Abstract
This study investigates the extent of bone marrow (BM) involvement at diagnosis of apparent isolated extramedullary (AIEM) relapses of childhood acute lymphoblastic leukemia (ALL) and its relation to prognosis. Sixty-four children with first AIEM relapse treated in Germany, Czech Republic, or France were included. Real-time quantitative polymerase chain reaction using T-cell receptor and immunoglobulin gene rearrangements provided a sensitive measure of submicroscopic BM involvement, which was detectable at a level of 10−4 or higher in 46 patients and less than 10−4 in 11 patients, and was nondetectable (sensitivity: 10−4) in 7 patients. In the total cohort, the probability of event-free survival (pEFS) for children with BM involvement of 10−4 or higher was 0.30 (0.09 ± SE) versus 0.60 (± 0.12) for those with less than 10−4 (P = .13). The cumulative incidence of subsequent relapse was 0.24 (± 0.01) for patients with BM involvement less than 10−4 and 0.65 (± 0.01) for those with 10−4 or higher (P = .012). Restricted to central nervous system (CNS) relapses, pEFS was 0.11 (± 0.09) for patients with BM involvement 10−4 or higher and 0.63 (± 0.17) for those with less than 10−4 (P = .053). CNS relapses were associated with a higher (≥ 10−4: 80%) submicroscopic BM involvement than testicular relapses (≥ 10−4: 57%, P = .08). In summary, we show marked heterogeneity of submicroscopic BM involvement at first AIEM relapse diagnosis in children with ALL, and demonstrate its possible prognostic relevance.
Introduction
Fifteen percent to 20% of all relapses in children with acute lymphoblastic leukemia (ALL) occur in an apparently isolated extramedullary (AIEM) compartment, defined as less than 5% leukemic blasts in the bone marrow (BM) aspirate. AIEM central nervous system (CNS) relapse accounts for two thirds of such patients.1-4 In patients with AIEM CNS relapse who are treated with combined radiochemotherapy, the relapse trials ALL-REZ BFM (ALL Relapse Berlin-Frankfurt-Münster) estimate the probability of event-free survival (pEFS) at 10 years to be approximately 40%.5 As in all ALL relapses, the pEFS of patients with AIEM relapse depends strongly on the time point of relapse diagnosis (ie, duration of first complete remission).6 In the majority of patients, AIEM CNS relapses occur either within 18 months of initial diagnosis of ALL (very early relapse) or 18 to 30 months after initial diagnosis (early relapse); relatively few occur 30 months or longer after initial diagnosis (late relapse). In the relapse trials ALL-REZ BFM, the pEFS rates at 10 years for patients with a very early, early, or late CNS relapse were 29%, 45%, and 67%, respectively.4
The testes are the second most frequent site of AIEM relapse, and account for most of the remaining third of relapses. AIEM testicular relapses occur significantly later than AIEM CNS relapses and have a relatively favorable outcome, having a pEFS rate of approximately 70% at 5 years.1,3,7 In the relapse trials of Children‘s Cancer Group, the pEFS rates at 6 years for patients with a very early (0-17 months after initial diagnosis), early (18-36 months), or late (≥ 36 months) testicular relapse were 52%, 57%, and 81%, respectively.1
Relapse at other extramedullary sites such as skin, breast, bone, muscle, abdominal organs, and eye is very rare.8
Until now, 3 prognostic parameters have been used for the stratification of first ALL relapses in BFM and other relapse trials: time from diagnosis, site of relapse, and leukemic immunophenotype. In patients with AIEM relapse, relapse time point is used as the single treatment stratification criterion in most current treatment protocols.4
On the basis of microscopic findings, site of relapse is divided into 3 categories with prognostic impact (a < b < c): (a) isolated BM relapse (equal or more than 25% ALL cells in the BM without extramedullary involvement), (b) combined relapse (equal or more than 5% ALL cells in the BM with extramedullary involvement), and (c) AIEM relapse.9,10 Assessment of BM involvement by cytology is limited to a detection sensitivity of approximately 5%. Although methods are now available that permit detection of minimal amounts of blasts with a very high sensitivity, there has been very little investigation of the extent and prognostic impact of submicroscopic BM involvement in AIEM relapses. More than 10 years ago, 4 groups reported on the detection of leukemic blasts in the BM at diagnosis of AIEM relapse using sensitive methods based on in vitro amplification (polymerase chain reaction [PCR]) of markers such as immunoglobulin (IG) or T-cell receptor (TCR) gene rearrangements. These analyses included only a very limited number of patients.7,11-13 We therefore decided to investigate in a larger group of patients whether submicroscopic BM involvement is a prognostic factor for patients with AIEM relapses.
Patients, materials, and methods
Patients
Our retrospective study included 64 patients with a first AIEM ALL relapse. Patients were treated either in the Czech Republic (n = 9; date of diagnosis: October 2001 to April 2004), in France (n = 16; date of diagnosis: December 1990 to June 2004), or in Germany (n = 39; date of diagnosis: September 1994 to February 2005). Treatment was given according to the national protocols: ALL-REZ BFM (Germany, Czech Republic) and Protocole Coopérateur de Traitement des Reschutes de Leucémies Aiguës Lymphoblastiques de L'enfant (COOPRALL; France). The mean follow-up time was 3.26 years (median, 2.7 years; minimum, 0.03 years; maximum, 16.2 years). For the total patient cohort included in the study, the pEFS was 0.38 (± 0.08) and the probability of overall survival (pOS) 0.48 (± 0.11).
Patients were included if they met the following criteria: (1) registration in the national trials for treatment of childhood relapsed ALL, (2) first AIEM relapse, (3) age younger than 18 years at relapse diagnosis, and (4) possibility of quantification of submicroscopic BM involvement using TCR/IG gene rearrangements identified as highly sensitive targets.
Informed consent was obtained from all patients and/or their guardians in accordance with the Declaration of Helsinki, and the investigation was approved by the institutional ethic committees of the participating institutions.
Diagnosis and definitions
AIEM relapses is defined as cytologically and/or histologically and/or immunologically proven leukemia appearing in an extramedullary compartment without overt BM involvement (BM blast lower than 5%, assessed by morphology).9,10 The time point of relapse is defined as “very early” if a relapse occurred within 18 months after initial diagnosis, “early” if the relapse occurred between 18 and less than 30 months, and “late” if the relapse occurred 30 months or later after initial diagnosis.14 The immunophenotype was defined according to EGIL criteria (European Group for the Immunological Characterization of Leukemias).15 A CNS relapse was diagnosed if lymphoblastic leukemic cells were identified in the cytospin preparation of cerebrospinal fluid (CSF), and if at least 5 nucleated cells per microliter of CSF were identified. The involvement of other extramedullary organs was proved by histologic assessment of a biopsy.
Treatment
In the ALL-REZ BFM 96/2002 trials, relapses were divided into the strategic groups S1 to S4, based on the time point from diagnosis and site of relapse, as well as leukemic immunophenotype. Patients with AIEM relapses were stratified into the groups S1 (late relapses) and S2 (early and very early relapses).4 In earlier trials (ALL-REZ BFM 85/87/90) AIEM relapses were combined in one group irrespective of the time point.9,14 In brief, the treatment schedule includes an intensive induction and consolidation with multidrug chemotherapy elements and repeated triple intrathecal therapy ([TIT]: methotrexate, cytarabin, prednisolone), local therapy including irradiation, and a conventional maintenance therapy. In patients with testicular relapse, a clinically involved testis is removed; a contralateral clinically not involved testis is irradiated.5 In comparison with treatment for S1, treatment for S2 patients is intensified and prolonged.4 In individual S2 patients, autologous or allogeneic stem cell transplantation (SCT) has been performed on the basis of center-specific decisions.16
According to COOPRALL, relapses are divided into the stratification groups G1 to G3. Patients with AIEM relapse are stratified into the groups G2 or G3. G2 includes early high-risk (chemoresistant during frontline therapy and/or displaying a t(9;22) or a t(4;11)) and T-cell relapses. G3, including only B-cell precursor and excluding early initially chemoresistant ALLs, subdivides in early (CR1 ≤ 24 months; G3a) and late (CR1 > 24 months; G3b) AIEM relapses. In group G3a, patients received a VANDA reinduction sequence. Patients in group G3a underwent SCT only if they had an intrafamilial donor. Patients in group G3b did not undergo a SCT. If no suitable related donor was available, patients received 3 series of chemotherapy, TIT, and a 2-year maintenance therapy. In group G3b, patients received the same 3 series of chemotherapy, TIT, and a 1-year maintenance therapy.17
Materials
Patients were retrospectively included in the study on the basis of the availability of the following samples: (1) Extramedullary sample at diagnosis of AIEM relapse (CSF, testis, lymph node, others) as first choice or/and material of initial diagnosis or second relapse diagnosis as second choice was necessary. In the second case, the presence of markers at the time of AIEM relapse had to be confirmed by real-time quantitative (RQ)–PCR on the small amount of extramedullary sample or by a positive quantitative result in BM at relapse diagnosis. If no marker from initial diagnosis or second relapse diagnosis was shown to be maintained at first relapse, the patient was excluded from the study (Table 1). (2) BM sample at time of diagnosis of AIEM relapse was necessary. Dilution series used for minimal residual disease (MRD) quantification were performed using the extramedullary sample of first relapse in 15 cases. In the other cases, BM (41 cases) or blood (4 cases) of initial diagnosis, or BM of a subsequent relapse (4 cases), was used after confirmation of valid and stable markers for disease detection at first relapse.
Material for identification and confirmation of clonal relation of clone-specific markers used for submicroscopic BM involvement quantification
| Material for marker screening . | Non-IEM sample . | |
|---|---|---|
| Initial diagnosis, n = 34 . | 2nd relapse, n = 3 . | |
| Method of stabilizing clonal markers | ||
| PCR in DNA extracted from cytospins or tissue slides | 7 | 0 |
| Positive quantitative result in first relapse BM | 27 | 3 |
| Material for marker screening . | Non-IEM sample . | |
|---|---|---|
| Initial diagnosis, n = 34 . | 2nd relapse, n = 3 . | |
| Method of stabilizing clonal markers | ||
| PCR in DNA extracted from cytospins or tissue slides | 7 | 0 |
| Positive quantitative result in first relapse BM | 27 | 3 |
For the IEM samples CSF (n = 14), testis (n = 11), and other (n = 2), there was no method of stabilization necessary.
Methods
Mononuclear cells (MNCs) were isolated by Ficoll density centrifugation. CSF samples were centrifuged, and DNA was isolated after removal of the supernatant. Testicular samples, which were not formalin fixed, were dried-cut into 10-μm slices, or passed through a strainer and digested with proteinase K (Roche Diagnostics, Mannheim, Germany) before DNA isolation. Assessment of common chromosomal translocations was performed with reverse-transcriptase PCR using standardized protocols.18,19
DNA was extracted from MNCs of BM, blood, and cells of CSF, as well as from cells of the extramedullary compartment, using the Puregene DNA-Isolation kit (Biozym Diagnostic, Oldendorf, Germany) and the QIAamp Blood Kit (QIAGEN, Hilden, Germany). If DNA was not isolated, nucleated cells were lysed and stored at −20°C until PCR was performed.
TCR (TCRD δ/TCRG γ/VD2JA [delta-alpha]) and IG (IGH [heavy chain]/IGK-Kde [kappa light chain]) gene rearrangements were used as clonal population-specific targets for quantification of submicroscopic BM involvement.20-23 “Clonality” of PCR products identified was assessed by homo-heteroduplex analysis or capillary electrophoresis of fluorescent PCR products (fragment analysis by Genescan, Applied Biosystems, Foster City, CA).24,25 IGH/TCR gene “monoclonal” rearrangements were sequenced, either directly or after polyacrylamide gel separation using the Big Dye terminator reaction mix (Applied Biosystems) according to the instructions of the manufacturer. Allele-specific primers were designed either manually or using the primer express 1.0 software (Applied Biosystems).
Sensitive quantification of submicroscopic BM involvement was performed using RQ-PCR with germ-line TaqMan probes and clone-specific primers, as described previously for IGH, IGK-Kde, VD2-JA, and TCRG.22,26-29 TCRD V2-D3 uncompleted rearrangement was analyzed using the forward primer VD2-F (5′-CGA GAA AAG GAC ATC TAT GGC C-3′) and probe VD2-P (5′-FAM TGG TTT CAA AGA CAA TTT CCA AGGTGA CAT TG TAMRA-3′) or as described by Eckert and Landt.28
Albumin or beta-globin gene quantification was performed in parallel, as previously described, to correct for the quantity and quality of DNA.30 Standard conditions were used for all systems, with 200-nM primers, 100-nM probes, and the Platinum quantitative PCR supermix-UDG kit (Invitrogen, Cergy Pontoise, France) or Platinum Taq Polymerase (Invitrogen Life Technologies, Karlsruhe, Germany). Different RQ-PCR detection machines were used in the 3 countries: Berlin, LightCycler (Roche Diagnostics); Prague, iCycler IQ (BIO-RAD, Hercules, CA); and Paris, ABI PRISM 7700 (Applied Biosystems).
The equivalent of 105 cells (corresponding to approximately 670 ng DNA) was analyzed for each sample. We tested serial 10-fold dilutions of diagnostic template (adjusted according to the blast content of the tumor sample to obtain 10−1 to 10−5 leukemia blast dilutions) in a pool (n ≥ 10) of normal peripheral blood MNCs to obtain a calibration curve and to determine the sensitivity obtained for each rearrangement. Peripheral blood MNCs were tested alone to evaluate the specificity of the assay. All samples were analyzed in duplicate or triplicate with the exception of peripheral blood MNCs, which were tested in quadruplicate or quintuplicate.
We aimed always to use at least one clone-specific marker with a sensitivity of 10−4, but, when the amount of DNA permitted, all available markers were tested. Sensitivity evaluation, interpretation of experimental data, and expression of results were done according to guidelines of the European Study Group on MRD detection in ALL (ESG-MRD).6
Statistical methods
Differences between patient subsets were assessed by chi-square test or Fisher exact test for categoric variables. A 2-tailed P value less than .05 was regarded as significant. Kaplan-Meier analyses were used to estimate probabilities of EFS or probabilities of OS differences were assessed by the log-rank test. The cumulative incidence of competing risks (death in remission, relapse) in different subgroups was assessed using the Kalbfleisch-Prentice method and the Gray statistics.31,32 EFS time was calculated from the date of relapse diagnosis to the date of the last recall or the date of an adverse event (death in remission, relapse). To test the independence of predictive factors for pEFS, multivariate Cox regression analysis and the Forward Wald test have been applied, including SCT as a time-dependent covariate. Mann-Whitney test and Kruskal-Wallis test were used as distribution-free test for a central value of a variable between 2 or more groups.
Results
Patient characteristics
In our patient cohort, 59% of relapses were located in the CNS (38/64), 33% in the testis (21/64), and 6% in other extramedullary compartments (2/64 in lymph nodes, 1/64 in the ovary, and 1/64 in the mediastinum). In one patient, 2 extramedullary sites were involved (CNS, testis). This patient is assigned to the CNS group in the following analyses.
Seven ALLs were TEL-AML1 fusion transcript positive; 4 ALLs, BCR-ABL fusion transcript positive; and 1 ALL, MLL/AF9 fusion transcript positive. Seventeen patients underwent a SCT in second complete remission; 8 patients received a transplant from allogeneic stem cell donors, and 9 patients received an autologous graft. All patients achieved a second complete remission. Among these, 47% (30/64) remained in second continuous complete remission (CCR) until the analysis, 6% (4/64) died during therapy, and 47% (30/64) suffered a second relapse. These 30 second relapses occurred in BM (40%, 12/30), CNS (33%, 10/30), testis (7%, 2/30), lymph node (3%, 1/30), mediastinum (3%, 1/30), BM/CNS (10%, 3/30), and BM/testis (3%, 1/30). Twenty-two of 39 with an initial CNS relapse suffered a second relapse. Notably, 55% (12/22) of the patients with a first AIEM relapse in the CNS had a second relapse in the BM (9 BM isolated, 3 BM combined), 41% (9/22) again in the CNS, and 4% (1/22) in the testis. After their second relapse, 21 children died, and 9 children are alive in third CCR. The clinical parameters and treatment regimens of the total study cohort are summarized in Tables 2 and 3.
Frequencies of clinical parameters in subgroups defined by submicroscopic BM involvement (cutoff: 10−4)
| Clinical parameters . | Submicroscopic BM involvement . | |||
|---|---|---|---|---|
| Total, no. (%) . | Less than 10−4, no. (%) . | At least 10−4, no. (%) . | P . | |
| Total | 64 (100) | 18 (100) | 46 (100) | — |
| Sex | .73 | |||
| Male | 52 (81) | 14 (78) | 38 (83) | — |
| Female | 12 (19) | 4 (22) | 8 (17) | — |
| Age | .68 | |||
| Less than 5 y | 10 (16) | 3 (17) | 7 (15) | — |
| 5 to 9 y | 24 (38) | 8 (44) | 16 (35) | — |
| 10 y or more | 30 (47) | 7 (39) | 23 (50) | — |
| Time point | .91 | |||
| Less than 18 mo | 24 (38) | 6 (33) | 18 (39) | — |
| 18 to less than 30 mo | 20 (31) | 6 (33) | 14 (30) | — |
| At least 30 mo | 20 (31) | 6 (33) | 14 (30) | — |
| Site | .18 | |||
| CNS* | 39 (61) | 8 (44) | 31 (67) | — |
| Testis | 21 (33) | 9 (50) | 12 (26) | — |
| Other | 4 (6) | 1 (6) | 3 (7) | — |
| Immunophenotype | .999 | |||
| BCP | 49 (80) | 13 (81) | 36 (80) | — |
| T lineage | 12 (20) | 3 (19) | 9 (20) | — |
| No data | 3 | 2 | 1 | — |
| Clinical parameters . | Submicroscopic BM involvement . | |||
|---|---|---|---|---|
| Total, no. (%) . | Less than 10−4, no. (%) . | At least 10−4, no. (%) . | P . | |
| Total | 64 (100) | 18 (100) | 46 (100) | — |
| Sex | .73 | |||
| Male | 52 (81) | 14 (78) | 38 (83) | — |
| Female | 12 (19) | 4 (22) | 8 (17) | — |
| Age | .68 | |||
| Less than 5 y | 10 (16) | 3 (17) | 7 (15) | — |
| 5 to 9 y | 24 (38) | 8 (44) | 16 (35) | — |
| 10 y or more | 30 (47) | 7 (39) | 23 (50) | — |
| Time point | .91 | |||
| Less than 18 mo | 24 (38) | 6 (33) | 18 (39) | — |
| 18 to less than 30 mo | 20 (31) | 6 (33) | 14 (30) | — |
| At least 30 mo | 20 (31) | 6 (33) | 14 (30) | — |
| Site | .18 | |||
| CNS* | 39 (61) | 8 (44) | 31 (67) | — |
| Testis | 21 (33) | 9 (50) | 12 (26) | — |
| Other | 4 (6) | 1 (6) | 3 (7) | — |
| Immunophenotype | .999 | |||
| BCP | 49 (80) | 13 (81) | 36 (80) | — |
| T lineage | 12 (20) | 3 (19) | 9 (20) | — |
| No data | 3 | 2 | 1 | — |
BM indicates bone marrow; CNS, central nervous system; BCP, B-cell precursor; and —, not applicable.
One patient with both CNS and testicular relapse.
Frontline treatment and relapse treatment in subgroups defined by submicroscopic BM involvement (cut-off: 10−4)
| Treatment . | Submicroscopic BM involvement . | P . | ||
|---|---|---|---|---|
| Total, no. (%) . | Less than 10−4, no. (%) . | At least 10−4, no. (%) . | ||
| Total | 64 (100) | 18 (100) | 46 (100) | — |
| Frontline protocol | .14 | |||
| ALL-BFM 90/96 | 20 (31) | 5 (28) | 15 (33) | — |
| ALL-BFM 2000 | 14 (22) | 3 (17) | 11 (24) | — |
| EORTC 58–881/952 | 16 (25) | 8 (44) | 8 (17) | — |
| COALL-92/97 | 14 (22) | 2 (11) | 12 (26) | — |
| Cranial irradiation frontline | .93 | |||
| No | 42 (78) | 11 (79) | 31 (78) | — |
| Yes | 12 (22) | 3 (21) | 9 (23) | — |
| No data | 10 | 4 | 6 | — |
| Relapse protocol | .023 | |||
| ALL-REZ BFM 90/96 | 16 (25) | 1 (6) | 15 (33) | — |
| ALL-REZ BFM P02/02 | 32 (50) | 9 (50) | 23 (50) | — |
| COOPRALL | 16 (25) | 8 (44) | 8 (17) | — |
| Cranial irradiation relapse | .30 | |||
| No | 19 (40) | 7 (50) | 12 (36) | — |
| Yes* | 18 (39) | 4 (29) | 14 (42) | — |
| TBI† | 10 (21) | 3 (21) | 7 (21) | — |
| No data | 17 | 4 | 13 | — |
| SCT | .30 | |||
| No | 47 (75) | 14 (78) | 33 (73) | — |
| Allogeneic‡ | 7 (11) | 2 (11) | 5 (11) | — |
| Autologous | 9 (14) | 2 (11) | 7 (16) | — |
| No data | 1 | 0 | 1 | — |
| Treatment . | Submicroscopic BM involvement . | P . | ||
|---|---|---|---|---|
| Total, no. (%) . | Less than 10−4, no. (%) . | At least 10−4, no. (%) . | ||
| Total | 64 (100) | 18 (100) | 46 (100) | — |
| Frontline protocol | .14 | |||
| ALL-BFM 90/96 | 20 (31) | 5 (28) | 15 (33) | — |
| ALL-BFM 2000 | 14 (22) | 3 (17) | 11 (24) | — |
| EORTC 58–881/952 | 16 (25) | 8 (44) | 8 (17) | — |
| COALL-92/97 | 14 (22) | 2 (11) | 12 (26) | — |
| Cranial irradiation frontline | .93 | |||
| No | 42 (78) | 11 (79) | 31 (78) | — |
| Yes | 12 (22) | 3 (21) | 9 (23) | — |
| No data | 10 | 4 | 6 | — |
| Relapse protocol | .023 | |||
| ALL-REZ BFM 90/96 | 16 (25) | 1 (6) | 15 (33) | — |
| ALL-REZ BFM P02/02 | 32 (50) | 9 (50) | 23 (50) | — |
| COOPRALL | 16 (25) | 8 (44) | 8 (17) | — |
| Cranial irradiation relapse | .30 | |||
| No | 19 (40) | 7 (50) | 12 (36) | — |
| Yes* | 18 (39) | 4 (29) | 14 (42) | — |
| TBI† | 10 (21) | 3 (21) | 7 (21) | — |
| No data | 17 | 4 | 13 | — |
| SCT | .30 | |||
| No | 47 (75) | 14 (78) | 33 (73) | — |
| Allogeneic‡ | 7 (11) | 2 (11) | 5 (11) | — |
| Autologous | 9 (14) | 2 (11) | 7 (16) | — |
| No data | 1 | 0 | 1 | — |
BM indicates bone marrow; TBI, total body irradiation; SCT, stem-cell transplantation; and —, not applicable.
For 12 Gy, n=2; for 15/18 Gy, n=17 patients.
For 12 Gy, n=4; for 15/17 Gy, n=6 patients.
Four matched sibling donor and 3 matched unrelated donor transplantations.
Heterogeneity in submicroscopic BM involvement at relapse diagnosis
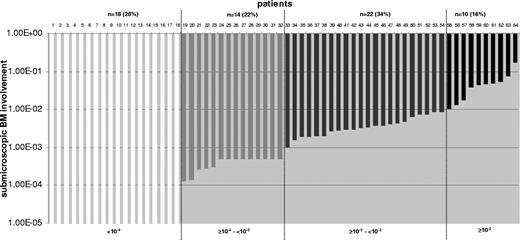
The levels of submicroscopic BM involvement were highly variable (Figure 1). A disease level of less than 10−4 was found in 28% (18/64) of the patients. Eleven of 18 patients showed a detectable disease level less than 10−4, whereas 7 of 18 patients showed no detectable disease (sensitivity: 10−4). Levels of 10−2 or higher were detected in 16% (10/64). CNS relapse was associated with a higher submicroscopic BM involvement than testicular relapse. Eighty percent (31/39) of the patients with a CNS relapse had submicroscopic BM involvement of 10−4 or higher versus only 57% (12/21) of the patients with a testicular relapse (P = .08).
Frequency of submicroscopic bone marrow involvement at relapse diagnosis in the total cohort (n = 64). Quantitative levels of submicroscopic BM involvement at relapse diagnosis are shown for all patients. Twenty-eight percent (18/64) of patients have a disease level of lower than 10−4; 22% (14/64), equal to or higher than 10−4 and lower than 10−3; 34% (22/64), equal to or higher than 10−3 and lower than 10−2; and 16% (10/64), with levels equal to or higher than 10−2.
Frequency of submicroscopic bone marrow involvement at relapse diagnosis in the total cohort (n = 64). Quantitative levels of submicroscopic BM involvement at relapse diagnosis are shown for all patients. Twenty-eight percent (18/64) of patients have a disease level of lower than 10−4; 22% (14/64), equal to or higher than 10−4 and lower than 10−3; 34% (22/64), equal to or higher than 10−3 and lower than 10−2; and 16% (10/64), with levels equal to or higher than 10−2.
For further analysis, patients are assigned to 1 of 2 subgroups according to their submicroscopic BM involvement (≥ 10−4 versus < 10−4). Our aim was to use the possible lowest cutoff that is allowed by our quantification method. A sensitivity of 10−4 for at least one specific TCR/Ig gene rearrangement can be reached for almost every patient. Lower sensitivities such as 10−5 are generally achieved for only a small proportion of patients (31% in our cohort).
Extent of submicroscopic BM involvement and relevant clinical parameters and treatment
As shown in Tables 2,3, there is no evidence of an association between submicroscopic BM involvement and sex, age, time point of relapse, site, or immunophenotype of the disease (Table 2) and frontline protocol, cranial irradiation during frontline treatment, cranial irradiation during relapse treatment, and SCT in second remission (Table 3). There is some evidence that patients treated according to ALL-REZ BFM relapse protocols especially ALL-REZ BFM 90/96 protocol showed higher proportions with a submicroscopic BM involvement of 10−4 or higher than patients of COOPRALL.
Submicroscopic BM involvement at relapse diagnosis is a prognostic parameter
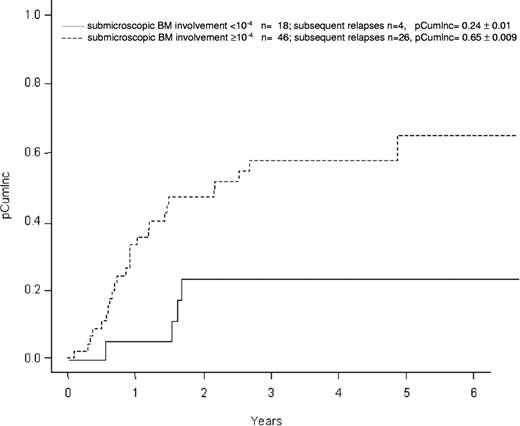
Performing a univariate Kaplan-Meier analysis in the total patient cohort (n = 64), the pEFS at 5 years in the 46 patients with BM involvement of 10−4 or higher is 0.30 (± 0.09 [SE]) versus 0.60 (± 0.12) in the 18 patients with BM involvement less than 10−4 (P = .13, Figure 2). The pOS is 0.39 (± 0.13) and 0.71 (± 0.10), respectively (P = .27). The cumulative incidence of subsequent relapse (pCumlnc) at 5 years was higher in patients with submicroscopic BM involvement of 10−4 or higher (0.65 ± 0.01) compared with those with BM involvement less than 10−4 (0.24 ± 0.01; P = .012; Figure 3).
Probability of event-free survival (pEFS ± standard error) according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; total group, n = 64. There is no evidence (P = .13) of different probability of event-free survival between both groups separated by the cutoff 10−4 in the total group of patients (n = 64).
Probability of event-free survival (pEFS ± standard error) according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; total group, n = 64. There is no evidence (P = .13) of different probability of event-free survival between both groups separated by the cutoff 10−4 in the total group of patients (n = 64).
Cumulative incidence (pCumlnc ± standard error) of subsequent relapses according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; total group, n = 64. There is a strong evidence (P = .012) of a different cumulative incidence of subsequent relapse in groups defined by the submicroscopic BM involvement cutoff 10−4.
Cumulative incidence (pCumlnc ± standard error) of subsequent relapses according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; total group, n = 64. There is a strong evidence (P = .012) of a different cumulative incidence of subsequent relapse in groups defined by the submicroscopic BM involvement cutoff 10−4.
When the analysis is restricted to CNS relapses (n = 39), the pEFS at 5 years is 0.63 (± 0.17) in patients with submicroscopic BM involvement less than 10−4 and 0.11 (± 0.09) in those with of 10−4 or higher (P = .053; Figure 4). Among patients with testicular relapse (n = 21), no difference of pEFS depending on the extent of BM involvement could be found.
Probability of event-free survival (pEFS ± standard error) according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; central nervous system group, n = 39. Considering only isolated CNS relapses (n = 39), there is some evidence (P = .053) toward lower probability of event-free survival in the group with submicroscopic BM involvement of 10−4 or higher.
Probability of event-free survival (pEFS ± standard error) according to submicroscopic bone marrow (BM) involvement at relapse diagnosis; central nervous system group, n = 39. Considering only isolated CNS relapses (n = 39), there is some evidence (P = .053) toward lower probability of event-free survival in the group with submicroscopic BM involvement of 10−4 or higher.
A multivariate Cox proportional hazard model for pEFS was fitted including the covariates sex, age at relapse diagnosis, immunophenotype, time point and site of relapse, submicroscopic BM involvement at relapse diagnosis, frontline treatment, relapse treatment, and SCT as time-independent variable. In this model, submicroscopic BM involvement, age at relapse diagnosis, site of relapse, and immunophenotype emerged as independent prognostic factors (Wald test: P < .05). Table 4 presents hazard ratios for included parameters. Patients with submicroscopic BM involvement of 10−4 or higher have 2.7 times the hazard (95% CI, 1.1-6.5) of dying or suffering a subsequent event than patients with a level less than 10−4.
Final multivariable Cox proportional hazard model for the outcome time to subsequent relapse or death
| Parameter . | No. of patients . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Immunophenotype | < .001 | |||
| BCP | 49 | 1 | — | — |
| T lineage | 12 | 5.3 | 2.1-13.1 | — |
| Submicroscopic BM involvement at relapse diagnosis | .04 | |||
| Less than 10−4 | 16 | 1 | — | — |
| At least 10−4 | 45 | 2.7 | 1.1-6.9 | — |
| Age at relapse diagnosis | .015 | |||
| 10 y or older | 28 | 1 | — | — |
| 5 to 9 y | 23 | 0.9 | 0.4-2.0 | — |
| Younger than 5 y | 10 | 3.9 | 1.5-10.3 | — |
| Site of relapse | .001 | |||
| CNS | 38 | 1 | — | — |
| Testis | 20 | 0.24 | 0.09-0.62 | — |
| Other | 3 | 0.43 | 0.09-2.04 | — |
| Parameter . | No. of patients . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| Immunophenotype | < .001 | |||
| BCP | 49 | 1 | — | — |
| T lineage | 12 | 5.3 | 2.1-13.1 | — |
| Submicroscopic BM involvement at relapse diagnosis | .04 | |||
| Less than 10−4 | 16 | 1 | — | — |
| At least 10−4 | 45 | 2.7 | 1.1-6.9 | — |
| Age at relapse diagnosis | .015 | |||
| 10 y or older | 28 | 1 | — | — |
| 5 to 9 y | 23 | 0.9 | 0.4-2.0 | — |
| Younger than 5 y | 10 | 3.9 | 1.5-10.3 | — |
| Site of relapse | .001 | |||
| CNS | 38 | 1 | — | — |
| Testis | 20 | 0.24 | 0.09-0.62 | — |
| Other | 3 | 0.43 | 0.09-2.04 | — |
CI indicates confidence interval; BCP, B-cell precursor; BM, bone marrow; CNS, central nervous system; and —, not applicable.
Extent of submicroscopic BM involvement, time point of relapse and prognosis
Separate analyses were done for patient groups with very early/early (n = 44) or late (n = 20) relapses using the cutoff of 10−4 for submicroscopic BM involvement. Among very early/early relapses, pEFS was 0.67 (± 0.14) versus 0.11 (± 0.89; P = .016) for patients with less than 10−4 versus of 10−4 or higher. No such difference was seen among late relapses (< 10−4 versus ≥ 10−4: pEFS 0.42 ± 0.22 versus 0.70 ± 0.13, P = .21). Regarding pOS, no difference was found between high- and low-level submicroscopic BM involvement for very early/early relapses (< 10−4 versus ≥ 10−4: 0.67 ± 0.13 versus 0.28 ± 0.13, P = .23) and late relapses (< 10−4 versus ≥ 10−4: 0.75 ± 0.22 versus 0.77 ± 0.12, P = .94).
There is no evidence that increasing submicroscopic BM involvement is associated with occurrence of second relapse in BM (Table 5).
Site of subsequent relapses and submicroscopic BM involvement at diagnosis of first relapse
| Site of subsequent relapse . | Submicroscopic BM involvement . | ||||
|---|---|---|---|---|---|
| Total . | Less than 10−4 . | At least 10−4 to less than 10−3 . | At least 10−3 to less than 10−2 . | At least 10−2 . | |
| Total | 30 (100) | 4 (100) | 7 (100) | 13 (100) | 6 (100) |
| BM | 12 (40) | 1 (30) | 1 (14) | 9 (69) | 1 (17) |
| BM/CNS | 3 (10) | 2 (40) | 0 (0) | 0 (0) | 1 (17) |
| BM/testis | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (17) |
| CNS | 10 (33) | 1 (30) | 4 (57) | 2 (15) | 3 (50) |
| Testis | 2 (7) | 0 (0) | 1 (14) | 1 (8) | 0 (0) |
| Other | 2 (7) | 0 (0) | 1 (14) | 1 (8) | 0 (0) |
| Site of subsequent relapse . | Submicroscopic BM involvement . | ||||
|---|---|---|---|---|---|
| Total . | Less than 10−4 . | At least 10−4 to less than 10−3 . | At least 10−3 to less than 10−2 . | At least 10−2 . | |
| Total | 30 (100) | 4 (100) | 7 (100) | 13 (100) | 6 (100) |
| BM | 12 (40) | 1 (30) | 1 (14) | 9 (69) | 1 (17) |
| BM/CNS | 3 (10) | 2 (40) | 0 (0) | 0 (0) | 1 (17) |
| BM/testis | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (17) |
| CNS | 10 (33) | 1 (30) | 4 (57) | 2 (15) | 3 (50) |
| Testis | 2 (7) | 0 (0) | 1 (14) | 1 (8) | 0 (0) |
| Other | 2 (7) | 0 (0) | 1 (14) | 1 (8) | 0 (0) |
Data are number (%).
BM indicates bone marrow; CNS, central nervous system.
Discussion
Heterogeneity of submicroscopic BM involvement
This collaborative study of a large group of children with relapsed ALL demonstrates that submicroscopic BM involvement is found in the majority of patients with AIEM relapse. It also shows that the level of submicroscopic BM involvement at the time of AIEM relapse is highly heterogeneous between patients. When using the sensitive quantitative MRD method, only 18 of our 64 patients display submicroscopic BM involvement less than 10−4. Because the stability of all applied markers was confirmed, the possibility of false-negative detection of leukemic cells can largely be excluded.
These data are consistent with the previous studies, which detected submicroscopic BM involvement in all or most patients included. However, these earlier studies were based on very small numbers of patients, did not confirm stability of initial MRD markers in extramedullary samples, and/or performed quantification with semiquantitative end-point PCR methods.7,11-13
Submicroscopic BM involvement and prognosis
Previous studies have not been able to assess the relationship between submicroscopic BM involvement and prognosis, mainly due to the very small number of patients included. In the current study, the cutoff of 10−4 divides the patient cohort of our study into 2 groups with significantly different cumulative incidences of subsequent relapse. However, there was no evidence of a different pEFS in the total group.
Redefinition of AIEM and combined relapse
Should patients with an AIEM and submicroscopic BM involvement equal to or higher than 10−4 be redefined as combined relapse? The level of 5% or more of leukemia blasts has been proposed to define combined relapses because it corresponds to the detection limit of the classical method used for blast content determination: cytomorphologic examination of BM smears. An IG/TCR-based RQ-PCR assay has a 1000-fold higher resolution in comparison with the assessment by light microscope.34-36 Therefore, a more precise and sensitive diagnosis of AIEM relapse is possible. We argue that on this basis it would be logical to redefine AIEM. Subject to confirmation by a prospective study, we would recommend implementing quantification of submicroscopic BM involvement in routine diagnostics for extramedullary relapses with less than 5% leukemic blasts in the BM smear. The quantification of submicroscopic BM involvement would divide patients with extramedullary involvement into 3 new groups: isolated extramedullary relapse: no BM involvement or BM involvement less than 10−4; combined relapse 1: with microscopic BM involvement of 5% or more; and combined relapse 2: microscopic BM involvement less than 5% and submicroscopic BM involvement of 10−4 or higher.
Logically, the shift from microscopic sensitivity in total BM cells (lower than 5%) to submicroscopic in MNCs (lower than 0.01%) should result in a similar shift in combined relapse definition. Such a definition implies that relapse diagnosis would then rely on a combination of light microscopy with a more sensitive technique (RQ-PCR methodology or flow cytometric immunophenotyping).
Does true isolated extramedullary relapse exist? Although more sensitive than cytomorphology, IG/TCR PCR also has a sensitivity threshold (10−4) and involvement lower than this threshold probably exists. The group of patients with AIEM and less than 10−4 BM involvement seem to have a better prognosis. It remains to be clarified whether this type of AIEM relapse (very low level or no BM involvement) is the result of a different pathophysiological process or is simply due to an early timing in relapse diagnosis.
Toward a new stratification of second relapse risk
In current relapse protocols such as ALL-REZ BFM and COOPRALL, patients with AIEM relapse receive not only local therapy but also systemic polychemotherapy that is given to children with BM and combined relapses.
In ALL-REZ BFM 96/2002 and COOPRALL, AIEM relapses have been stratified according to time point of relapse. Patients with a late relapse receive a less intensive therapy than patients with early or very early relapse (see “Treatment”). The poor outcome in the group with submicroscopic BM involvement of 10−4 or higher (pCumlnc: 0.65 ± 0.01; pEFS: 0.30 ± 0.09) indicates an insufficient therapy for this group and time point of relapse as single stratification criterion used until now may not be sufficient. On the basis of these findings, we suggest that the quantitative assessment of submicroscopic BM involvement is useful as an additional stratification criterion. A more intensified therapy may be necessary with high-dose polychemotherapy and/or autologous/allogeneic SCT for patients with submicroscopic BM involvement.37
Contribution to pathogenesis of extramedullary relapses
Strong evidence exists for an association between the extent (cutoff 10−4) of submicroscopic BM involvement at relapse diagnosis and prognosis (pEFS) in very early/early relapse, whereas no such association was seen in late relapse. This observation might be explained by different biologic mechanisms for very early/early versus late relapses. Early/very early relapses occur during or very shortly after antileukemic treatment. An early/very early combined relapse derives most probably from highly resistant leukemic cells progressing at several sites at the same time. It is evident, that a higher load of resistant leukemic cells must lead to an inferior prognosis. In contrast, late combined relapses may derive from leukemic cells, in a protected extramedullary compartment persisting despite initial chemotherapy, which than recede into the BM. These cells had a less intensive exposition to initial chemotherapy and would remain drug sensitive, even at a higher tumor load.38 This explanation is supported by the observation that early or late relapses occurring in both an extramedullary compartment and BM have a better prognosis than those occurring in isolation in the BM.9,39 Our data support the hypothesis that the pathogenesis of BM involvement in case of extramedullary relapses might be different between early and late AIEM relapses.40
Directions for future research
We have shown a significant heterogeneity regarding submicroscopic BM involvement in patients with AIEM relapse and indicated the adverse prognostic significance of 10−4 or higher leukemic cells in a normal morphologic marrow. The study is retrospective and included patients only according to availability of material, and a selection bias cannot be excluded. It is therefore necessary to corroborate these results in a prospective study.
As in frontline relapse therapy, it may be important to follow the change in submicroscopic BM involvement during therapy to determine prognosis.
Measuring submicroscopic BM involvement at relapse diagnosis and its reduction during therapy of AIEM relapses will enhance the characterization of these relapses, and may redefine AIEM disease and identify those patients who are likely to suffer a subsequent relapse. The early intensification of treatment in patients with submicroscopic BM involvement might reduce the probability of subsequent relapses, and might improve the overall survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Kinderkrebsstiftung in Germany; Kompetenznetz of the Pediatric Oncology, BMBF in Germany; Programme Hospitalier de Recherche Clinique (PHRC-AOM02 003) in France; and MSM0021620813/MZO 00064203 in the Czech Republic.
We thank all those involved in the study in Paris, Prague, and Berlin for their excellent technical assistance. We are grateful to the study documentation officers for the data management of the ALL-REZ BFM trial (Berlin, Prague) and COOPRALL trial (Paris). We gratefully thank Anna Goodman for critical reading and helpful comments on the paper.
Authorship
Contribution: N.H., C.A., C.E., H.C., E.F., G.H., A.v.S., A.S., and U.S. were responsible for the concept of the study, and the coordination and the interpretation of the results: N.H., C.E., C.A., H.C., E.F., J.T., and K.S. were responsible for the laboratory assessments and analysis of the data and the overall study coordination; G.H., A.v.S., A.B., N.G., and K.S. were responsible for the treatment protocol designs; N.H., C.E., H.C., A.v.S., G.H., and K.S. prepared the paper; A.B., C.A., N.G., A.S., E.F., J.T., and U.S. provided advice; A.S. and U.S. provided samples of initial diagnosis; E.F., C.A., and N.H. collected the samples from isolated extramedullary compartment; A.B. and A.v.S. were responsible for the statistical analysis.
N.H. and C.A. contributed equally to this work.
A complete list of the participants in the International Berlin-Frankfurt-Münster study group can be found in Document S1, available on the Blood website; see the Supplemental Appendix link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cornelia Eckert, Charité Medical University, CVK, Department of Pediatric Oncology/Hematology, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: cornelia.eckert@charite.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal