Abstract
Our interest in chronic lymphocytic leukemia (CLL) derives primarily from the exploitation of human diseases as strategic models for defining the in vivo biological roles of CD38. Using this model, we showed that CD38 triggers robust proliferation/survival signals modulated through the interactions with the CD31 ligand expressed by nurselike cells and by the stromal/endothelial components. By analyzing a cohort of 56 patients with clinically and molecularly characterized CLL, we show that (1) patients with CD38+/ZAP-70+ are characterized by enhanced migration toward Stromal derived factor-1α (SDF-1α)/CXCL12; (2) CD38 ligation leads to tyrosine phosphorylation of ZAP-70, showing that these markers are functionally linked; (3) ZAP-70 represents a limiting factor for the CD38 pathway in the CLL context, as shown by studying CD38-mediated signal transduction in 26 molecularly characterized patients; and (4) the CLL subgroup of patients defined on the basis of migratory potential is marked by a specific genetic signature, with a significant number of differentially expressed genes being involved in cell-cell interactions and movement. Altogether, the results of this work provide biological evidence for why the combined analysis of CD38 and ZAP-70 expression as determined in several clinical trials results in more dependable identification of patients with CLL who have aggressive disease.
Introduction
Human CD38 is a pleiotropic membrane glycoprotein with ectoenzymatic properties1,2 mediating cell-cell interactions by binding the nonsubstrate CD31 ligand.3,4 CD38 is also a powerful negative prognostic marker for patients with chronic lymphocytic leukemia (CLL): CD38+ CLL cells are characterized by a greater proliferative potential and by diminished sensitivity to chemotherapies (reviewed in Matrai5 ). CD38 expression by leukemic cells is higher in peripheral lymphoid organs and in bone marrow (BM) than in circulating CLL cells of the same patient.6 Recent data indicate that peripheral lymphoid organs host the proliferative core of the disease: results from in vivo measurements of B-cell kinetics showed that patients with CLL have definable and often substantial birth rates, varying from 0.1% to more than 1% of the entire clone per day.7 Along with histologic findings that have demonstrated the presence of proliferation centers in lymph nodes and in BM,8 the aforementioned study also substantiates the notion that the bulk of the leukemic clone resides in solid lymphoid organs
The lesson inferred from these observations is that CLL cells recirculate from blood to peripheral lymphoid organs, where a favorable microenvironment provides growth and survival signals mediated—at least in part—by CD38.9 Chemokines and their receptors regulate CLL cell trafficking between the 2 compartments.10-12 Stromal derived factor-1α (SDF-1α)/CXCL12,13 which is abundantly produced by stromal and nurselike cells (NLCs),14 is effective in recruiting circulating CLL lymphocytes toward secondary lymphoid organs via the specific CXCR4 receptor.15
Together with CD38, ZAP-70, a member of the syk tyrosine kinase family, has recently been recognized as a reliable negative prognostic marker for patients with CLL.16,17 Here, we show that CD38+/ZAP-70+ patients are characterized by enhanced migration toward SDF-1α. Further, CD38 ligation leads to phosphorylation of the activatory tyrosines in ZAP-70 in both a panel of 12 patients with CLL and in an ad hoc modified B-cell line model. These results show that the 2 markers are functionally linked and confirm previous data in T and natural killer (NK) cell models.18,19 ZAP-70 represents a limiting factor for the CD38 pathway in the CLL context, likely serving as a cross-point where migratory signals mediated via CXCR4 receptor intersect with growth signals mediated via CD38. Last, a specific genetic signature marking migratory potential was identified with microarray studies. Many of the differentially expressed genes modulate cell-cell interactions and movement.
Altogether, the results of this work provide biological evidence for why the combined analysis of CD38 and ZAP-70 expression experienced in clinical trials20 results in more dependable identification of patients with CLL who have aggressive disease.
Patients, materials, and methods
Patients and cells
All samples used in this study were acquired after informed consent was obtained in accordance with the Declaration of Helsinki and with Institutional Review Board approval from Molinette University Hospital. Cells obtained from 56 patients with CLL presenting with typical morphology and immunophenotype were studied. Sample analyses included CD38 and ZAP-70 stainings, IgV mutational status,9 and fluorescence in situ hybridization (FISH) for chromosomes 11, 12, 13, and 17 by using a CLL panel of multicolor probe sets (Vysis, Downers Grove, IL).21 To determine ZAP-70 expression, peripheral blood mononuclear cells (PBMCs) were labeled with anti-CD2–PE, anti-CD19–Cy5, and anti-CD5–APC (Caltag-Invitrogen, Burlingame, CA). Cells were then fixed, permeabilized (Fix&Perm kit), and stained with anti–ZAP-70 Alexa 488 (all from Caltag). Fluorescence-activated cell sorter (FACS) analysis was performed following published methods.17 In selected cases, ZAP-70 expression was also confirmed by Western blot using purified B cells.
PBMCs were purified from blood samples by Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) centrifugation. Where indicated, B cells were purified by negative selection using anti-CD3, anti-CD14, and anti-CD16 antibodies (all produced and in-house purified) and Dynal magnetic beads (Invitrogen, Milan, Italy), with a purity constantly greater than 95%, as checked by double staining with CD5 and CD19.
The BJAB–ZAP-70+ cells and control BJAB-mock were obtained as described.22
Reagents
Antibodies used to treat cells were agonistic anti-CD38 (clone IB423 ), anti-gp120 (clone JAS), polyclonal anti-surface IgM (Southern Biotech, Birmingham, AL), donkey anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) and blocking anti-CXCR4 (R&D Systems, Minneapolis, MN). Antibodies used for immunoprecipitation and Western blot were anti-CD38 (clone SUN-4B7), anti-myc (clone 9E10), locally produced or purified; antiphosphotyrosine, anti–phospho-ERK1/2, anti–pan-ERK (BD Biosciences, Palo Alto, CA), and anti-syk (Cell Signaling Technologies, Danvers, MA). The anti–ZAP-70 antibodies evaluated were clone 29 (BD Biosciences), clone 2F3.2 (Upstate-Millipore, Charlottesville, VA), and clone 99F2 (Cell Signaling Technologies). Secondary reagents were goat anti-mouse IgG horseradish peroxidase (HRP)–conjugated (Perkin Elmer, Wellesley, MA) and goat anti-rabbit HRP-conjugated (Santa Cruz Biotechnology, Santa Cruz, CA). Antibodies used for cell-surface staining were anti-CD2–PE-Cy5, anti-CD56–PE-Cy5, anti-CD5–PE, anti-CD19–PE-Cy7, anti–ZAP-70–Alexa Fluor 488, anti-CXCR3–FITC, anti-CCR7–FITC (Caltag); anti-CXCR4 (R&D Systems); anti-CD38–PE, anti-CD5–FITC, and anti-CD19–PE (BD Biosciences); and anti-CD38–FITC and anti-CD100–FITC (in-house).
Chemotaxis assays
Freshly isolated PBMCs from patients with CLL were resuspended in 100 μL RPMI-1640 plus 0.5% bovine serum albumin (BSA; both from Sigma, Milan, Italy) and plated in the upper chamber of a 6.6-mm diameter Transwell culture insert in bare polycarbonate with a 5-μm pore size (Sigma). The lower chamber of each well contained 600 μL of medium with CXCL12/SDF-1α (100 ng/mL; R&D Systems) or medium alone. All the assays were performed in triplicate (ie, measuring chemotaxis in 3 separate wells for each condition). After 4 hours of incubation (37°C, 5% CO2), 3 aliquots of 100 μL were recovered from each lower chamber. These samples were counted for 1 minute under a defined flow rate using a FACSort flow cytometer (BD Biosciences) in order to obtain a median of the relative number of total transmigrated cells. Reading parameters were constant in each acquisition (forward scatter [FSC], 1.49; side scatter [SSC], 381). The remaining 300 μL of the lower chamber were labeled with anti-CD5–FITC and anti-CD19–PE and used to determine the percentage of transmigrated CLL cells. Where indicated, purified anti-CXCR4 or isotype-matched irrelevant mAb were used to block migration by incubation (30 minutes, 4°C) with CLL cells prior to the beginning of the assay.
Migration index was calculated as number of CD5+/CD19+ cells transmigrating in presence of the chemokine per number of CD5+/CD19+ cells transmigrated in absence of the chemokine.24 Student 2-sample, 2-tailed t test was used to determine the statistical significance of the results.
Immunoprecipitation and Western blot
CLL cells were used immediately after purification, while BJAB–ZAP-70+ and mock-transfected cells were starved for 4 hours in serum-free RPMI-1640 medium. Cells were treated with the relevant antibodies (37°C for indicated time points), lysed (150 mM NaCl, 20 mM HEPES [pH 7.4], 50 mM NaF, 1 mM Na3VO4, 1 mM EGTA, 50 μM phenylarsine oxide, 10 mM iodoacetamide, 1 mM PMSF, and 1% NP-40) and nuclear debris was discarded by centrifugation (20 000g for 15 minutes).
After a preclearing step with Protein-A–agarose (Sigma), supernatants were incubated with antiphosphotyrosine mAb (4 hours at 4°C) followed by Protein-A–agarose. Immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF membranes (Upstate-Millipore), reacted with the indicated antibodies, and developed using enhanced chemiluminescence reagents (Perkin Elmer). Total lysates for direct phosphotyrosine and phospho-ERK1/2 detection were resolved by SDS-PAGE and analyzed by Western blot.
Densitometric analyses were performed on scanned films using the public domain National Institutes of Health ImageJ software (Bethesda, MD). The percentage of increase in p-ERK1/2 tyrosine phosphorylation was calculated as: [(p-ERK1/2 band intensity of stimulated cells / phospho-ERK1/2 band intensity of cells treated with irrelevant mAb) −1].
Culture conditions
Freshly separated PBMCs or purified B cells from patients with CLL were cultured in RPMI-1640 plus 5% fetal calf serum (FCS) with recombinant human IL-2 (100 IU/mL) for 1 to 5 days. To induce CLL activation and plasma blast differentiation, cells were cultured with IL-2 and agonistic anti-CD38 mAb (10 μg/mL) for 5 days.
Gene profiling, data analysis, and gene selection
RNA from each sample and from the human universal reference (Human Universal Reference Total RNA; Clontech, Mountain View, CA) was amplified by means of the Amino Allyl MessageAmp I aRNA Kit (Ambion, Austin, TX) to obtain amino allyl antisense RNA (aaRNA). A total of 2 rounds of amplification were performed to obtain the amount of aaRNA required for labeling. Labeling (at least 5 μg of mRNA) was performed using NHS ester Cy3 or Cy5 dyes (Amersham Biosciences, Arlington Heights, IL) reactive with the modified RNA. Total RNA quality was checked by means of RNA 6000 picochip assays and run on the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). mRNA quality and labeling were checked by means of RNA 6000 nanochip assays. Total RNA and amplified and labeled mRNA concentrations were calculated using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Equal amounts (0.75 μg) of labeled specimens from each sample and reference were combined, fragmented, and hybridized to oligonucleotide glass arrays representing 41 000 human unique genes and transcripts (Human Whole Genome Oligo Microarray Array; Agilent Technologies). Each step was performed using the In Situ Hybridization Kit-Plus (Agilent Technologies) and following the 60-mer oligo microarray processing protocol. Slides were then washed with the SSPE wash procedure and scanned using an Agilent B dual-laser microarray scanner. A dye-swap replicate was performed for each sample.
Images were analyzed using the Feature Extraction software version 7.6 (Agilent Technologies); raw data files containing feature and background intensities and related statistical parameters for red and green signals were then loaded onto the Resolver SE System (Rosetta Biosoftware, Seattle, WA) together with the scan images and the Agilent Human Whole Genome pattern file. Data processing and normalization were performed using the Agilent Human Whole Genome platform-specific error model. A total of 2 replicated expression profiles for each sample were created, associating each gene to an expression fold-change and a P value to assess the statistical significance of its modulation in the sample compared with the reference.
Virtual intensity experiments were then designed by applying the “ratio-splitting with common reference” transformation available within the Rosetta Resolver to the entire set of ratio profiles and combining (weighted average) the 2 resulting intensity profiles for each patient.
Single-ratio profiles were used to compare class expression patterns. Analyses were first performed on the set of ratio profiles obtained applying 1 kind of labeling, then on the “dye-swap” set. One-way weighted analysis of variance (ANOVA) with Benjamini-Hoechberg correction for false discovery rate was applied to extract genes differentially expressed between the classes of interest. The intersection of the 2 gene lists obtained for each comparison was then created. Divisive hierarchic clustering and cosine correlation as metrics was applied to the gene-per-patient matrices, using intensity experiment values. This algorithm was chosen as the divisive approach retaining the overall structure of the data, meaning that the root of “upper” levels of the dendrogram is highly representative of the original structure of the data. New ratios were created by dividing the intensity of each sample by the weighted average of the intensities of all other samples analyzed, to obtain more accurate interpretation of the gene expression results for up- and down-regulation.
To assess the performance of cluster separation, the minimum number of significant genes obtained in all the class comparison assays was maintained. On the basis of this number, only the most significant genes from each list were used to calculate intercluster correlation and intracluster homogeneity indexes. The former is the cosine correlation of the first 2 nodes of the divisive hierarchic clustering process, while the latter is the average of the cosine correlation parameters of the first 2 subnodes associated to the top nodes.
Results
Patient characteristics
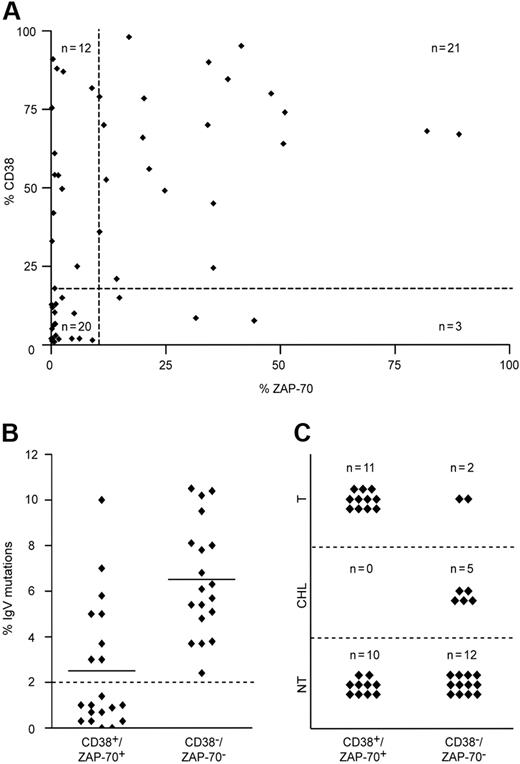
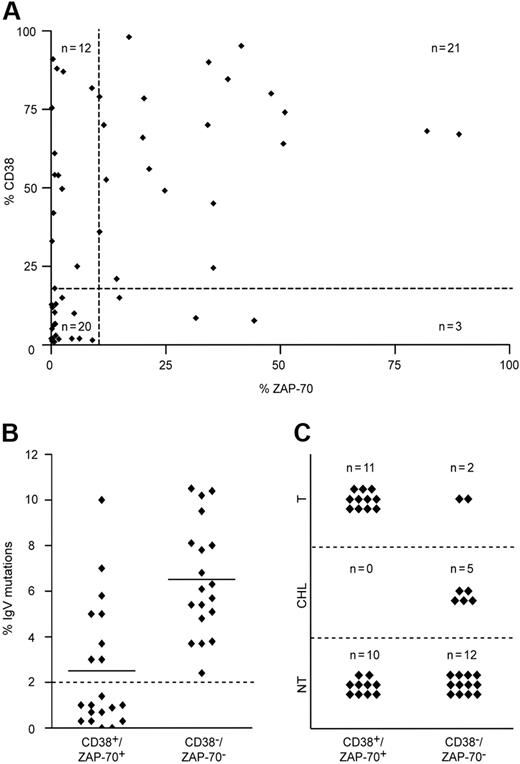
The expression of CD38 and ZAP-70 was examined in a cohort of 56 patients with CLL by flow cytometry, with a cut-off positivity value of 20% and higher and 10% and higher, respectively (Table 1). The cut-off values were experimentally defined as functional thresholds (ie, values below which CD38-mediated ZAP-70 tyrosine phosphorylation was undetectable; see third paragraph in “Results”). The same group was analyzed for mutations in the IgV genes and for abnormalities in chromosomes 11, 12, 13, and 17. Within this group, a clear association was found between CD38 and ZAP-70 (39% of patients CD38+/ZAP-70+ and 35% CD38−/ZAP-70−; P < .001), while the remaining 26% were discordant (Figure 1A). CD38+/ZAP-70+ patients were characterized by a low incidence of IgV gene mutations (Figure 1B); by more frequent abnormalities in chromosomes 11, 12, and 17; by a shorter lymphocyte doubling time (LDT); and by the need for more intensive treatments than the other subsets of patients (Figure 1C). In contrast, CD38−/ZAP-70− patients were homogeneously characterized by the presence (> 2%) of mutations in the IgV genes (Figure 1B). Most of these patients (89%) were either left untreated or received only a chlorambucil-based regimen, which is the first-line therapy for nonaggressive CLL at our institution (Figure 1C).
Molecular and clinical features of the CLL cohort. (A) Relation between CD38 and ZAP-70 in 56 patients with CLL, measured with cut-off values of 20% and 10%, respectively (dotted lines). A strong association between both predictive markers was observed in most (74%; n = 41) patients (Fisher exact test; P < .001). Discordant results were detected in 26% (n = 15) of patients. (B) IgV mutational status in CD38+/ZAP-70+ (n = 21) and CD38−/ZAP-70− (n = 20) patients with CLL, with a cut-off value of 2% (dotted line). CD38−/ZAP-70− patients were characterized by heavily mutated IgV genes, whereas 57% (n = 12) of CD38+/ZAP-70+ samples were unmutated. Horizontal lines represent the mean of all measured values in the corresponding group. (C) CD38+/ZAP-70+ patients required more treatment compared with CD38−/ZAP-70− patients. While 85% (n = 17) CD38−/ZAP-70− patients were either untreated (NT) or treated with chorambucil monotherapy (CHL), CD38+/ZAP-70+ patients required polychemo- or combined immunochemotherapy (T) in 52% (n = 11) of patients.
Molecular and clinical features of the CLL cohort. (A) Relation between CD38 and ZAP-70 in 56 patients with CLL, measured with cut-off values of 20% and 10%, respectively (dotted lines). A strong association between both predictive markers was observed in most (74%; n = 41) patients (Fisher exact test; P < .001). Discordant results were detected in 26% (n = 15) of patients. (B) IgV mutational status in CD38+/ZAP-70+ (n = 21) and CD38−/ZAP-70− (n = 20) patients with CLL, with a cut-off value of 2% (dotted line). CD38−/ZAP-70− patients were characterized by heavily mutated IgV genes, whereas 57% (n = 12) of CD38+/ZAP-70+ samples were unmutated. Horizontal lines represent the mean of all measured values in the corresponding group. (C) CD38+/ZAP-70+ patients required more treatment compared with CD38−/ZAP-70− patients. While 85% (n = 17) CD38−/ZAP-70− patients were either untreated (NT) or treated with chorambucil monotherapy (CHL), CD38+/ZAP-70+ patients required polychemo- or combined immunochemotherapy (T) in 52% (n = 11) of patients.
CD38+/ZAP-70+ CLL cells show enhanced migration in response to SDF-1α
We reported that CD38 is a receptor mediating interactions with NLCs, which sustain proliferation and survival.9 Other groups showed that CD38 is an essential mediator of migration and homing, acting through the modulation of chemokine signaling.25-27 The knowledge that NLCs produce high amounts of SDF-1α to attract CLL cells,15 and that ZAP-70 is a key element in CXCR4 signaling,28 were starting points in our analysis of the influence of the coexpression of CD38 and ZAP-70 in CLL cell migration.
PBMCs from 43 patients with CLL were placed in the upper chamber of a 24-transwell plate, using SDF-1α as chemoattractant in the lower chamber. The experiments were stopped after 4 hours, and the number of CLL cells present in both chambers were calculated as described.24 A total of 5 patients were excluded from the analyses because of lack of reproducibility in the results (each condition was examined in triplicate).
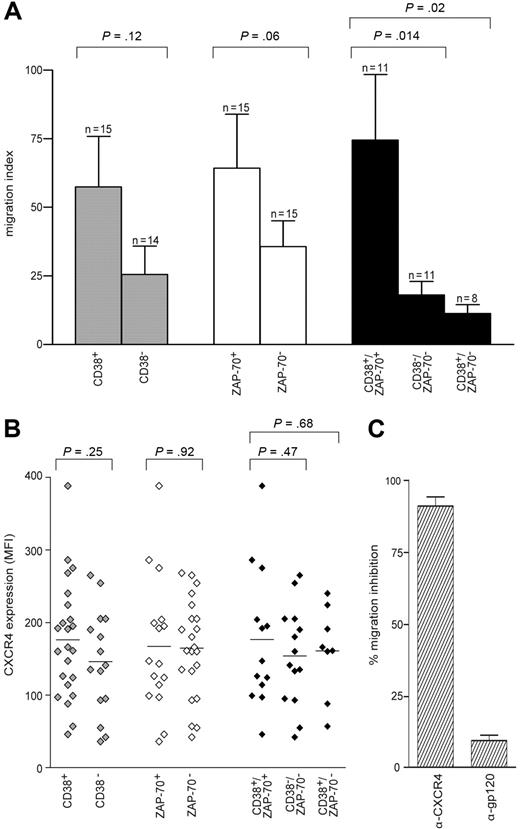
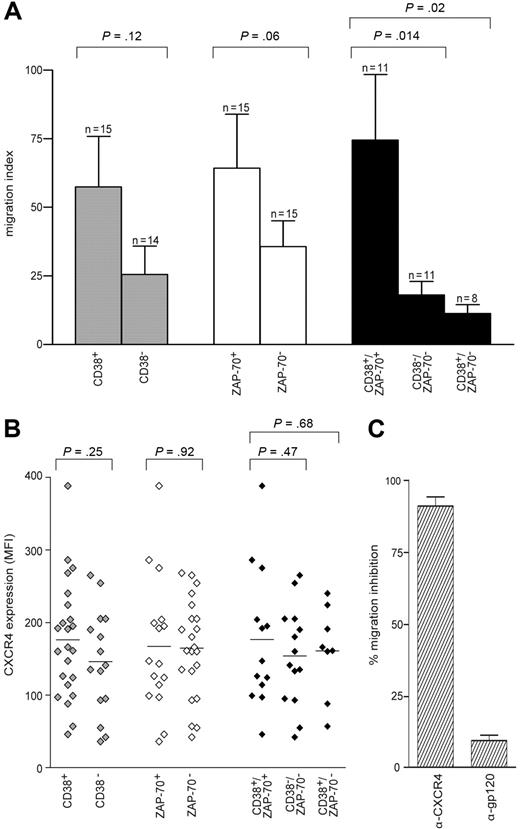
Migration indexes were compared among the different sets of patients, first grouped according to CD38 expression. In CD38+ patients, cells migrate better than in CD38− patients in response to SDF-1α, although statistical significance is not reached (P = .12; Figure 2A). A similar approach based on ZAP-70 expression showed that ZAP-70+ cells migrate better than ZAP-70−, again not significantly (P = .06), and confirming in a larger cohort published data.24 However, the combination of both parameters, obtained by selecting CD38+/ZAP-70+ and CD38−/ZAP-70− groups, rendered the difference highly significant (P = .014; Figure 2A). In CD38+/ZAP-70− patients, cells migrated poorly in response to SDF-1α, scoring an index slightly inferior to the CD38−/ZAP-70− group (P = .02). The CD38−/ZAP-70+ sample was too small (n = 3) to allow any conclusion to be drawn (not shown; Figure 2A); however, none of the 3 showed relevant migration indexes.
CD38+/ZAP-70+ CLL cells show enhanced migration in response to SDF-1α. (A) PBMCs from 38 patients with CLL were assayed in the bare-filter chemotaxis assay for migration toward SDF-1α (100 ng/mL). The comparison between CD38+ (n = 15) and CD38− (n = 14) patients (▩) or between ZAP-70+ (n = 15) and ZAP-70− (n = 15) patients (□) is not statistically significant. On the contrary, the results scored by comparing CD38+/ZAP-70+ (n = 11) with CD38−/ZAP-70− (n = 11) or with CD38+/ZAP-70− (n = 8) patients (■) are significantly different. Results are expressed as the mean of the migration indexes, while the error bars indicate the SEM. Statistical difference was calculated according to the Student 2-sample, 2-tailed t test. (B) MFI values for CXCR4 expressed by the 38 patients studied for migration. The comparison between CD38+ (n = 23) and CD38− (n = 16) ( ) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.
) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.
CD38+/ZAP-70+ CLL cells show enhanced migration in response to SDF-1α. (A) PBMCs from 38 patients with CLL were assayed in the bare-filter chemotaxis assay for migration toward SDF-1α (100 ng/mL). The comparison between CD38+ (n = 15) and CD38− (n = 14) patients (▩) or between ZAP-70+ (n = 15) and ZAP-70− (n = 15) patients (□) is not statistically significant. On the contrary, the results scored by comparing CD38+/ZAP-70+ (n = 11) with CD38−/ZAP-70− (n = 11) or with CD38+/ZAP-70− (n = 8) patients (■) are significantly different. Results are expressed as the mean of the migration indexes, while the error bars indicate the SEM. Statistical difference was calculated according to the Student 2-sample, 2-tailed t test. (B) MFI values for CXCR4 expressed by the 38 patients studied for migration. The comparison between CD38+ (n = 23) and CD38− (n = 16) ( ) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.
) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.
Analysis of cell-surface expression of CXCR4 in the same patient cohort indicated uniformly high percentages of expression, although with marked differences in mean fluorescence intensities (MFIs). In general, CD38+ and ZAP-70+ subsets independently considered featured a MFI slightly higher than the negative counterparts, in agreement with published data indicating increased CXCR4 expression in patients with advanced CLL29 (Figure 2B). In all instances, CXCR4 exerted a dominant role in the migratory response, as pretreatment of the cells with a specific blocking mAb completely abrogated the effect in all samples studied (Figure 2C).
These results suggest that CXCR4 expression is necessary for SDF-1α sensing. They also indicate that neither CD38 nor ZAP-70 independently considered is significantly associated with migration. However, their combination identifies a subset of patients with CLL that has enhanced migratory potential, suggesting that the association between CD38 and ZAP-70 is functional in nature.
CD38 ligation directly triggers ZAP-70 tyrosine phosphorylation
If their association is functional in nature, it may be hypothesized that the cell-surface receptor CD38 and the cytoplasmic tyrosine kinase ZAP-70 form part of the same signaling pathway or that they intersect in some steps.
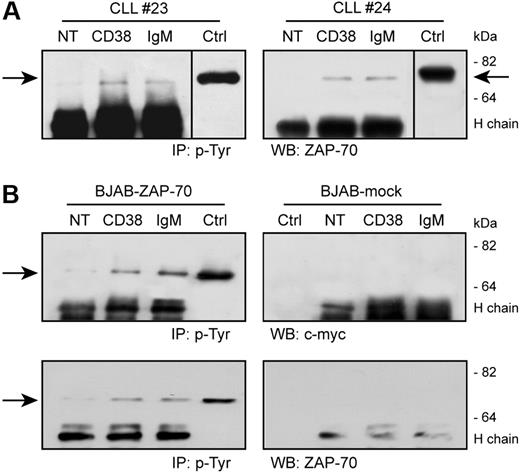
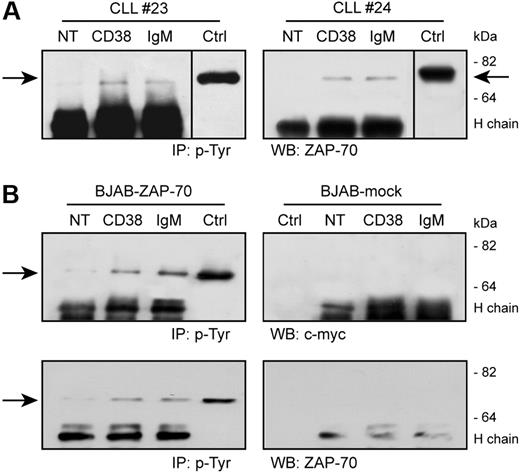
CD38 activation results in a direct tyrosine phosphorylation of ZAP-70 in CD38+/ZAP-70+ CLL cells, as detected after immunoprecipitation of specific lysates with an antiphosphotyrosine mAb followed by Western blot using an anti–ZAP-70 reagent (Figure 3A). ZAP-70 activation is detectable 5 minutes after receptor engagement and returns to background levels after 10 minutes (not shown). IgM ligation also induces ZAP-70 tyrosine phosphorylation at comparable levels, as reported.30,31
CD38 ligation induces ZAP-70 tyrosine phosphorylation. (A) CLL cells were purified from patients with CLL and ligated with the indicated Ab for 5 minutes at 37°C. Lysates were immunoprecipitated (IP) using an antiphosphotyrosine mAb, followed by Western blot (WB) with an anti–ZAP-70 reagent. The arrows indicate the relevant band; molecular weights are shown on the right. Ctrl indicates control total lysate of BJAB–ZAP-70–transfected cells; NT, not treated; H chain; heavy chain of the precipitating Ab. Vertical line has been inserted to indicate a repositioned gel lane. (B) BJAB–ZAP-70–transfected cells and control BJAB-mock were treated as in panel A. Top panel shows the results of the Western blot with an anti–c-myc mAb, which is confirmed in the bottom panel using an anti–ZAP-70 reagent. No tyrosine phosphorylation of ZAP-70 is observed in the mock-transfected cells. Ctrl indicates control total lysate of BJAB–ZAP-70+ or mock-transfected cells.
CD38 ligation induces ZAP-70 tyrosine phosphorylation. (A) CLL cells were purified from patients with CLL and ligated with the indicated Ab for 5 minutes at 37°C. Lysates were immunoprecipitated (IP) using an antiphosphotyrosine mAb, followed by Western blot (WB) with an anti–ZAP-70 reagent. The arrows indicate the relevant band; molecular weights are shown on the right. Ctrl indicates control total lysate of BJAB–ZAP-70–transfected cells; NT, not treated; H chain; heavy chain of the precipitating Ab. Vertical line has been inserted to indicate a repositioned gel lane. (B) BJAB–ZAP-70–transfected cells and control BJAB-mock were treated as in panel A. Top panel shows the results of the Western blot with an anti–c-myc mAb, which is confirmed in the bottom panel using an anti–ZAP-70 reagent. No tyrosine phosphorylation of ZAP-70 is observed in the mock-transfected cells. Ctrl indicates control total lysate of BJAB–ZAP-70+ or mock-transfected cells.
Critical to these experiments is the specificity for ZAP-70 of the antibodies used, excluding any potential cross-reactivity with syk. Therefore, BJAB, a constitutively syk+ human B-cell line, was modified to contain ZAP-70 in a trackable form (ZAP-70/c-myc). This model mimics the scenario occurring in a ZAP-70+ CLL cell and allows a straightforward identification of ZAP-70 tyrosine phosphorylation.
CD38 ligation in ZAP-70–transfected BJAB cells is followed by direct tyrosine phosphorylation of ZAP-70, as detected by an anti-myc reagent (Figure 3B). The kinetics of activation coincides with that described for CLL cells. No specific bands are highlighted when using the mock-transfected cells (Figure 3B). The identity of the bands observed in the CLL cells was confirmed by using the same anti–ZAP-70 mAb (Figure 3B).
Out of the 3 tyrosines present in the ZAP-70 molecule (namely, Y315–319 activatory and Y292 inhibitory), Y292 was not activated (not shown), suggesting that the signal leads to activation rather than inhibition.22
CD38 signaling is dependent on the presence of ZAP-70
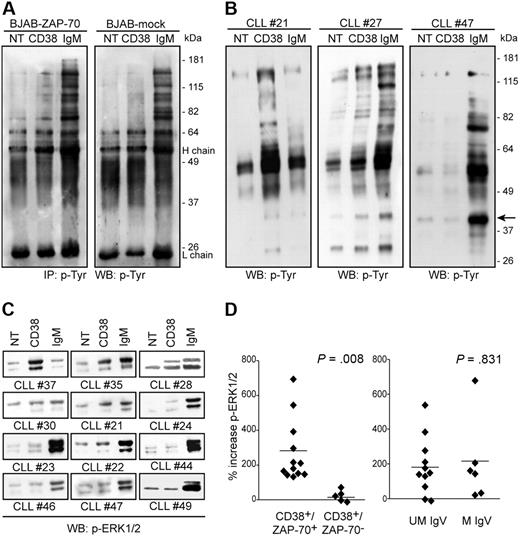
The results obtained using patients with CLL and confirmed with the BJAB line model indicate that ZAP-70 is a component of the CD38 signaling pathway. We therefore turned to investigate the relevance and contribution of ZAP-70 to the chain of events marking CD38 signals. Protein tyrosine phosphorylation was selected as an indicator of the early steps of signal transduction. ZAP-70–transfected BJAB cells display a distinct protein tyrosine phosphorylation profile upon CD38 cross-linking. The signal becomes detectable after 3 minutes (Figure 4A) and is completely down-regulated after 10 minutes (not shown), with prominent bands in the range of approximately 60 to 180 kDa. The signal via IgM results in the phosphorylation of a pattern of proteins, as already reported (Figure 4A). Conversely, CD38 ligation in mock-transfected BJAB cells fails to culminate in any significant protein tyrosine phosphorylation. The signal mediated by IgM is apparent, although less intense (Figure 4A), in agreement with published data.22,30
CD38 signaling is dependent on the presence of ZAP-70. (A) CD38 signaling benefits from the presence of ZAP-70. BJAB–ZAP-70+ and mock-transfected cells were treated with the indicated antibodies, and the lysates were immunoprecipitated and blotted using antiphosphotyrosine antibodies. H and L chain refer to the heavy and light chains of the precipitating Ab. (B) Total protein tyrosine phosphorylation profile of 3 patients with CLL upon CD38 or IgM ligation (5 minutes, 37°C). CLL no. 21 and no. 27 were CD38+/ZAP-70+, while no. 47 was CD38+/ZAP-70−. The arrow shows a band likely corresponding to ERK1/2. No increase in protein tyrosine phosphorylation was observed with CLL no. 47 cells. (C) Analysis of ERK1/2 phosphorylation in a subset of 12 patients with CLL upon CD38 and IgM ligation. CD38+/ZAP-70+ cells show a clear increase in phosphorylation compared with CD38+/ZAP-70− cells. (D) ERK1/2 phosphorylation was studied in 26 patients with CLL; gels were scanned and the band intensities were measured by the ImageJ software and plotted by dividing the patients according to different molecular parameters. The only statistically significant difference is observed by dividing CD38+ patients (n = 17) on the basis of ZAP-70 expression (left panel). No difference is scored when using IgV mutations (right panel). Data are expressed as percentage of increase in ERK1/2 phosphorylation compared with background; the horizontal line represents the mean of all samples. Statistical difference was calculated according to the Student 2-sample, 2-tailed t test.
CD38 signaling is dependent on the presence of ZAP-70. (A) CD38 signaling benefits from the presence of ZAP-70. BJAB–ZAP-70+ and mock-transfected cells were treated with the indicated antibodies, and the lysates were immunoprecipitated and blotted using antiphosphotyrosine antibodies. H and L chain refer to the heavy and light chains of the precipitating Ab. (B) Total protein tyrosine phosphorylation profile of 3 patients with CLL upon CD38 or IgM ligation (5 minutes, 37°C). CLL no. 21 and no. 27 were CD38+/ZAP-70+, while no. 47 was CD38+/ZAP-70−. The arrow shows a band likely corresponding to ERK1/2. No increase in protein tyrosine phosphorylation was observed with CLL no. 47 cells. (C) Analysis of ERK1/2 phosphorylation in a subset of 12 patients with CLL upon CD38 and IgM ligation. CD38+/ZAP-70+ cells show a clear increase in phosphorylation compared with CD38+/ZAP-70− cells. (D) ERK1/2 phosphorylation was studied in 26 patients with CLL; gels were scanned and the band intensities were measured by the ImageJ software and plotted by dividing the patients according to different molecular parameters. The only statistically significant difference is observed by dividing CD38+ patients (n = 17) on the basis of ZAP-70 expression (left panel). No difference is scored when using IgV mutations (right panel). Data are expressed as percentage of increase in ERK1/2 phosphorylation compared with background; the horizontal line represents the mean of all samples. Statistical difference was calculated according to the Student 2-sample, 2-tailed t test.
These results suggest that CD38 signals in CLL cells depend on the presence of ZAP-70. This hypothesis was tested in the CLL model by analyzing CD38-mediated signal transduction in a panel of 26 patients with CLL characterized for CD38 and ZAP-70 expressions, mutations in the IgV genes, and chromosomal abnormalities (Table 1). Total protein tyrosine phosphorylation upon CD38 engagement revealed different patterns of response, ranging from pronounced activation to total lack of response (Figure 4B). A consistent finding in the samples responding to CD38-mediated signals is the presence of a phosphorylated band of approximately 44 kDa, likely corresponding to ERK1/2 (Figure 4B), a common target in the CD38 signaling pathway.1 The use of a specific mAb confirms different levels of CD38-mediated ERK1/2 phosphorylation in the same subset of CLL samples (Figure 4C). The intensities of the bands corresponding to ERK1/2 were quantified using the ImageJ software and correlated with the prognostic parameters available. Results indicate a clear-cut association between CD38-mediated ERK1/2 phosphorylation and the presence of ZAP-70 (P = .008; Figure 4D). CD38+/ZAP-70+ CLL cells (n = 12) were constantly associated with significantly higher phosphorylation levels, while the CD38+/ZAP-70− (n = 5) group consistently failed to induce ERK1/2 activation. No association was found with IgV mutational status, likely due to the presence of a subgroup of CD38+/ZAP-70+ patients with mutated IgV genes (n = 4; Table 1; Figure 4D).
CD38 and IL-2 signaling upmodulate ZAP-70
These results indicate that CD38 and ZAP-70 are not merely unrelated negative prognostic markers, but form part of a single signaling pathway leading to cell activation. This finding prompted us to rethink our understanding of the requisites and characteristics of CD38 signaling in the growth and survival of CLL cells. Our previous work with CLL indicated that IL-2 exposure is a key requisite for CD38-mediated signals.32 These effects were originally attributed to a demonstrated increase in cell surface expression of CD38, likely setting a signaling threshold. We then set out to test the hypothesis that IL-2 also affects the levels of ZAP-70, the substrate shown to be necessary for CD38 signaling.
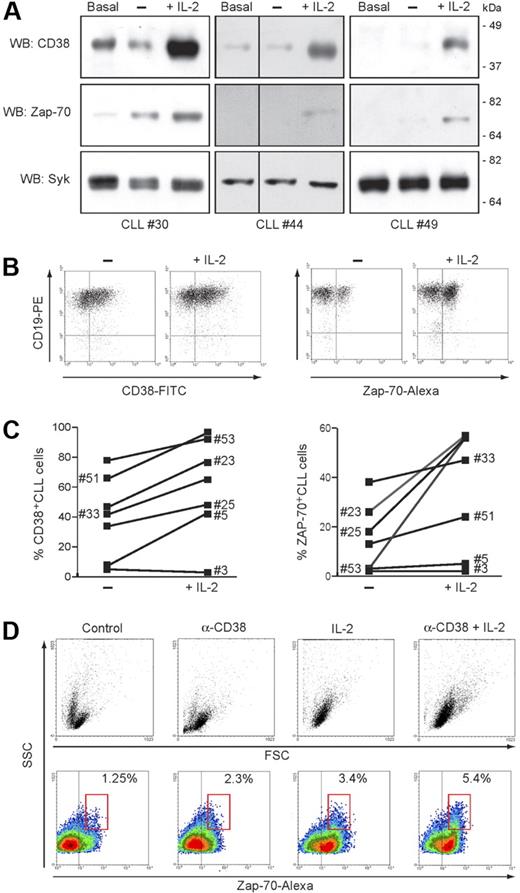
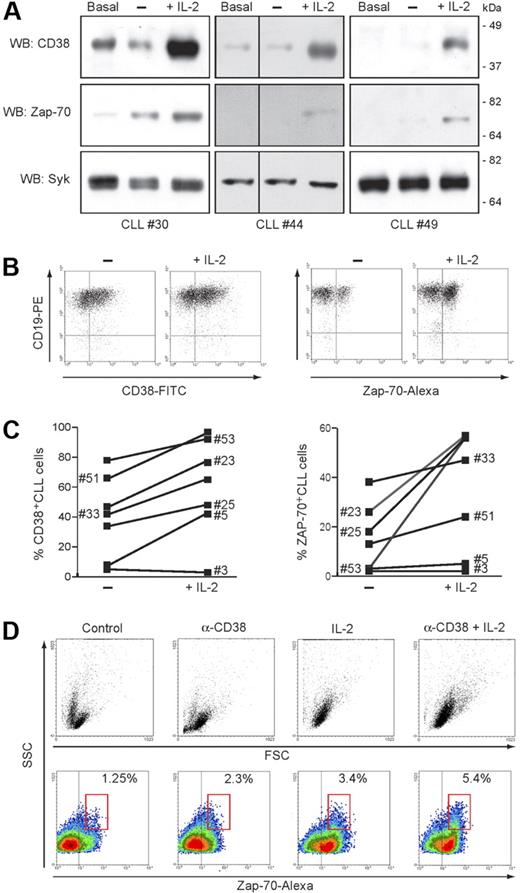
Treatment of purified CLL cells with IL-2 (100 IU/mL) induces a marked upmodulation of ZAP-70 expression, apparent after48 hours, peaking at day 3 and remaining stable until day 5 (Figure 5A). The behavior of CD38 confirmed the sensitivity of the molecule to IL-2, both in CD38+ patients and also in a subset of CD38− patients. Conversely, no increase in syk levels could be detected, indicating that the effect is specific for the ZAP-70 signaling pathway (Figure 5A). Up-regulation of ZAP-70 by potentially contaminating T cells was excluded by double staining for CD19 (Figure 5B). IL-2 effects were studied in 19 patients, and the sensitivity of CD38 and ZAP-70 proved to be generally consistent. A representative sample is shown in Figure 5C.
CD38 and IL-2 signaling upmodulate ZAP-70. (A) Purified CLL cells from 3 representative patients were cultured for 72 hours in the presence of IL-2 (100 IU/mL). Lysates were probed for CD38, ZAP-70, and syk using specific antibodies. Basal refers to freshly purified cells, while “−” refers to cells cultured for 72 hours without IL-2. Vertical lines have been inserted to indicate a repositioned gel lane. (B) PBMCs from 19 patients with CLL were cultured as in panel A, and CD38 and ZAP-70 was assayed by intracellular staining. Specificity for B cells was confirmed by counterstaining with an anti-CD19 mAb, as shown in the dot plot. (C) Effects induced by IL-2 exposure on the number of CD38+ and ZAP-70+ CLL cells in 7 representative patients. (D) ZAP-70 expression is increased following CD38 signaling in the presence of IL-2. Purified CLL cells from 5 patients were cultured for 5 days in the presence of agonistic anti-CD38 mAb, IL-2, or a combination of the 2 (CD38 + IL-2). The top panel shows the morphologic changes in the population as a dot plot of forward scatter (FSC) and side scatter (SSC). Bottom panels show density plots of ZAP-70 and SSC. A marked increase in ZAP-70 expression is apparent after treatment with anti-CD38 mAb or IL-2 and is boosted by the combination of the 2. Percentages indicate ZAP-70+ CLL cells within the red gate, highlighting cells with highest SSC values.
CD38 and IL-2 signaling upmodulate ZAP-70. (A) Purified CLL cells from 3 representative patients were cultured for 72 hours in the presence of IL-2 (100 IU/mL). Lysates were probed for CD38, ZAP-70, and syk using specific antibodies. Basal refers to freshly purified cells, while “−” refers to cells cultured for 72 hours without IL-2. Vertical lines have been inserted to indicate a repositioned gel lane. (B) PBMCs from 19 patients with CLL were cultured as in panel A, and CD38 and ZAP-70 was assayed by intracellular staining. Specificity for B cells was confirmed by counterstaining with an anti-CD19 mAb, as shown in the dot plot. (C) Effects induced by IL-2 exposure on the number of CD38+ and ZAP-70+ CLL cells in 7 representative patients. (D) ZAP-70 expression is increased following CD38 signaling in the presence of IL-2. Purified CLL cells from 5 patients were cultured for 5 days in the presence of agonistic anti-CD38 mAb, IL-2, or a combination of the 2 (CD38 + IL-2). The top panel shows the morphologic changes in the population as a dot plot of forward scatter (FSC) and side scatter (SSC). Bottom panels show density plots of ZAP-70 and SSC. A marked increase in ZAP-70 expression is apparent after treatment with anti-CD38 mAb or IL-2 and is boosted by the combination of the 2. Percentages indicate ZAP-70+ CLL cells within the red gate, highlighting cells with highest SSC values.
Previously, we showed that combined IL-2– and CD38-mediated signals lead to plasma blast transformation of a subset (10%-30%) of CLL cells.32 The appearance of this subset may be easily monitored by FACS analysis using morphologic parameters (Figure 5D). In light of the present data, we now show that these plasma blasts are characterized by levels of ZAP-70 uniformly higher than in the unmodified cells of the same CLL clone (Figure 5D), suggesting that plasma blast transformation occurs preferentially in CD38+/ZAP-70+ cells.
Comparison of molecular and functional parameters in CLL gene profiling
The next step was the identification of a discrete population expressing both CD38 and ZAP-70, and at the same time capable of migrating in response to SDF-1α. Attention was focused on seeking a specific genetic signature at the molecular level. Total RNA was obtained from 20 individuals and processed for hybridization on whole-genome oligonucleotide arrays. Class comparison algorithms were applied to extract differentially expressed genes between the classes of interest. To assess the performance of class separation, divisive hierarchic clustering was applied, yielding 2 indexes to measure intercluster correlation and intracluster homogeneity (indexes range from −1 to 1, where 1 represents complete genetic overlap, 0 means lack of correlation, and −1 indicates total genetic anti-correlation).
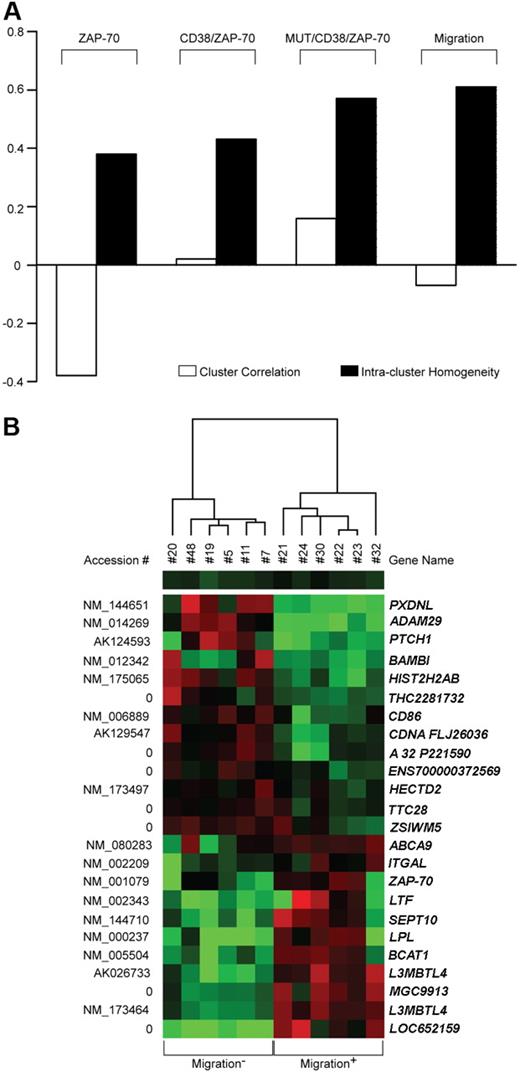
Patients were grouped according to their molecular characteristics, first on the basis of CD38 and/or ZAP-70 expression. Using this approach and a stringent gene selection criterion including correction for false discovery rates, no separation was observed when classifying the patient cohort according to CD38 expression. Instead, when focusing on ZAP-70 expression, 12 genes were found differentially expressed in the 2 classes, with intercluster correlation and intracluster homogeneity indexes of −0.38 and 0.38, respectively (Figure 6A). CD38 and ZAP-70 were then assessed in combination with 51 genes differentially expressed by the CD38+/ZAP-70+ (n = 9) and CD38−/ZAP-70− (n = 6) subgroups. To compare class separation results, the first 12 significant genes were taken into account, yielding an intercluster correlation index of 0.01 and an intra-cluster homogeneity index of 0.48 (Figure 6A). Factoring in IgV mutational status (UM/CD38+/ZAP-70+ = 6; MUT/CD38−/ZAP-70− = 6) resulted in 36 differentially expressed genes and a further improved intracluster homogeneity index of 0.57 (Figure 6A).
Comparison of molecular and functional parameters in CLL gene profiling. (A) Supervised analysis of microarray data obtained from 20 CLL samples distinguished on the basis of molecular (ZAP-70, CD38/ZAP-70, MUT/CD38/ZAP-70) or functional (Migration) characteristics. The performance of each class separation was calculated by measuring intercluster correlation (□) and intracluster homogeneity (■). Scores range from −1 to 1, where 1 represents complete genetic overlap, 0 means lack of correlation, and −1 indicates total genetic anticorrelation. (B) Representation of the expression pattern of 24 genes differentially expressed between 6 most- and 6 least-migrating samples. Colored squares refer to intensity ratios created by dividing the gene intensities of each sample with the weighted average of the gene intensities of all other samples analyzed. Red and green colors indicate up- and down-regulation, respectively. Accession numbers are listed on the left, while gene names are on the right.
Comparison of molecular and functional parameters in CLL gene profiling. (A) Supervised analysis of microarray data obtained from 20 CLL samples distinguished on the basis of molecular (ZAP-70, CD38/ZAP-70, MUT/CD38/ZAP-70) or functional (Migration) characteristics. The performance of each class separation was calculated by measuring intercluster correlation (□) and intracluster homogeneity (■). Scores range from −1 to 1, where 1 represents complete genetic overlap, 0 means lack of correlation, and −1 indicates total genetic anticorrelation. (B) Representation of the expression pattern of 24 genes differentially expressed between 6 most- and 6 least-migrating samples. Colored squares refer to intensity ratios created by dividing the gene intensities of each sample with the weighted average of the gene intensities of all other samples analyzed. Red and green colors indicate up- and down-regulation, respectively. Accession numbers are listed on the left, while gene names are on the right.
Next, patients were divided purely on the basis of the functional response to SDF-1α by comparing the 6 most (migration+) and 6 least (migration−) migrating samples, according to migration index. This approach identified 24 significant genes and resulted in the overall best class separation performance (Figure 6A,B). Of these, ZAP-70, LPL, ADAM29, and SEPT10 have already been identified by microarray technology and independently confirmed as indicators of unfavorable prognosis, thus offering indirect confirmation of the present results.16,33,34 A statistically significant number of differentially expressed genes are known to be involved in the control of cell motility and adhesion, either through the modulation of cell-cell contacts (ZAP-70, ADAM29, CD86, CD11a) or through the control of cytoskeletal organization (BAMBI, SEPT10). A total of 5 genes appear to be involved in the control of lipid metabolism (LPL, ABCA9, BCAT1, LTF, and PXDNL), 2 have reported transcription factor/transcription regulation activities (L3MBTL4 and HIST2H2AB), and 1 (PTCH1) is a hypothetical tumor suppressor. The remaining 8 genes code for hypothetical proteins or for proteins of yet unknown function (Table 2).
Discussion
A full understanding of CLL continues to elude researchers despite seminal progress in the field.35 The main issues to be resolved concern the cell of origin36 and the identification of pathogenetic chromosomal and/or molecular defects.37 The most widely credited view hypothesizes that CLL originates from a marginal zone T-independent B lymphocyte, which undergoes variable degrees of activation.38,39 The resulting neoplastic clone has distinct proliferative and antiapoptotic properties, which translate into a wide spectrum of clinical behaviors. According to this view, a central role is played by the antigen and by the signals of varying strengths implemented by its presentation to and ligation by the B-cell receptor.40 Attempts to identify antigens potentially providing pathogenetic clues41,42 are flanked by analyses of the microenvironment, which is considered to be a crucial element for the expansion of leukemic cells.43-45 Operationally, the microenvironment hosts a bidirectional network of signals running back and forth between the CLL cells and the surrounding nonneoplastic cells, thus representing a potential therapeutic target.
Our interest in CLL derives primarily from the exploitation of human diseases as strategic models for defining the in vivo biological role of CD38.46 Using this model, we showed that CD38 triggers robust proliferation/survival signals modulated through the interactions with the CD31 ligand expressed by NLCs and by the stromal/endothelial components.9,32 The present work represents a follow-up of those studies, and the results may offer insights of mutual interest for clinicians and basic researchers.
This study originated from an attempt to conciliate 2 apparently unrelated observations, namely that (1) CD38 is involved in the intricate web of interactions between CLL and NLCs, and that (2) NLCs are the source of chemotactic signals attracting CLL cells to BM and peripheral lymphoid organs, niches for further growth.47
We sought to determine the migratory potential of CLL cells by examining chemotaxis toward SDF-1α (a critical chemokine produced by NLC15 ) in a large cohort of freshly collected CLL samples, and to correlate this potential to the distinctive molecular features of the patients. A migration index was thus determined for each of the 46 participating patients; as expected, migration was strictly dependent on CXCR4 expression, given that a functional block of the receptor completely abrogated the response. In agreement with previously published data,24 ZAP-70+ patients displayed a migration index higher than the negative counterparts, although not statistically significant. The same observation was repeated when the patients were categorized according to CD38 expression. Migration differences were greatly enhanced when the 2 molecules were simultaneously evaluated, resulting in the separation of CD38+/ZAP-70+ and CD38−/ZAP-70− subgroups. The conclusion from this part of the work is that CD38+/ZAP-70+ patients are endowed with a greater ability to migrate toward SDF-1α. This phenomenon cannot be simply attributed to CXCR4 expression because a quantitative correlation between CXCR4 MFI values and the migration index is not apparent.
These results and clinical data showing that the combined expression of CD38 and ZAP-70 is a more precise diagnostic tool for identifying high-risk patients prompted us to evaluate the existence of a functional link between the 2 molecules in neoplastic B cells. CD38 engagement leads to a transient but significant tyrosine phosphorylation of ZAP-70 in CD38+/ZAP-70+ CLL as well as in a genetically modified human B-cell–line model. The lack of activation of tyrosine 292 suggests that this pathway is activatory rather than inhibitory. Further, ZAP-70 expression seems to be a limiting factor for CD38-mediated functions. Extensive analysis of CD38 signals in a panel of 26 patients with CLL proved that the CD38 pathway is selectively active in CD38+/ZAP-70+ cells. On the contrary, CD38+/ZAP-70− patients were consistently unable to signal via CD38. This observation is clinically relevant because it provides a molecular rationale for simultaneous testing of CD38 and ZAP-70 in risk stratification of patients.
Still to be determined is whether ZAP-70 is a stable disease marker,48 at variance with CD38, whose expression can apparently change over time.49 The issue of stability was addressed from a biological perspective: a previous work identified IL-2 as a potent inducer of CD38 expression in CLL cells, paralleled by the acquisition of signaling properties.32 Here, we show that IL-2 can also significantly enhance ZAP-70 expression, thus acting on the signaling pathway as opposed to just the single player. This result may be interpreted by hypothesizing that (1) CD38 (and likely ZAP-70) can change during the course of the disease, and also (2) that their levels result from different microenvironmental pressures, which vary according to organ or district. A recent report suggests that CLL cells may lose CD38 when entering the circulation, and that a high expression of CD38 simply reflects an increased cellular turnover.50 It is possible that similar results hold true for ZAP-70 and that CLL cells with the greatest proliferative potential are those that coexpress CD38 and ZAP-70, as in our in vitro model.
In all instances, preliminary data obtained in vitro8,45 and in vivo7 indicate that recirculation to and from BM and lymph nodes is critical for the maintenance and progression of the disease. Genetic evidence supporting this notion comes from genome-wide analyses performed on 20 patients with molecularly and functionally characterized CLL. Indeed, patients with high migratory potential, identified purely on the basis of the migration index and independently of any molecular marker, are characterized by a specific homogeneous genetic signature. A significant number of the differentially expressed genes is involved in modulating cell-cell interactions and movement both at the cell surface (CD86, CD11a, ADAM29, BAMBI) or at the cytoskeletal level (ZAP-70, SEPT10).
This work offers a molecular explanation of why combined CD38 and ZAP-70 assessment provides a more accurate identification of patients with high-risk CLL. Furthermore, it indicates that a higher migratory potential may be (or may contribute to) the reason why CD38+/ZAP-70+ patients with CLL are characterized by an unfavorable prognosis. How can the enhanced ability to recirculate be explained? The obvious answer is that the genes defined by microarrays confer the CLL cell with more efficient migratory machinery, and that the CD38 pathway subsequently delivers proliferation/survival signals mediated via ZAP-70 phosphorylation. The alternative hypothesis is that the CD38/ZAP-70 pathway is directly involved in the migratory response. Supporting evidence comes from independent observations indicating that ZAP-70 is part of the CXCR4 signaling apparatus,28,51,52 and from the fact that CD38 can mediate selectin-type interactions by binding the endothelial-expressed CD31 ligand.3,53 Furthermore, a direct role of CD38 in chemokine-driven migration (including SDF-1α) was recently demonstrated using Cd38−/− mice.26,27 What remains to be seen is whether these findings also apply for normal and neoplastic human B cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The excellent technical help of Ms Francesca Cottino is gratefully acknowledged.
This work was supported by grants from Chronic Lymphocytic Leukemia Global Research Foundation (CLL-GRF; S.D.), Associazione Italiana Ricerca Cancro (AIRC; S.D. and F.M.), Progetti di Ricerca di Interesse Nationale (PRIN) (S.D. and F.M.), University of Turin (S.D. and F.M.), and Regione Piemonte (F.M.). Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) and Compagnia di SanPaolo provided financial contributions. T.V. is supported by a Federazione Italiana Ricerca Cancro (FIRC) fellowship. S.A. was a Fellow of the European Society for Medical Oncology (ESMO) and is now supported by Fondazione ISI (Institute for Scientific Interchange)–Lagrange Project, Fondazione CRT (Cassa di Risparmio di Torino).
Authorship
Contribution: S.D. designed experiments and wrote the manuscript; T.V. performed tyrosine phosphorylation experiments; S.A. performed migration experiments; L. Bergui provided patient samples; G.D'A. provided patient samples; L. Bonello performed IgV mutation studies; P.O. performed ZAP-70 staining; M.S. performed gene expression experiments; O.J. provided patient samples; G.C. analyzed gene expression experiments; D.E. contributed vital reagents and helped in data interpretation; and F.M. designed experiments and together with S.D. wrote the paper.
T.V. and S.A. contributed equally to the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvia Deaglio or Fabio Malavasi, Department of Genetics, Biology and Biochemistry, Via Santena 19, 10126 Torino, Italy; e-mail: silvia.deaglio@unito.it; fabio.malavasi@unito.it.


 ) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.
) is not statistically significant, as opposed to the results scored by comparing ZAP-70+ (n = 17) with ZAP-70− (n = 22) patients (◇) and CD38+/ZAP-70+ (n = 13) with CD38−/ZAP-70− (n = 14) patients (◆). CD38+/ZAP-70− patients (n = 8) featured CXCR4 MFI values not significantly different from CD38−/ZAP-70− patients. (C) Migration was completely abrogated by anti (α)–CXCR4 treatment. Pretreatment with the irrelevant anti (α)–gp120 mAb excluded a nonspecific block. Results are presented as percentage of inhibition of migration in the absence of antibodies and are the means plus or minus SEM of 5 experiments.