, 5, and 10 years of follow-up (Table 2). MM patients had a significantly increased risk of any thrombosis (venous and arterial) at 1, 5, and 10 years of follow-up after diagnosis, with HR 2.6 (95% CI, 2.4-2.8), 1.9 (95% CI, 1.8-2.0), and 1.8 (95% CI, 1.7-1.9), respectively. Analyses stratified by sex resulted in similar risk estimates (Table 2). When we allowed the impact of case control status to differ for the first year using a time-dependent variable, risk was significantly higher during the first year after diagnosis than subsequently for all outcomes studied in Table 2 (P < .001).
Cumulative risk of arterial and venous thrombosis in patients with MM and MGUS compared with matched controls.
Cumulative risk of arterial and venous thrombosis in patients with MM and MGUS compared with matched controls.
Hazard curves for arterial and venous thrombosis among MGUS and MM patients compared with matched controls.
Hazard curves for arterial and venous thrombosis among MGUS and MM patients compared with matched controls.
The HR for venous thrombosis and arterial thrombosis in MM patients diagnosed before compared with after the year 2000 were not significantly different (data not shown).
Arterial and venous thrombosis in MGUS patients
MGUS patients had an HR of 3.4 (95% CI, 2.5-4.6), 2.1 (95% CI, 1.7-1.5), and 2.1 (95% CI, 1.8-2.4) for venous thrombosis at 1, 5, and 10 years of follow-up, respectively (Figure 1). The risks of DVT and PE were also significantly increased for all follow-up periods (Table 3). Risks for arterial thrombosis were significantly increased, with HR 1.7 (95% CI, 1.5-1.9), 1.3 (95% CI, 1.2-1.4), and 1.3 (95% CI, 1.3-1.4) at 1, 5, and 10 years of follow-up after diagnosis, respectively (Figure 1). The same increased risks were seen in Figure 2. Risks for coronary artery disease and cerebrovascular events were increased for all follow-up periods (Table 3). MGUS patients had a significantly higher risk of any thrombosis than matched controls at 1, 5, and 10 years of follow-up after diagnosis, with HR 1.9 (95% CI, 1.7-2.1), 1.4 (95% CI, 1.3-1.5), and 1.4 (95% CI, 1.4-1.5). When we allowed the impact of case-control status to differ for the first year using a time-dependent variable, risk was significantly higher during the first year after diagnosis than subsequently for all outcomes reported in Table 3 (P < .001). In sensitivity analyses, excluding MGUS patients with thrombosis within the first 6 months after MGUS diagnosis, the observed risks remained significantly elevated. In addition, when restricted to MGUS patients diagnosed before age 40, risk was still significantly elevated (data not shown).
When we assessed risk by MGUS isotype (Table 4), patients with IgG/IgA MGUS had significantly increased risk of both venous and arterial thrombosis based on 1-year follow-up (HR = 4.2; 95% CI, 2.6-6.8 and HR 1.6; 95% CI, 1.3-1.9, respectively) and 5-year follow-up after diagnosis (HR = 2.1; 95% CI, 1.6-2.7 and HR 1.2; 95% CI, 1.1-1.4, respectively). Patients with IgM MGUS did not have an increased risk of venous or arterial thrombosis compared with controls (Table 4).
Risks for thrombosis did not vary by M-protein concentration (> 10.0 g/L or < 10.0 g/L) at diagnosis (Table 5).
MGUS, thrombosis, and subsequent MM
Ten years after MGUS diagnosis, there were 170 (6.2%) IgG/IgA patients who progressed to MM. The risk of progression to MM was not different in patients with venous or arterial thrombosis compared with those without (Table 6). A total of 28 (0.5%) patients progressed to Waldenström macroglobulinemia.
Survival in relation to thrombosis in MM and MGUS
Survival in MM patients with versus without thrombosis was not statistically different at 5 (HR = 1.0; 95% CI, 0.3-3.5) or 10 years (HR = 0.8; 95% CI, 0.4-1.6). MGUS patients with thrombosis had inferior survival compared with MGUS patients without thrombosis at 5 (HR = 1.7; 95% CI, 1.3-2.2) and 10 years (HR = 1.6; 95% CI, 1.3-2.0).
Discussion
In this large study, including more than 5000 MGUS patients, 18 000 MM patients, and their matched controls, we found that both MGUS and MM patients had an increased risk of venous as well as arterial thrombosis. Among MGUS patients, an excess risk of thrombosis was observed in patients with IgG/IgA MGUS, but not IgM. In contrast to a prior smaller study,18 we did not find thrombosis to predict for MM progression among patients diagnosed with MGUS. In accordance with other studies, thrombosis in MM patients had no effect on survival.33 However, thrombosis in MGUS patients was associated with an inferior survival. Our findings are important and provide novel clues for future studies designed to explore underlying mechanisms of myelomagenesis and thromboembolism. In addition, these results may impact the development of thrombosis prophylaxis strategies in MM and possibly MGUS patients.
In accordance with prior smaller studies18,19 as well as our recent study on veterans in United States,21 we found MGUS to be associated with an increased risk of DVT and PE. In the present study, we found a slightly higher risk of DVT during the first year and a stable increased risk thereafter. This pattern is quite consistent with the results from our previous study in which we found a constant excess risk of DVT over time.21 We found no difference in the risk of MM progression among MGUS patients with (vs without) a diagnosis of venous thrombosis, which is in contrast to the study by Sallah et al.18 Interestingly, we found that patients with IgM MGUS did not have an increased risk of thrombosis, whereas patients with IgG/IgA MGUS had a 4-fold increased risk of venous thrombosis. Although the exact mechanisms remain unclear, this is consistent with previous studies on patients with Waldenström macroglobulinemia treated with IMiDs, where no increased risk of venous thrombosis was observed.34-36 Thus, our study and these prior observations suggest that there might be a biologic difference between IgG/IgA and IgM MGUSwith regard to risk of thromboembolism. In contrast to the study by Sallah et al,18 we found no association between concentration of M-protein and risk of thrombosis.
In accordance with the literature, we found MM patients to have a significantly increased risk of venous thromboembolism, with the highest risk during the first year after diagnosis.13,16,21 The reason for the observed increased risk of venous thrombosis in MM is not completely understood. Factors such as immobilization, surgery, infections, indwelling central venous catheters, use of erythropoietin, and acquired and inherited hypercoagulable state are known risk factors for venous thrombosis and have probably contributed to the excess risk.10,37-39 However, because the highest risk of venous thrombosis was observed during the first year after diagnosis, it seems reasonable that the hypercoagulable state, at least in part, also reflects accelerated neoplastic activity, high tumor burden, and perhaps active MM therapy.13 Indeed, studies focusing on IMiDs and risk of venous thrombosis in MM have reported the cumulative incidence to vary between approximately 2% and 75%, with the greatest risk in previously untreated patients receiving combination therapy.13,40 The fact that we observed no increase in risk of thrombosis in the period after 2000 (compared to before) is probably explained by the fact that very few MM patients in Sweden have received treatment with IMiDs, as first-line therapy during the study period as, in accordance with national guidelines, younger patients were treated with the VAD regimen until the year 2005, when cyclophosphamide and dexamethasone became standard. Similarly, elderly patients in Sweden, not included in clinical trials, have been treated with the MPT regimen only for the last 3 to 4 years.41
Our findings of an increased risk of arterial thrombosis in both MM and MGUS are novel. More specifically, we found the risk of coronary artery disease and cerebrovascular disease to be elevated among MGUS and MM patients. In patients with MM, the risk of arterial thrombosis was significantly elevated throughout the study period and did not differ statistically significantly between the time period before and after the introduction of the IMiDs. However, further investigations with longer follow-up are needed.
For the first time, we show that thrombosis is a predictor of survival in MGUS patients diagnosed in a clinical setting. The same did not apply to MM patients, as in accordance with another study.33 In our previous study, we found life expectancy in MGUS patients to be decreased compared with the general population, and predictors of poor survival were high age at diagnosis and IgG or IgA isotype. Furthermore, the risk for death in ischemic heart disease was 30% higher than in matched controls.42 Thrombosis in MGUS is thus a new predictor of survival but not MM progression.
Our study adds substantially to the limited literature on thrombosis among patients with plasma cell dyscrasias. For the first time, we provide a quantitative measure of arterial and venous thrombosis risk among patients with MGUS and MM. We have speculated that the elevated risk of thrombosis among MGUS patients is less likely caused by accelerating neoplastic activity but is rather a result of ongoing clonal plasma cell activities, as has been suggested by other authors.39 Factor VIII and von Willebrand factor levels were found to be increased among MGUS cases in a recent study; and in good accordance with our clinical findings, the observed increase was similar to that of untreated MM patients.39 Our observations of an excess risk of arterial thromboembolism among MGUS and MM patients may influence clinical management and the development of improved prophylactic regimens. Furthermore, they are of importance for our understanding of the pathogenesis of thromboembolism in plasma cell dyscrasias. Indeed, the observed excess risk of both arterial and venous thrombosis suggests that there might be some shared biologic features, most probably involving platelet activation. This is further supported by the indication that aspirin is an effective prophylactic agent in venous thrombosis in MM.17,43,44 In addition, some studies have found evidence of platelet aggregation43 and activation caused by thalidomide, which is abrogated by aspirin.45 Future investigations are needed to clarify underlying mechanisms of our observations.
Our study has several strengths, including its large size as well as the application of high-quality data from Sweden, with its stable population with access to standardized medical care during the entire study period. In our study, we used a register-based cohort design, which ensured a population-based setting and generalization of our findings. As reported previously,46 the MGUS patients in our study were diagnosed at hematology/oncology outpatient units. In accordance with clinical practice in Sweden, most MGUS patients typically underwent a bone marrow examination as part of the clinical workup. In a recent validation study, we have reported that ascertainment and diagnostic accuracy for lymphoproliferative disorders are very high (> 90%-95%) in Sweden.30 Limitations include the lack of information on potential confounders (although the matched design and analyses ensured adjustment for sex, age, and geography), and lack of detailed clinical data, including information on subtype of MM and underlying diseases. Because our data do not come from an MGUS screening study, some of the controls might have an undiagnosed MGUS, and also the observed excess risks among MGUS patients may partly reflect various underlying medical illnesses that lead to the medical workup and the detection of the M-protein. To minimize such influences, MGUS patients with a diagnosis of a lymphoproliferative malignancy within 6 months after MGUS diagnosis were excluded from our analyses. In addition, when excluding MGUS patients with a thrombosis within 6 months after MGUS diagnosis, the risks were still elevated. Another limitation is the potential inaccuracy and lack of independent validation of thromboembolic diagnosis obtained from the centralized Patient Registry. However, because we compared MM/MGUS cases to matched controls, using data from the same registries, the ascertainment should be nondifferential and any bias should be toward a null association.
In conclusion, in a population-based clinical setting (compared with controls), we found patients with MM and IgG/IgA MGUS to have a significantly increased risk of both arterial and venous thrombosis. In contrast, IgM MGUS patients had no excess risk of thrombosis. MGUS patients with thrombosis had an inferior survival compared with those without. We did not find thromboembolism to be a predictor of MM progression among patients diagnosed with MGUS. Future studies are needed to clarify underlying mechanisms of our findings. Such efforts will be of relevance for physicians managing MGUS and MM patients and have potential implications for the development of better thrombosis prophylaxis strategies.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Medical Product Agency in Sweden, Ms Shiva Ayobi, the National Board of Health and Welfare, Stockholm, Sweden; Medical Products Agency, Uppsala, Sweden; Ms Susanne Dahllöf, Statistics Sweden, Örebro, Sweden; and Ms Charlotta Ekstrand, Ms Molly Collin, and Ms Lisa Camner, Karolinska Institutet, Stockholm, Sweden, for invaluable ascertainment of MGUS data.
This work was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute, the Swedish Cancer Society, Stockholm County Council, and the Karolinska Institutet Foundations.
Authorship
Contribution: S.Y.K., M.B., I.T., and O.L. designed the study, obtained data, and initiated this work; R.M.P. performed all statistical analyses; S.Y.K., R.M.P., and O.L. wrote the report; all the authors were involved in the interpretation of the results; read, gave comments, and approved the final version of the manuscript; had full access to the data in the study; and take responsibility for the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sigurdur Y. Kristinsson, Department of Medicine, Division of Hematology, Karolinska University Hospital Solna, SE-171 76 Stockholm, Sweden; e-mail: sigurdur.kristinsson@karolinska.se.