Abstract
Mounting evidence suggests that dynamic interactions between a tumor and its microenvironment play a critical role in tumor development, cell-cycle progression, and response to therapy. In this study, we used mantle cell lymphoma (MCL) as a model to characterize the mechanisms by which stroma regulate cell-cycle progression. We demonstrated that adhesion of MCL and other non-Hodgkin lymphoma (NHL) cells to bone marrow stromal cells resulted in a reversible G1 arrest associated with elevated p27Kip1 and p21 (WAF1) proteins. The adhesion-mediated p27Kip1 and p21 increases were posttranslationally regulated via the down-regulation of Skp2, a subunit of SCFSkp2 ubiquitin ligase. Overexpression of Skp2 in MCL decreased p27Kip1, whereas inhibition of Skp2 by siRNA increased p27Kip1 and p21 levels. Furthermore, we found cell adhesion up-regulated Cdh1 (an activating subunit of anaphase-promoting complex [APC] ubiquitin ligase), and reduction of Cdh1 by siRNA induced Skp2 accumulation and hence p27Kip1 degradation, thus implicating Cdh1 as an upstream effector of the Skp2/p27Kip1 signaling pathway. Overall, this report, for the first time, demonstrates that cell-cell contact controls the tumor cell cycle via ubiquitin-proteasome proteolytic pathways in MCL and other NHLs. The understanding of this novel molecular pathway may prove valuable in designing new therapeutic approaches for modifying tumor cell growth and response to therapy.
Introduction
Mantle-cell lymphoma (MCL) is now recognized as a distinct clinicopathologic subtype of B-cell non-Hodgkin lymphoma (NHL) and constitutes approximately 5% to 8% of all NHLs.1 It carries the poorest prognosis among NHL subtypes; the median survival is 3 to 4 years.1 Mounting evidence now suggests that dynamic interactions between the lymphoma cell and its local microenvironment may play a critical role in tumor development. In this study, we used MCL as a model to characterize the mechanisms by which stroma regulate cell-cycle progression.
Lymphoma cells home to the bone marrow, suggesting that the bone marrow microenvironment provides components necessary for survival of lymphoma cells. The incidence of bone marrow involvement by NHL varies from 40% to 80% in MCL and from 20% to 60% in intermediate- and high-grade lymphomas.2 The mere presence of residual lymphoma in the marrow is predictive for a poor outcome.2 Undetectable subpopulations of tumor cells that survive drug treatment consequently lead to relapse.3 Previous studies4,5 demonstrated that adhesion of myeloma cell lines to fibronectin (FN) induced cell-cycle arrest and protected cells from apoptosis via increased p27Kip1 protein. A more recent study has shown that cell-cycle arrest can be induced in myeloma cells following adhesion to bone marrow stromal cells via up-regulation of p21 protein.6 These findings suggest cell-adhesion–mediated regulation of cell-cycle progression and the induction of a dormant state by bone marrow stroma through the cyclin-dependent kinase inhibitors (CDKIs) p21 or p27Kip1. However, how bone marrow stroma regulates cell-cycle progression (p21 or p27Kip1), and the underlying molecular mechanisms involved, are unclear to date.
Accumulating evidence shows that the cell cycle is regulated by the cyclin-dependent kinases (Cdks) and their inhibitors (CDKIs).7 The Cdks (CDK2, CDK4) and their regulatory partners, the cyclins (cyclin E, cyclin D) control progression through the cell cycle.8 The activity of the CDKs is further regulated by the CIP/Kip family and the INK family of CDKIs.7,8 A large body of research has documented that the CDKI protein p27Kip1 negatively regulates the transition from the G1- to the S-phases of the cell cycle by inhibiting Cdk2-cyclin E and Cdk2-cyclin A complexes.9 Quiescent cells or cells exposed to certain antimitogenic and differentiation signals such as contact inhibition, serum deprivation, and TGF-β have high levels of the p27Kip1 protein.10 Mitogenic signals cause a rapid decrease in p27Kip1 by inducing the polyubiquitination and proteasome-dependent degradation of the protein.1-12 Recently, several lines of evidences have supported the idea that the intracellular concentration of p27Kip1 is regulated predominantly through the ubiquitin-mediated degradation of the proteolytic pathway.12 Linkage of ubiquitin to p27Kip1 requires the activities of the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3). The E3 ligase activity in the ubiquitination of p27Kip1 has been provided by the stem cell factor (SCF) complex, composed of Skp1, Cullin-1, F-box protein, and the p27Kip1-specific F-box protein Skp2. Skp2 specifically interacts with the extreme COOH terminus of p27Kip1 when Thr187 is phosphorylated by cyclin E/CDK2.13 This association of Skp2 with p27Kip1 results in the recruitment of p27Kip1 to the SCF core complex, thereby promoting its ubiquitination and degradation.14,15 Anaphase-promoting complex/cyclosome (APC/C) is a multisubunit complex that functions as a ubiquitin ligase specific for various cell-cycle proteins, including cyclin B, cyclin A, mitotic kinases, inhibitors of anaphase, spindle-associated proteins, and inhibitors of DNA replication.16 The activity of APC is tightly regulated to control cell-cycle progression, with high levels from late mitosis until late in the G1-phase, but low levels in S-phase, G2-phase, and early mitosis. The APC ubiquitinates regulatory proteins such as securin and cyclin B and thereby targets them for destruction by the 26S proteasome. Activation of the APC depends on the activator proteins Cdc20 and Cdh1, which are thought to recruit substrates to the APC.17 Two recent studies18,19 suggest that Skp2 accumulation is determined by the APC/C (Cdh1) ubiquitin ligase. It is not clear whether this mechanism is also used to stop cell proliferation when cells are adhered to stromal cells. In this study, we used MCL as a model to elucidate the mechanisms by which cell-adhesion–regulated cell-cycle progression and degradation of p27Kip1 in MCL cell lines and primary MCL cells. We demonstrated that adhesion of MCL and other NHL cells to bone marrow stromal cell (HS-5) resulted in a reversible growth arrest and elevated p21 and p27Kip1 protein levels through down-regulation of the SCFSkp2 ubiquitin ligase, Skp2. Furthermore, we provide evidence demonstrating that Cdh1 (APC ubiquitin ligase activator) was strongly induced by cell adhesion to stroma, and inhibition of Cdh1 by siRNA triggered Skp2 accumulation and hence p27Kip1 and p21 degradation, implicating Cdh1/APC ubiqutin ligase as an upstream effector of the Skp2/p27Kip1 signaling pathway. Overall, our study, for the first time, describes that cell-cell adhesion controls cell-cycle progression via ubiquitin-proteasome proteolytic pathways in MCL and in other NHLs.
Materials and methods
Cell lines, cell cultures, and patient lymphoma samples
The human lymphoma cell lines SUDH-10 and SUDH-4 were provided by Dr M. Raffeld (National Cancer Institute, Bethesda, MD). The human bone marrow stromal cell line, HS-5, was obtained from the American Type Culture Collection (Rockville, MD). The cells were grown in suspension in RPMI 1640 (Cellgro; Fischer Scientific Pittsburgh, PA), supplemented with 5% fetal bovine serum (Omega Scientific, Tarzana, CA), and 1% (vol/vol) penicillin (100 U/mL), streptomycin (100 U/mL), and 1% (vol/vol) l-glutamine (GIBCO-BRL, Grand Island, NY). Cells were fed after 4 days by adding one-half volume of complete medium, and then at weekly intervals by complete replacement of the medium, and were maintained at 37°C in a 5% CO2/95% air atmosphere.
Diffuse large B-cell lymphoma (DLBL) and MCL cells were obtained from fresh biopsy-derived lymphoma tissues (lymph nodes) after informed consent was obtained, in accordance with the Declaration of Helsinki, and after approval by the institutional review board of the University of South Florida. The lymph nodes were cut and gently dispersed. For preparation of viable, sterile, single-cell suspensions, the lymph node tissue was diced and forced through a metal sieve into RPMI tissue culture medium. Cells, obtained after low-speed centrifugation, were resuspended in media. After Ficoll-Paque purification, the CD19+ cells were positively isolated by using CD19 microbeads with the autoMACS magnetic cell sorter according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Either lymphoma cell lines or CD19-sorted lymphoma cells (from diffuse large-cell lymphomas; 1.5 × 106 cells/mL) were adhered to a preestablished monolayer of HS-5 or kept in suspension for 12 hours. MCL cells were used for experiments without CD19 sorting since all of our MCLs had more than 80% of lymphoma cells in purity. Lymphoma cells were then carefully removed with the monolayer of HS-5 kept intact. The purity of the lymphoma cell population was greater than 90% by flow cytometry. Cells were then analyzed. In transwell experiments, lymphoma cells were separated from the HS-5 by transwell inserts.
Antibodies, Western blotting, and Skp2 degradation assay
The following monoclonal antibodies were purchased: p27Kip1, p21Cip1/WAF1, cyclin D1 (BD Transduction Laboratory, San Diego, CA), Skp2 (Zymed Laboratories, South San Francisco, CA), and Cdh1 (Sigma, St Louis, MO). At the indicated time, cells were collected, washed twice with ice-cold PBS, lysed in ice-cold lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 0.1% Tween-20, 2 mM EDTA, 2 mM EGTA, 1 mM NaF, 0.1 mM NaVO4, 5 μg/mL leupeptin, 0.2 mM PMSF, and 1 mM dithiotreitol. Following 30 minutes of incubation on ice, the lysates were sonicated and centrifuged at 12 000g for 15 minutes at 4°C. The lysates were quantified using Biorad reagent (Biorad, Hercules, CA). A total of 50 μg whole-cell lysate was separated by 4% to 12% NuPage gel (Invitrogen, Carlsbad, CA), transferred to polyvinylidenefluoride (PVDF) membrane, and blocked with 5% milk in TBS/T. The membrane was then probed with with either a p27Kip1, Skp2, p21Cip1/WAF1, or Cdh1 monoclonal antibody, washed with TBS/T, and incubated with horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody for 1 hour. Specific bands were developed by means of a chemiluminescence reagent kit (Amersham Life Sciences, Arlington Heights, IL). The blot was subsequently stripped (100 mM betamercaptoethanol, 2% SDS, and 62.5 mM Tris [pH 6.7] for 30 minutes at 50°C) and reprobed for β-actin (Sigma). Protein bands were quantified by the Image Quant software program (Molecular Dynamics, Sunnyvale, CA).
For measurement of Skp2 degradation, Jeko-1 cells in suspension versus HS-5 adhesion were treated with cycloheximide (Sigma), which was added to the culture medium (final concentration: 100 μM, 2 hours) to inhibit protein synthesis. At the times indicated following cell adhesion, cells were lysed, and Skp2 protein levels were determined by Western blot analysis for determination of Skp2 protein stability.
Cell-cycle analysis
Lymphoma cells that were either adhered to HS-5 or kept in suspension for 24 hours were treated for 1 hour with 30 μg/mL bromodeoxyuridine (BrdU; Sigma), collected, and fixed in ice-cold 66% ethanol. Cells were stained with anti-BrdU and propidium iodide (PI) as previously described5 and analyzed by flow cytometry (Becton Dickinson, San Jose, CA).
RNase protection assay
RNA expression levels of CDKIs, including p27Kip1, were measured by an RNase protection assay. RNA was isolated from either Jeko-1 or SUDH-10 cells using RNeasy columns according to the manufacturer's instructions (Qiagen, Valencia, CA), following adherence to the HS-5 stroma cell line for 24 hours. RNase protection assays were performed using the Pharmingen RiboQuant cell-cycle kit according to the manufacturer's protocol (Pharmingen, San Diego, CA). The multiprobe template was prepared by 32P incorporation in an in vitro transcription reaction, and free nucleotide was removed on a G50 column. Purified probe (1 × 106 cpm specific activity) was combined with 20 μg of total RNA. The hybridization reaction was carried out overnight at 56°C. Samples were treated with RNase for 45 minutes at 30°C and then with proteinase K for 15 minutes at 37°C. Hybridized probes were extracted with phenol:chloroform:isoamyl alcohol and precipitated using ethanol. Samples were separated on a 5% denaturing gel (7 mM urea), and protected fragments were quantified by phosphorimaging analysis using Image Quant software (Molecular Dynamics).
Transient transfection of siRNA
Skp2 siRNA, p27, and Cdh1siRNA were purchased from Dharmacon (Chicago, IL). Control (nonsilencing) siRNA was purchased from Qiagen. It is important that the negative control siRNA is incorporated into RNA-induced silencing complex (RISC), and that it goes through the same biological process of entry into RISC as the gene-specific siRNA. Thus, we also used control-RISC siRNA (siCONTROL, a sequence processed by RISC; Dharmacon) as another negative control siRNA and made comparison with the control (nonsilencing) siRNA from Qiagen. We observed the same result when applied to siRNA experiments for p27, Skp2, and Cdh1. The control-RiSC siRNA is a siCONTROL nontargeting pool siRNA, which was designed to have no gene targets in human, mouse, and rat cells. For transient expression, cell lines were transfected by electroporation using Nucleofector (Amaxa, Gaithersburg, MD) according to the manufacturer's instructions and commercially available transfection instrument (Amaxa, Koeln, Germany). Briefly, cells (5 × 106) from each lymphoma cell line were suspended in 100 μL Nucleofector T solution with siRNA duplex (final concentration, 1 μM) and then electroporated using Amaxa program T-001. Cells were cultured in 5 mL of 37°C prewarmed medium containing 10% FCS. Based on the expression of green fluorescent protein (GFP) analyzed by flow cytometry, the transfection efficiency was between 50% and 75%, with a median of 60% (5 independent experiments).
qRT-PCR analysis
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) analysis for p27, Skp2, and Cdh1 was performed as described previously.20 Briefly, total RNA was extracted using the RNeasy Mini Kit (Qiagen) and used for cDNA synthesis (Invitrogen first-strand cDNA synthesis kit; Invitrogen, Frederick, MD). Expressions of Cdh1, Skp2, p27Kip1, and GAPDH were analyzed using inventoried primers: TaqMan gene expression assay Hs 00212373_m1, Hs 00261857_m1, Hs 00153277_m1, and Hs 99999905_m1, respectively (Applied Biosystems [ABI], Foster City, CA). Real-time PCR reactions were performed using the ABI 7900 Sequence Detection System (ABI). All cDNA samples were synthesized in parallel, and PCR reactions were run in triplicate. mRNA levels were derived from standard curves, and the gene-expression level was normalized using the endogenous control gene GAPDH (the relative gene-expression level was determined using 2-(-delta delta C (T)) (ΔΔCT) methods.20 In addition, knockdown of mRNA for p27, Skp2, and Cdh1 by their respective siRNAs are confirmed by using this qRT-PCR method (data not shown).
Establishment of SUDH-4 and Jeko-1 stable clones
Jeko-1 lymphoma cells were transfected with 10 μg of pcDNA3-Skp2 plasmid for the establishment of Skp2 stable clones or with pcDNA3 as the vector control as described previously.21 All of the stable clones were obtained through selection in medium containing 400 μg/mL G418 for a period of about 4 weeks, followed by clonal isolation with glass cylinders as described previously.21
Statistical analysis
The significance of differences between experimental conditions was determined using the 2-tailed Student t test.
Results
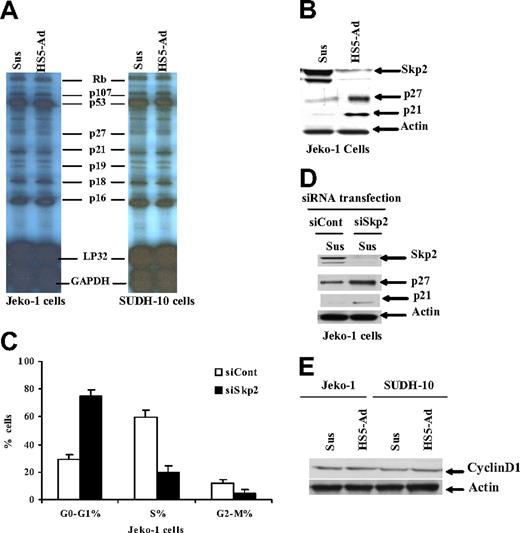
Cell adhesion to bone marrow stroma induces a reversible cell-cycle arrest and increases p27Kip protein level
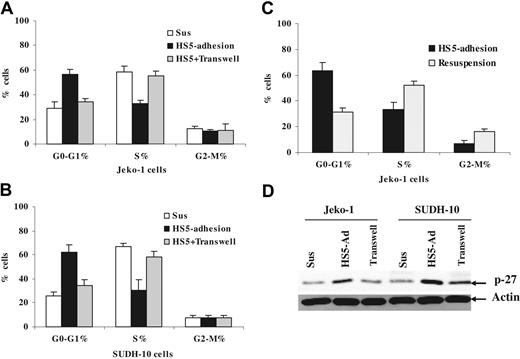
It has been demonstrated that adhesion of myeloma cells to the extracellular matrix (ECM) component fibronectin results in a reversible growth arrest and elevated p27Kip1 protein levels.8 In this study, we extended our investigations to lymphoma adherent to stromal cells in an attempt to further elucidate the molecular mechanism by which stroma regulates lymphoma cell-cycle progression. We asked whether adhesion of lymphoma cells to bone marrow stromal cells results in growth inhibition of lymphoma cells. Following 12 hours of adhesion, we observed approximately 2-fold more cells residing in G1-phase compared with cells in suspension for both the MCL cell line (Jeko-1) and the large-cell lymphoma cell line (SUDH-10), as illustrated in Figure 1. The increase in the number of cells in G1-phase correlated with the presence of 2-fold fewer cells residing in S-phase (Figure 1A). Evidence has accumulated suggesting that the tumor microenvironment can influence tumor cells in at least 2 different ways: via cell-cell contact or by soluble factors. To examine the contribution of soluble factors independent of physical cell contact, we used transwell inserts to allow lymphoma cells to be juxtaposed to HS-5 stromal cells without cell-cell contact. In order to include the soluble factors produced via dynamic interaction between bone marrow stroma and lymphoma cells, we performed the experiments by incubating lymphoma cells in a transwell insert (upper chamber) with coculture of HS-5 and lymphoma in the lower chamber. As shown in Figure 1A, lymphoma cells that were in direct contact with the HS-5 cell line, but not cells exposed to soluble factors (in transwell insert), were cell-cycle arrested. To determine if the growth arrest initiated by adhesion to stroma was reversible, cells were detached following 12 hours of adhesion and then grown in suspension for 12 to 24 hours prior to cell-cycle analysis. As shown in Figure 1B, disruption of adhesion to stroma resulted in the rapid increase in S-phase cells. This indicates that adhesion to stroma did not induce terminal growth arrest in these lymphoma cells, and that the adherent population appears to accumulate at the G1/S interphase.
Cell adhesion to bone marrow stroma induces a reversible cell-cycle arrest and increases p27Kip protein levels. Jeko-1 (A) or SUDH-10 (B) lymphoma cells (105/mL) were incubated for 12 hours either in the absence of HS-5 bone marrow stromal cells (Sus), or on a confluent HS-5 monolayer (HS5-adhesion), or in the same well with a confluent HS-5 monolayer but separated by cell culture inserts (HS-5 + Transwell). (C) Jeko-1 cells were resuspended for 12 hours after adhering to HS-5 (Resuspension). After the indicated time, lymphoma cells were followed by pulsing with BrdU for 40 minutes and staining with anti-BrdU-FITC for detection of S-phase cells and PI for cell-cycle distribution. Data shown are the mean of 3 experiments. (D) Direct cell-cell contact but not soluble factor(s) increased p27Kip1 expression analyzed by Western blot in Jeko-1 and SUDH-10 lymphoma cells. All data are representative of at least 4 experiments (means ± SD).
Cell adhesion to bone marrow stroma induces a reversible cell-cycle arrest and increases p27Kip protein levels. Jeko-1 (A) or SUDH-10 (B) lymphoma cells (105/mL) were incubated for 12 hours either in the absence of HS-5 bone marrow stromal cells (Sus), or on a confluent HS-5 monolayer (HS5-adhesion), or in the same well with a confluent HS-5 monolayer but separated by cell culture inserts (HS-5 + Transwell). (C) Jeko-1 cells were resuspended for 12 hours after adhering to HS-5 (Resuspension). After the indicated time, lymphoma cells were followed by pulsing with BrdU for 40 minutes and staining with anti-BrdU-FITC for detection of S-phase cells and PI for cell-cycle distribution. Data shown are the mean of 3 experiments. (D) Direct cell-cell contact but not soluble factor(s) increased p27Kip1 expression analyzed by Western blot in Jeko-1 and SUDH-10 lymphoma cells. All data are representative of at least 4 experiments (means ± SD).
We next investigated whether the observed increase in the percentage of cells residing at the G1/S boundary correlated with increased expression of the cell-cycle regulator, p27Kip1 in lymphoma cells. We examined the protein level of p27Kip1 in MCL and SUDH-10 lymphoma cells adherent to stroma, cultured in the transwell system, and in suspension. Western blot analysis showed that the p27Kip1 protein was markedly increased when Jeko-1 and SUDH-10 lymphoma cells were adherent to stromal cells (Figure 1D). Taken together, our data indicate that adhesion of lymphoma cells to the HS-5 cell line causes reversible cell-cycle arrest at the G1/S boundary, a finding that is associated with increased p27Kip1 expression. Furthermore, inhibition of cell-cycle progression was not the result of soluble factors produced by the coculture system, but rather requires direct cell-to-cell contact.
Cell-adhesion–mediated p27Kip1 protein level is posttranslationally regulated through down-regulation of the SCFSkp2 ubiquitin ligase, Skp2
To investigate the molecular mechanism for the changes observed in levels of p27Kip1, we used RNase protection assay (RPA) to evaluate the gene expression of a number of cell-cycle family members. Using this assay, we did not observe any significant changes in RNA expression of p27Kip1, p21, or other cell-cycle-related molecules when either Jeko-1 or SUDH-10 cells were adhered to stroma, compared with cells cultured in suspension (Figure 2A). These observations indicate that the cell-adhesion–mediated change in p27 is dependent on a posttranslational regulatory mechanism. Recent evidence indicates that the F-box protein Skp2 mediates the ubiquitin-dependent degradation of several negative regulators of cell proliferation in cancer,22 and the level of Skp2 was found to be the rate-limiting regulator for the degradation of p27Kip1, p21, p57, and cMyc. We therefore hypothesized that Skp2 was a likely candidate for regulating p27Kip1 ubiquitination and proteasomal degradation in adherent lymphoma cells. We examined the levels of Skp2 with and without coculture with HS-5 cells, and assessed the association with p27 and p21 protein levels. A strong inverse relation was found between p27Kip1, p21, and Skp2 levels when lymphoma cells (Jeko-1) were cocultured with HS-5. Decreased levels of p27Kip1 and p21 were associated with increased levels of Skp2, whereas low levels of Skp2 coincided with high levels of p27Kip1 and p21 as shown in Figure 2B. The reduction of Skp2 might represent an important mechanism in the up-regulation of p27Kip1, contributing to the inhibition of cell-cycle progression in adherent lymphoma cells. We next determined the importance of Skp2 in the regulation of p27 levels. Two different approaches were used, aimed at providing direct evidence for a specific role of Skp2 in cell-adhesion–mediated cell-cycle arrest and p27Kip1 elevation. First, we attempted to inhibit Skp2 expression in Jeko-1 and SUDH-10 lymphoma cells by a siRNA strategy. For this purpose, Skp2 activity was blocked by direct and specific inhibition of Skp2 expression using siRNA against Skp2. As depicted in Figure 2C,D, transfection of siRNA against Skp2 resulted in Skp2 protein depletion, and induced cell-cycle arrest in G1-phase (Figure 2C) as well as concomitant p27Kip1 and p21 accumulation in Jeko-1 cells (Figure 2D). Similar results were observed in Mino MCL cells and SUDH-10 lymphoma cells as well as in serum-free medium (data not shown). We next sought to determine whether the increase in Skp2 protein levels leads to cell-cycle progression and the decrease of p27Kip1 following adhesion of lymphoma cells onto HS-5 cells. We established an Skp2 clone that stably expresses Skp2 at higher levels. As expected, transfection of pcDNA3-Skp2 increased Skp2 protein, and likely promoted p27Kip1 degradation and cell-cycle progression. The level of p27 protein in the Skp2 clone was much lower than that in the control empty vector clone, and the number of cells in G1-phase was lower in the stable Skp2 cell line. We also examined the role of Skp2 in cell-adhesion–mediated p27 accumulation and cell-cycle arrest. The result by overexpression and knockdown of Skp2 would be presented in the following sections (Figures 3,4). We next examined the change of cyclin D1 levels upon lymphoma cell adhesion since cyclin D1 plays a critical role in cell entry into S-phase. Figure 2E reveals that cell adhesion has no effect on the level of cyclin D1, indicating that cell-adhesion–mediated cell arrest is mainly through CDKI p21 and p27 proteins.
Cell adhesion–mediated p27Kip1 protein levels are posttranslationally regulated through down-regulation of the SCFSkp2 ubiquitin ligase Skp2. (A) An RPA for gene expression of p27Kip1, p21, and other cell-cycle–related molecules in Jeko-1 and SUDH-10 cells adhered to HS-5 versus those placed in suspension media. (B) The p27Kip1 and Skp2 expression in Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. Cell-cycle distribution (C) and p27Kip1 and Skp2 expression (D) 24 hours after transfection with either Skp2 siRNA (siSkp2) or control siRNA (siCont) in Jeko-1 cells in suspension. (E) Cyclin D1 expression in Jeko-1 and SUDH-10 lymphoma cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. All data are representative of at least 3 experiments (means ± SD).
Cell adhesion–mediated p27Kip1 protein levels are posttranslationally regulated through down-regulation of the SCFSkp2 ubiquitin ligase Skp2. (A) An RPA for gene expression of p27Kip1, p21, and other cell-cycle–related molecules in Jeko-1 and SUDH-10 cells adhered to HS-5 versus those placed in suspension media. (B) The p27Kip1 and Skp2 expression in Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. Cell-cycle distribution (C) and p27Kip1 and Skp2 expression (D) 24 hours after transfection with either Skp2 siRNA (siSkp2) or control siRNA (siCont) in Jeko-1 cells in suspension. (E) Cyclin D1 expression in Jeko-1 and SUDH-10 lymphoma cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. All data are representative of at least 3 experiments (means ± SD).
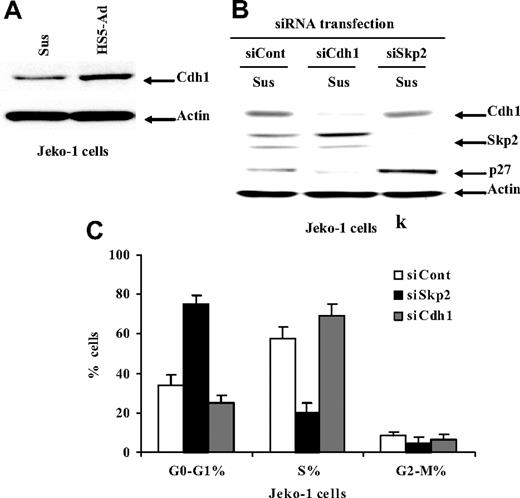
Cell-adhesion–mediated down-regulation of Skp2 requires Cdh1
We next tested whether Cdh1-APC is involved in the regulation of the Skp2/p27Kip1 pathway as well as cell-adhesion–mediated cell-cycle arrest. To investigate the role played by Cdh1 in cell- adhesion–dependent regulation of Skp-2, we examined the change in Cdh1 level upon Jeko-1 cell adhesion to HS-5. As depicted in Figure 3A, Jeko-1 lymphoma cells adherent to HS-5 triggered Cdh1 protein expression. We then proceeded to investigate whether manipulation of Cdh1 levels had any effect on the levels of Skp2 and p27Kip1 and cell-cycle progression. To address this issue, we again used the siRNA strategy to deplete Cdh1 in Jeko-1 and SUDH-10 cells. Cell extracts from Jeko-1 cells transfected with Cdh1 siRNA or mock transfectants were prepared and followed by Western blotting analysis. As shown in Figure 3B, Cdh1 knockdown by siRNA resulted in a dramatic Skp2 protein elevation and concomitant p27Kip1 depletion. In contrast, Skp2 depletion by Skp2 siRNA caused a significant increase in p27Kip1, but had no effect on the protein level of Cdh1. Furthermore, we observed that Cdh1 silencing rapidly triggered cells entering the cell cycle (S-phase) and led to cell-cycle progression (Figure 3C).
Cell adhesion–mediated down-regulation of Skp2 requires Cdh1. (A) The Cdh1 expression in Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. Cell-cycle distribution (B) and protein levels of p27Kip1, Skp2, and Cdh1 (C) 24 hours after transfection with Skp2 siRNA (siSkp2), Cdh1 siRNA (siCdh1), or control siRNA (siCont) in Jeko-1 lymphoma cells in suspension. All data are representative of at least 3 experiments (means ± SD).
Cell adhesion–mediated down-regulation of Skp2 requires Cdh1. (A) The Cdh1 expression in Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by Western blot. Cell-cycle distribution (B) and protein levels of p27Kip1, Skp2, and Cdh1 (C) 24 hours after transfection with Skp2 siRNA (siSkp2), Cdh1 siRNA (siCdh1), or control siRNA (siCont) in Jeko-1 lymphoma cells in suspension. All data are representative of at least 3 experiments (means ± SD).
Cell-adhesion–mediated p27Kip accumulation is required for cell-cycle arrest and is dependent on ubiquitin-proteasome APC/Skp2 ligase pathways
We first addressed whether p27 up-regulation is required for the G1 accumulation to occur upon cell adhesion to the stromal cell HS-5. We performed p27 knockdown experiments using siRNA and examined the changes of p27 protein and cell-cycle distribution upon lymphoma cell (Jeko-1) adhesion to HS-5 cells. Figure 4A,B demonstrates that p27 depletion by siRNA markedly reduced p27 accumulation upon cell adhesion and restored lymphoma cell growth on bone marrow stroma. This result provides direct evidence that p27 accumulation plays a critical role in cell- adhesion–mediated cell-cycle arrest.
p27Kip1 depletion, Skp2 overexpression, and Cdh1 knock down abolished cell-adhesion–mediated p27Kip1 induction and cell-cycle arrest. (A) The p27Kip1 expression in control siRNA entering RISC-transfected (siCont.RISC[ + ]) or siP27Kip1 RNA-transfected (siP27) Jeko-1 cells in suspension (Sus) versus 12 hours of HS-5 adhesion (HS5-Ad) analyzed by Western blot, and (B) corresponding cell-cycle distribution in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by flow cytometry. (C) The Skp2 and p27Kip1 expression in nontransfected [pCont(−)] Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) or stably mock (pCont( + ))- or plasmid Skp2-transfected (pSkp2) Jeko-1 cells in suspension versus to HS-5 adhesion for 12 hours analyzed by Western blot and (D) corresponding cell-cycle distribution in stably mock- or plasmid Skp2-transfected Jeko-1 cells in suspension (pCont( + )-Sus or pSkp2-Sus) versus HS-5 adhesion (pCont( + )-Ad or pSkp2-Ad) for 12 hours analyzed by flow cytometry. (E) Cdh1, Skp2, and p27Kip1 expressions in nonsilencing siControl RNA-transfected (siCont) or siCdh1 RNA-transfected (siCdh1)-Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) for 12 hours analyzed by Western blot, and (F) corresponding cell-cycle distribution in control siRNA- or Cdh1 siRNA-transfected Jeko-1 cells in suspension (siCont-Sus or siCdh1-Sus) versus HS-5 adhesion (siCont-Ad or siCdh1-Ad) for 12 hours analyzed by flow cytometry. Each blot is representative of 3 experiments, and the bar graphs show means plus or minus SD of 3 experiments.
p27Kip1 depletion, Skp2 overexpression, and Cdh1 knock down abolished cell-adhesion–mediated p27Kip1 induction and cell-cycle arrest. (A) The p27Kip1 expression in control siRNA entering RISC-transfected (siCont.RISC[ + ]) or siP27Kip1 RNA-transfected (siP27) Jeko-1 cells in suspension (Sus) versus 12 hours of HS-5 adhesion (HS5-Ad) analyzed by Western blot, and (B) corresponding cell-cycle distribution in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by flow cytometry. (C) The Skp2 and p27Kip1 expression in nontransfected [pCont(−)] Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) or stably mock (pCont( + ))- or plasmid Skp2-transfected (pSkp2) Jeko-1 cells in suspension versus to HS-5 adhesion for 12 hours analyzed by Western blot and (D) corresponding cell-cycle distribution in stably mock- or plasmid Skp2-transfected Jeko-1 cells in suspension (pCont( + )-Sus or pSkp2-Sus) versus HS-5 adhesion (pCont( + )-Ad or pSkp2-Ad) for 12 hours analyzed by flow cytometry. (E) Cdh1, Skp2, and p27Kip1 expressions in nonsilencing siControl RNA-transfected (siCont) or siCdh1 RNA-transfected (siCdh1)-Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) for 12 hours analyzed by Western blot, and (F) corresponding cell-cycle distribution in control siRNA- or Cdh1 siRNA-transfected Jeko-1 cells in suspension (siCont-Sus or siCdh1-Sus) versus HS-5 adhesion (siCont-Ad or siCdh1-Ad) for 12 hours analyzed by flow cytometry. Each blot is representative of 3 experiments, and the bar graphs show means plus or minus SD of 3 experiments.
To determine whether Skp2 is indeed involved in cell-adhesion–mediated p27Kip1 accumulation and cell-cycle arrest, we transfected Jeko-1 cells using pcDNA3-Skp2 and examined the changes in p27Kip1 and cell-cycle progression upon cell adhesion to HS-5 in pcDNA3-Skp2- and control empty vector-transfected lymphoma cells. Cell extracts from Jeko-1 cells transfected with Skp2 DNA or control vector were prepared with or without cell adhesion to HS-5, followed by Western blotting analysis. As shown in Figure 4C, cell adhesion significantly decreased Skp2 levels and increased p27Kip1 in nontransfected and control vector–transfected cells. However, in pcDNA3-Skp2-transfected Jeko-1 cells, cell adhesion increased the level of the p27Kip1 in the Skp2 clone, but the amount was not increased above the basal level in control clones, indicating that the Skp2 expression is required for p27Kip1 up regulation. Corresponding cell-cycle analysis (Figure 4D) demonstrated that Skp2 overexpression partially abolished cell-adhesion–mediated cell-cycle arrest. The partial effect could be attributed to persistent activity of the upstream proteins APC/Cdh1 and/or only a portion of lymphoma cells (50%-75%) being transfected. We therefore next increased the level of Skp2 by Cdh1 siRNA knockout. We examined the levels of Skp2 and p27Kip following cell adhesion to HS-5 in Cdh1-depleted Jeko-1 cells. Figure 4E,F reveals that Cdh1 depletion significantly blocked cell-adhesion–induced Spk2 degradation and p27Kip accumulation as well as cell-adhesion–mediated cell arrest. In addition, our experiment showed that cell-adhesion–induced change of the cell cycle is minimal in Skp2 RNA1-transfected versus control Jeko-1 cells (data not shown), supporting the important role of Skp2 in cell-adhesion–induced response. These results, for the first time, demonstrate that the level of p27Kip1 and cell-cycle progression are tightly controlled by the interaction with surrounding stromal cells via ubiquitin-proteasome APC/Skp2 ligase pathways.
Cell adhesion induces Cdh1 up-regulation at the mRNA level
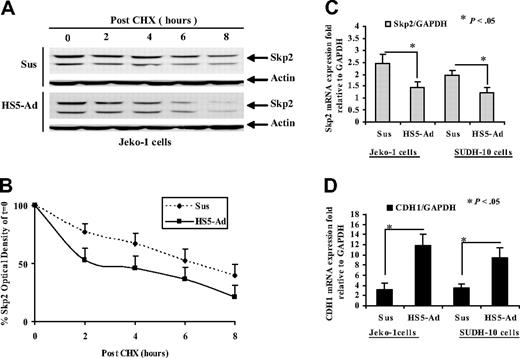
Furthermore, we investigated the molecular signal transduction for the observed Skp2 changes mediated by cell adhesion. We treated lymphoma cells (Jeko-1) with cycloheximide, a protein synthesis inhibitor, and examined the change of Skp2 in cycloheximide-treated suspension and cell adhesion lymphoma cells. Figure 5A reveals that treatment of cells with cycloheximide significantly increases the rate of Skp2 degradation following cell adhesion. Half-life analysis was performed from 3 independent experiments (Figure 5B). The half-life of Skp2 was 6.0 plus or minus 0.5 hours in suspension cells, while in HS-5-adhered cells it was 2.4 plus or minus 0.7 hours. This result suggests that cell adhesion activates a proteasomal degradation pathway that subsequently degrades Skp2. To test the possibility that cell-adhesion–mediated Skp2 can also be regulated at the level of mRNA, we also examined the expression of Skp2 mRNA in Jeko-1 and SUDH-4 lymphoma cells in suspension and 12 hours after cell adhesion. Semiquantitative RT-PCR showed that Skp2 mRNA was expressed at a higher level (Figure 5C). In addition, we observed that the steady-state mRNA level of Skp2 after cycloheximide treatment was unchanged (data not shown). Together with the observed differential expression patterns of Skp2 mRNA and protein, these findings suggest that cell adhesion to stromal cells regulated Skp2 at both translational and posttranslational levels.
The molecular mechanisms for cell-adhesion–mediated Skp2 and Cdh1 changes. (A,B) Effect of cell adhesion on Skp2 degradation. Jeko-1 cells were treated with cycloheximide (100 μM) with and without HS-5 cells adhesion for the indicated time periods. At the times indicated, cells were lysed, and Skp2 protein levels were determined by Western blot analysis (A) and quantified (from 3 independent experiments) using densitometry. The data shown (B) represent mean values and standard deviations of the average percentage of Skp2, compared with the amount of Skp2 at time 0 for each treatment group. (C,D) Cell-adhesion–induced changes of Skp2 and CDH1 mRNA expressions measured by real-time qRT-PCR. Fold values were obtained by externally standardizing against identical amplifications in Jeko-1 and SUDH-10 cells in suspension versus HS-5 adhesion and by internally standardizing against GAPDH in each cell line. The mean values and standard deviations from 3 independent experiments are shown. Student t test was used for statistical analysis. *P < .05.
The molecular mechanisms for cell-adhesion–mediated Skp2 and Cdh1 changes. (A,B) Effect of cell adhesion on Skp2 degradation. Jeko-1 cells were treated with cycloheximide (100 μM) with and without HS-5 cells adhesion for the indicated time periods. At the times indicated, cells were lysed, and Skp2 protein levels were determined by Western blot analysis (A) and quantified (from 3 independent experiments) using densitometry. The data shown (B) represent mean values and standard deviations of the average percentage of Skp2, compared with the amount of Skp2 at time 0 for each treatment group. (C,D) Cell-adhesion–induced changes of Skp2 and CDH1 mRNA expressions measured by real-time qRT-PCR. Fold values were obtained by externally standardizing against identical amplifications in Jeko-1 and SUDH-10 cells in suspension versus HS-5 adhesion and by internally standardizing against GAPDH in each cell line. The mean values and standard deviations from 3 independent experiments are shown. Student t test was used for statistical analysis. *P < .05.
To better understand the mechanism by which cell adhesion leads to up-regulation of Cdh1, we measured the mRNA level of Cdh1 by semiquantitative RT-PCR. Figure 5D shows that Cdh1 mRNA was significantly increased in lymphoma cells adhered to HS-S compared with those in suspension, thus implicating that cell adhesion induces Cdh1 up-regulation at the mRNA level.
Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in primary MCL and other non-Hodgkin B-cell lymphomas
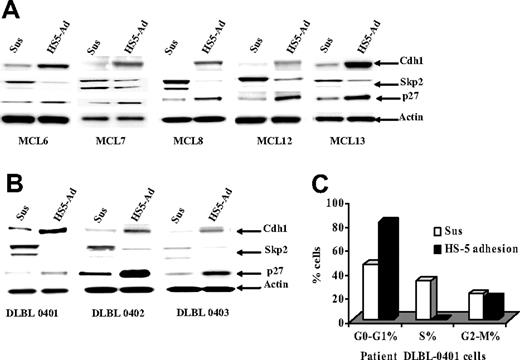
Finally we determined whether cell-cycle arrest, p27Kip1 protein, and the ubiquitin ligases (Skp2 and Cdh1) observed in lymphoma cell lines were operative in primary MCL and other NHL specimens. We examined the changes of p27Kip1, Skp2, and Cdh1 following adhesion of the primary lymphoma cells to HS-5 cells. Figure 6A shows that all 10 lymphoma samples (including 5 MCL samples) had similar changes to those observed in lymphoma cell lines (Jeko-1, Mino, and SUDH-10). We also determined the effect of cell adhesion on cell-cycle progression in primary lymphoma cells. As shown in Figure 6B, in a representative sample of primary lymphoma cells, similar to the cultured lymphoma cell lines, adhesion of primary lymphoma cells to bone marrow stromal cells (HS-5) induced cell-cycle arrest at G1-phase as determined by flow cytometry. Overall, this study implicated bone marrow stromal control of tumor cell-cycle progression via ubiquitin-proteasome proteolytic pathways in MCL and other NHLs.
Cell-adhesion–induced p27Kip1-associated cell-cycle arrest through down-regulating the SCFSkp2 ubiquitin ligase pathway in primary MCL and other non-Hodgkin B-cell lymphomas. (A,B) The Cdh1, Skp2, and p27Kip1 expressions in primary MCL (A) and DLBCL (B) cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) were analyzed by Western blot. (C) Cell-cycle distribution in DLBCL (DLBL0401) cells in suspension (Sus), or on a confluent HS-5 monolayer (HS5-Ad; 12 hours).
Cell-adhesion–induced p27Kip1-associated cell-cycle arrest through down-regulating the SCFSkp2 ubiquitin ligase pathway in primary MCL and other non-Hodgkin B-cell lymphomas. (A,B) The Cdh1, Skp2, and p27Kip1 expressions in primary MCL (A) and DLBCL (B) cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) were analyzed by Western blot. (C) Cell-cycle distribution in DLBCL (DLBL0401) cells in suspension (Sus), or on a confluent HS-5 monolayer (HS5-Ad; 12 hours).
Discussion
This study, for the first time, demonstrates that the SCFSkp2 ubiquitin ligase pathway is an important part of the mechanism by which cell adhesion regulates the level of p27Kip1 and cell-cycle progression in B-cell lymphomas. Another crucial finding is that Cdh1, an activating subunit of APC ubiquitin ligase, serves as an upstream effector in cell-adhesion–mediated Skp2 degradation, p27Kip1 accumulation, and hence lymphoma cell quiescence when lymphoma cells are attached to their stroma. This study reveals a novel molecular and signaling mechanism-regulating interaction between lymphoma cells and the bone marrow microenvironment. Similar to our findings for myeloma cells,5,6 here we show that cell-cell adhesion (contact) to the bone marrow stromal cell HS-5 causes a reversible growth arrest that correlated with p27Kip1 protein levels in MCL and other NHLs. The cell-cycle–arrested phenotype was reversible upon detachment of cells from stroma. Together, these data suggest that lymphoma cells are kept dormant, protected by cell adhesion, but remain competent to reenter the cell cycle, leading to disease recurrence upon unleashing the lymphoma cells from stroma.
Cell-adhesion–mediated p27Kip1 elevation and its association with cell-cycle arrest has been less emphasized. The molecular mechanism of how stroma controls the level of p27Kip1 and hence the cell cycle has not yet been understood. In the present study, we demonstrated a cell-adhesion (not soluble factor)–mediated increase in p27Kip1 in MCL cell lines and primary lymphoma cells. Furthermore, RNA protection analysis did not reveal any increase in p27Kip1 mRNA levels, suggesting that stabilization in p27Kip1 was responsible for the intracellular accumulation in p27Kip1 following cell adhesion to stroma. More important, our data reveal that levels of p27Kip1 were posttranslationally up-regulated by stroma and were dependent on the activity of the SCFSkp2 ubiquitin ligase pathway. First, we showed that the Skp2 protein was markedly reduced and became undetectable upon lymphoma cell adherence to the stromal cell line HS-5 in both lymphoma cell lines and primary lymphoma cells. The decrease of Skp2 protein correlated with reciprocal up-regulation of p27Kip1. Second, inhibition of Skp2 expression by specific siRNA increased p27Kip1 levels and had no effect on the level of Cdh1. Third, overexpression of Skp2 by dominant Skp2 decreased p27Kip1 and induced lymphoma cell proliferation. Finally, we presented that ectopic expression of Skp2 by transfection of Skp2 pcDNA or depletion of Cdh1 by siRNA abolished cell-adhesion–dependent p27Kip1 elevation when lymphoma cells adhered to stromal cells. Taken together, these data imply that regulation of Skp2 expression by stroma likely plays a significant role in the regulation of lymphoma cell-cycle progression. Cdh1/APC is an important upstream signaling molecule for the Skp2/ p27Kip1 pathway and cell-cycle progression.
Depending on the cellular context and type of tumor, cell adhesion is reported to either negatively or positively regulate cell-cycle progression.23,24 It has been reported that cellular adhesion of fibroblasts is necessary for the progression past the G1 restriction point. Furthermore, recent studies demonstrated that tumor cell adhesion induces Skp2 and results in p27Kip1 degradation as well as cell proliferation in fibroblasts and prostate cancer cells.25,26 In contrast, adhesion of lymphoma cells to stroma resulted in growth arrest. The inhibition of cell growth correlated with reduced Skp2 activity and elevated p27Kip1. This difference is most likely attributed to the nature (embryonic derivation) of tumor cells, stromal context, integrin receptors, and/or Skp2 upstream signaling events. Our findings add to an increasing body of evidence suggesting that Skp-2 functions as a modulator of the cell cycle as well as p27Kip1. Depletion of Skp2 protein upon lymphoma cell adhesion to stroma likely occurs through protein degradation. However, the precise mechanisms involved had not been elucidated before. By using cycloheximide treatment and quantitative real-time RT-PCR analysis, we demonstrated here that cell adhesion regulated Skp2 through both translational and posttranslational levels. To analyze the requirement of APC/C in cell-adhesion–promoted Skp2 degradation, we used RNA interference to knock down the expression of Cdh1 in Jeko-1 and SUDH-10 cells. As expected, silencing of Cdh1 expression with siRNA resulted in the accumulation of Skp2 and cell-cycle progression in these cells. Thus, expression of Skp2 appears to be controlled by at least partially posttranscriptional Cdh1/APC ubiquitin ligase-dependent mechanisms. In the present study, we report that this event is mediated through the ADP/Cdh1 ubiquitin ligase complex. Indeed, down-regulation of Cdh1 by siRNA was sufficient to rescue Skp2-reversing cell-adhesion–mediated cell-cycle arrest. The present study provides the first demonstration that bone marrow stroma regulate the lymphoma cell cycle through the F-box protein Skp2, its upstream signaling molecule Cdh1/APC, and the downstream molecule p27Kip1. These observations are in agreement with 2 more recent reports implicating that Skp2 is targeted for ubiquitination and destruction in G1-phase by APC/C and its activator Cdh1.18,19 Furthermore, this study, for the first time, provides the molecular basis for the observed change of Cdh1 upon cell adhesion, implicating cell-adhesion–mediated Cdh1 up-regulation at the mRNA level.
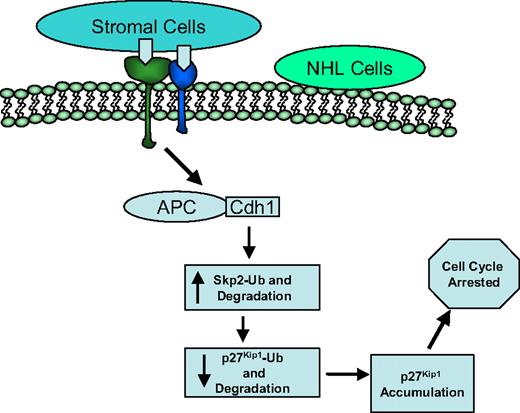
In summary, our studies have demonstrated that Skp2 directly interacts with and promotes the degradation of p27Kip1 and regulates the G1-S transition of the cell cycle. As depicted in Figure 7, this process requires APC/Cdh1 ubiquitin ligase complex activation initiated by cell adhesion, followed by a hypothetically ordered series of signaling events: cell-adhesion–dependent activation of APC/Cdh1 ubiquitin ligase complex at mRNA level, increased ubiquitination and degradation of Skp2 through both mRNA and protein stability, subsequent decreased ubiquitination and degradation of p27Kip1 via protein stability, and ultimately p27Kip1 accumulation, resulting in cell-cycle arrest. Clinically, elimination of lymphoma in the bone marrow is a more arduous task than in the lymph node and peripheral blood, again emphasizing the importance of the microenvironment. The residual lymphoma in the bone marrow after therapy is the result of subpopulations of lymphoma cells being rendered resistant to cytotoxic drugs. The bone marrow microenvironment induces lymphoma cells into a dormant state, promotes lymphoma cell survival, and confers drug resistance. The bone marrow would permit surviving cells to repopulate the host either without further genetic changes or with gene mutations because of inherent genetic instability and/or instability resulting from the DNA-damaging therapy that they received. Therefore, understanding this molecular pathway may prove valuable for elucidation of this important physiopathologic process (lymphoma and stroma interaction) in designing new therapeutic approaches modifying lymphoma cell growth and response to therapy.
Model for cell-adhesion–mediated cell-cycle arrest. Hypothetically ordered series of signaling events: cell-adhesion–dependent activation of APC/Cdh1 ubiquitin ligase complex, increased ubiquitination and degradation of Skp2, subsequent decreased ubiquitination and degradation of p27Kip1, and ultimately p27Kip1 accumulation, resulting in cell-cycle arrest.
Model for cell-adhesion–mediated cell-cycle arrest. Hypothetically ordered series of signaling events: cell-adhesion–dependent activation of APC/Cdh1 ubiquitin ligase complex, increased ubiquitination and degradation of Skp2, subsequent decreased ubiquitination and degradation of p27Kip1, and ultimately p27Kip1 accumulation, resulting in cell-cycle arrest.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the American Cancer Society (IRG 032 ACS to J.T.), National Cancer Institute Aging and Cancer Pilot Research Grant (to J.T.), National Institutes of Health P01 grant CA76292 (to W.S.D.), and Lymphoma Research Foundation of America (to S.D.).
National Institutes of Health
Authorship
Contribution: T.L. designed and performed the experiments, analyzed data, and wrote the paper. L.A.H. designed and analyzed data and reviewed the paper. L.M., S.D., and E.S. provided patient samples, analyzed data, and reviewed the paper. R.L., W.B. contributed vital cell lines and new reagents and reviewed the paper. W.S.D. was the co-senior author, analyzed data, reviewed the paper, and contributed funds. J.T. designed and performed the experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jianguo Tao, Department of Interdisciplinary Oncology and Experimental Therapeutics Program, H. Lee Moffitt Cancer Center and Research Institute at the University of South Florida, MCC-LAB 2071, 12902 Magnolia Dr, Tampa, FL 33613; e-mail: taoj@moffitt.usf.edu.

![Figure 4. p27Kip1 depletion, Skp2 overexpression, and Cdh1 knock down abolished cell-adhesion–mediated p27Kip1 induction and cell-cycle arrest. (A) The p27Kip1 expression in control siRNA entering RISC-transfected (siCont.RISC[ + ]) or siP27Kip1 RNA-transfected (siP27) Jeko-1 cells in suspension (Sus) versus 12 hours of HS-5 adhesion (HS5-Ad) analyzed by Western blot, and (B) corresponding cell-cycle distribution in suspension (Sus) versus HS-5 adhesion (HS5-Ad) analyzed by flow cytometry. (C) The Skp2 and p27Kip1 expression in nontransfected [pCont(−)] Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) or stably mock (pCont( + ))- or plasmid Skp2-transfected (pSkp2) Jeko-1 cells in suspension versus to HS-5 adhesion for 12 hours analyzed by Western blot and (D) corresponding cell-cycle distribution in stably mock- or plasmid Skp2-transfected Jeko-1 cells in suspension (pCont( + )-Sus or pSkp2-Sus) versus HS-5 adhesion (pCont( + )-Ad or pSkp2-Ad) for 12 hours analyzed by flow cytometry. (E) Cdh1, Skp2, and p27Kip1 expressions in nonsilencing siControl RNA-transfected (siCont) or siCdh1 RNA-transfected (siCdh1)-Jeko-1 cells in suspension (Sus) versus HS-5 adhesion (HS5-Ad) for 12 hours analyzed by Western blot, and (F) corresponding cell-cycle distribution in control siRNA- or Cdh1 siRNA-transfected Jeko-1 cells in suspension (siCont-Sus or siCdh1-Sus) versus HS-5 adhesion (siCont-Ad or siCdh1-Ad) for 12 hours analyzed by flow cytometry. Each blot is representative of 3 experiments, and the bar graphs show means plus or minus SD of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2006-11-060350/6/m_zh80170706220004.jpeg?Expires=1769089697&Signature=kZoxbafsMNA8moROyE3VZtLShnLm0tGxcc4TKDHPaflDzat8t7Kp6p6ixW4kRvgwSHAcEo5eP0cnLA96gP~rnDLHrIKl1IFdBoPbelpEEX38npIasp42lcO-IJQW05Y4sR1WAY32m6acKFUmmBBgXrfYxP-u1fA6eC3S2Gi-NOkV6PR3eMA7KccjRRixjwg9tiVsPhs5sXIEkKznH9NDy49zQhrt3ZPgb9~AQbW6pG4N-JLllLp~Mw6S9kmj7SUNddzq4-WkON9KWPWmkvlLEMgIFQ1OemIIIBcW~BGRSXiVG3FyzTYniude0CIKSw~2Wg3CBnLUDg5CyxyHesAkrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)