In this issue of Blood, Kristinsson and colleagues report an increased risk of venous and arterial thrombosis in MGUS and multiple myeloma in a population-based study including 18 627 patients with multiple myeloma, 5326 patients with MGUS, and 70 991 controls.1
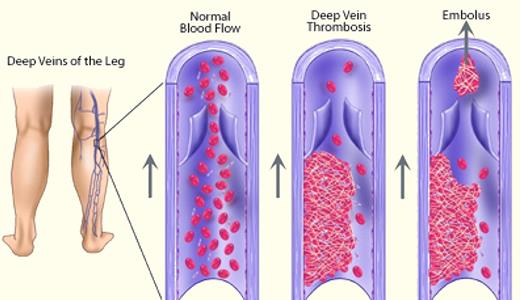
Since the early days of Rudolf Karl Ludwig Virchow (1821-1902), the occurrence of thrombosis is thought to be due to one or more of 3 basic changes: alterations in the vessel wall, decreased blood flow, and changes in the cellular and/or soluble components of the blood. Thrombosis usually is associated with disruptive symptoms, may lead to a postthrombotic syndrome, and can sometimes become life-threatening (see figure). The age-adjusted incidence of venous thrombosis in the general population is 1 per 1000 person-years2 and is similar to the incidence of stroke.3 For myocardial infarction, incidence rates of 0.18 per 1000 persons of an age-standardized population have been reported.4 Hence, venous and arterial thrombosis are frequent complications with their incidence rising with age.
In this issue, Kristinsson et al provide substantial evidence for a significantly increased incidence of venous thrombosis, and interestingly also for arterial thrombosis, both in patients with monoclonal gammopathy of undetermined significance (MGUS) as well as in those with multiple myeloma.1 The reported risk for venous thrombosis at 1 year after diagnosis was twice as high in myeloma compared with patients with MGUS, whereas for arterial thrombosis, this risk seems similarly increased in both patient groups. As for myeloma, a significantly increased risk for venous thrombosis in patients with MGUS has previously already been reported by the same authors5 and summarized by others,6 although it remained unclear to what extent this increased risk can be attributed to the MGUS itself, or to the underlying medical problems prompting laboratory testing for monoclonal proteins. This question now seems to be resolved by the documentation of a continued elevated thrombotic risk even after 10 years of follow-up, diminishing or excluding a role of confounding comorbidities.
In addition, several other new findings in the MGUS cohort are notable. Thrombosis in MGUS was associated with increased mortality but not with transformation to multiple myeloma. This indicates that the underlying premalignant process activates biological pathways, putting the affected person with MGUS and thrombosis at a higher risk for mortality. Increased secretion of proinflammatory cytokines, elevated levels of factor VIII and von Willebrand factor, decreased protein S, resistance to activated protein C, impaired fibrinolysis, platelet activation, and endothelial damage have all been described in MGUS.7 Whether these or some other as yet not identified mechanisms account for the higher mortality in MGUS patients with thrombosis remains to be resolved. In myeloma, high levels of factor VIIIc and von Willebrand factor antigen are associated with disease activity, probably reflecting increased bone marrow angiogenesis.8
The risk for thrombosis was highest during the first year after diagnosis of MGUS, an observation reported for myeloma patients as well. The thrombotic risk reduction with further course of the disease is mainly due to better disease control after initiation of antimyeloma therapy. However, this argument does not apply to MGUS patients as they are not receiving any disease-specific therapy. Which factor(s) then could account for the decline in thrombosis incidence even at 1 year after MGUS diagnosis? There are no convincing data providing an answer available as yet. It may be speculated that the underlying medical problems that motivate a patient to undergo clinical investigation increased the risk for thrombosis. Alternatively, the interaction between the monoclonal cell population and the host may be more intense shortly after initiation of the clonal plasma cell expansion. Thereafter, contra-regulatory mechanisms may dampen the stimulatory activity and thereby reduce, but not efface, the thrombotic risk.
Only patients with an IgG or IgA isotype, but not those with an IgM paraproteinemia, were at increased risk for thrombosis. This is an important observation pinpointing a substantial difference in the underlying biological processes in patients with different paraprotein isotypes. However, most likely not the paraprotein itself but rather the clonal cells and their interactions with the components of the host account for all or most of the different biological and clinical sequels.
Patients with MGUS had an almost 2-fold increased risk of arterial thrombosis compared with matched controls, an observation not reported before, although its explanation needs still to be elucidated. The analysis in patients with myeloma revealed for the first time also an increased risk for arterial thrombosis, of similar magnitude as in MGUS. These latter findings are substantiated by a prospective cohort study of the HOVON group, which included patients receiving induction therapy either with TAD (thalidomide-adriamycin-dexamethasone), PAD (bortezomib-adriamycin-dexamethasone), or VAD (vincristine-adriamycin-dexamethasone), followed by stem cell transplantation.9 These authors observed arterial thrombosis in 11 of 195 patients (5.6%) followed for 522 patient-years. The incidence of arterial thrombosis was not different in the 3 treatment cohorts (VAD: 5.9%, TAD: 4.5%, PAD: 6.4%), with 5 of the 11 events occurring during induction therapy. In this study, multivariate analysis revealed increased factor VIII levels, together with hypertension and smoking as critical risk factors for arterial thrombosis. Importantly, 4 patients developed arterial thrombosis while on therapy with vitamin K antagonists, and 2 despite low-molecular-weight heparin prophylaxis, indicating that platelet inhibitory treatment might be more effective and appropriate in reducing the incidence of arterial thrombosis.
What are the consequences for clinical management? For MGUS patients, applying the widely accepted guidelines for antithrombotic prophylaxis should suffice, therefore restricting therapy only to persons with additional risk factors for thrombosis.10 In multiple myeloma, the situation is more complex because the thrombotic risk depends not only on disease-related and conventional risk factors, but even more on the type of antimyeloma therapy applied. Thalidomide, high-dose dexamethasone, doxorubicin, erythropoietins, and lenalidomide all increase the risk for thrombosis. Guidelines for prophylactic interventions have been published,6 but several of the recommendations are based on expert opinions rather than on solid clinical data.
In the future, we should aim at elucidating the underlying causes of increased thrombosis risk in greater detail, establish more precise risk models for patient selection for prophylactic treatment, and elucidate the value of novel oral antithrombin and antifactor Xa inhibitors in this setting.
Conflict-of-interest disclosure: H.L. received honoraria for lectures from Celgene, Ortho-Biotech, and Mundipharma, and research funding from Ortho-Biotech, Celgene, and Mundipharma. M.D. received honoraria for lectures from Celgene and Ortho-Biotech. ■