Abstract
Acute graft-versus-host disease (GVHD) is a major limiting factor in allogeneic hematopoietic stem cell transplantation (HSCT), and the timing of acute GVHD may affect patient outcomes. We evaluated the incidence, risk factors, clinical manifestations, and outcomes of hyperacute GVHD, defined as that occurring within 14 days after transplantation, among 809 consecutive HSCTs at the University of Texas M. D. Anderson Cancer Center. Of 265 patients with grade II-IV acute GVHD, 27% had biopsy-proven hyperacute GVHD. Skin involvement was significantly more common (88% versus 44%) and more severe (stage III-IV, 88% versus 66%) in the hyperacute group compared with acute GVHD diagnosed after day 14. On multivariate analysis, significant risk factors for hyperacute GVHD included a mismatched related or matched unrelated donor, a myeloablative conditioning regimen, more than 5 prior chemotherapy regimens, and donor-recipient sex mismatch. Hyperacute GVHD was associated with a significantly lower response rate to first-line therapy and a higher rate of nonrelapse mortality in patients with a mismatched related or matched unrelated donor graft. In conclusion, hyperacute GVHD accounts for a substantial proportion of grade II-IV acute GVHD after HSCT. Patients at high risk or with a diagnosis of hyperacute GVHD should be included in clinical studies.
Introduction
Acute graft-versus-host disease (GVHD) is one of the major limiting factors in successful allogeneic hematopoietic stem cell transplantation (HSCT).1,2 Traditionally, acute GVHD has been defined as a syndrome occurring within the first 100 days following HSCT, with neutrophil engraftment assumed as a condition for the diagnosis. Acute GVHD defined in this way affects at least a third of the patients undergoing a matched related (Mrel) donor transplantation and a higher proportion of mismatched and unrelated donor (MUD) transplantations.1 As our transplantation practices evolve, it becomes clearer that acute GVHD is better defined as a clinical syndrome that can occur both early,3 even before engraftment, and late, beyond day 100.4-6 The timing of acute GVHD may affect the outcome of the disease, with late acute GVHD having a better outcome than “classic” acute GVHD.6 On the other side of the spectrum, early-onset, or hyperacute, GVHD, initially identified in allogeneic transplant recipients without GVHD prophylaxis,7 may be associated with a higher nonrelapse mortality rate, particularly in unrelated or mismatched transplant recipients.3 In this retrospective study, we defined acute GVHD as a clinical syndrome that can occur any time after an allogeneic infusion, independent of neutrophil engraftment, and we evaluated the risk factors for and outcomes of hyperacute GVHD
Patients, materials, and methods
Patient eligibility
We identified all consecutive patients who underwent an allogeneic HSCT as part of prospective clinical trials at the University of Texas M. D. Anderson Cancer Center between January 1998 and September 2002. The majority of the patients received transplants from HLA-compatible related or unrelated donors serologically matched for HLA-A and -B and matched for HLA-DRB1 by high-resolution molecular methods. Patients who received an umbilical cord transplant or a T cell-depleted graft were not eligible for this analysis. For patients who received multiple allogeneic transplants during the study period, only the first transplantation was considered for this analysis, and censoring was performed at the time of the second transplantation.
All patients were treated on clinical protocols, which were reviewed and approved by the M. D. Anderson Cancer Center Institutional Review Board (IRB). All patients provided written informed consent in accordance with the Declaration of Helsinki before being enrolled in the protocols. IRB approval for this retrospective chart review was obtained according to institutional guidelines. Demographic and clinical data were retrieved from the Department of Stem Cell Transplantation and Cellular Therapy electronic database, which is prospectively updated according to standardized data entry criteria.
Conditioning regimen and GVHD prophylaxis
Conditioning regimens included total body irradiation (TBI)–based myeloablative regimens, high-dose chemotherapy-based myeloablative regimens, and reduced-intensity regimens. Preparative regimens were considered myeloablative if they were expected to produce profound pancytopenia for more than 28 days without transplantation and if, after transplantation, hematopoietic recovery was completely donor derived. Reduced-intensity regimens were defined as those in which hematopoietic recovery was expected to occur within 28 days without transplantation and, after transplantation, chimerism could be documented in most patients.8
Almost all patients (98%) received tacrolimus (0.015-0.3 μg/kg/d starting on day −2, with dose adjustments to maintain blood levels of 5-15 ng/dL); methotrexate 5 mg/m2 was given on days 1, 3, 6, and 11 in bone marrow (BM) recipients and on days 1, 3, and 6 in peripheral blood (PB) recipients. Tacrolimus was continued for about 180 days in the absence of disease progression or acute GVHD. Pentostatin, steroids, or antithymocyte globulin (ATG) were added to the GVHD prophylaxis regimen as part of studies involving unrelated donor or HLA-mismatched transplants.
Engraftment and chimerism
Engraftment day was defined as the first of 3 consecutive days in which the patient had an absolute neutrophil count more than 0.5 × 109/L. Failure to engraft by day 30 was considered primary graft failure. Chimerism analysis was performed on days 30 and 100 after transplantation and every 3 months thereafter, according to standard methods.9
Assessment of GVHD
The diagnosis of acute GVHD was based on clinical signs, positive biopsy results from at least one involved organ, and exclusion of other causes of rash, diarrhea, liver function abnormalities, or other potential manifestations of acute GVHD. The staging and grading of acute GVHD were performed using the modified Glucksberg consensus criteria.10 Considering a median time to engraftment of 12 days (range, 7-37 days) in recipients of peripheral blood grafts and 13 days (range, 5-56 days) in recipients of BM grafts (overall median, 12 days), we defined hyperacute GVHD as one occurring within the first 14 days after SCT to encompass time to engraftment for both PB and BM grafts. The term “other acute GVHD” was used for cases developing after day 14, unless otherwise specified.
Treatment of acute GVHD
All patients with grade II-IV acute GVHD (except one) were started on methylprednisolone 2 mg/kg/d according to our institutional guidelines. Tacrolimus was continued at blood levels between 5 and 15 ng/dL. A total of 27 patients received one or more additional immunosuppressants, including infliximab (n = 14), daclizumab (n = 6), ATG (n = 6), and basiliximab (n = 3).
Evaluation of response to therapy for grade II-IV acute GVHD
Responses were assessed for each organ involved. Complete responses (CRs) and partial responses (PRs) were assessed 14 days after the initiation of therapy. A CR was defined as the resolution of all manifestations of acute GVHD. A PR was a decrease in organ stage by 1. Progressive disease (PD) was defined as an increase in organ stage by 1 and was evaluated 48 hours (gastrointestinal [GI] and liver) or 72 hours (skin) after the initiation of corticosteroids. Patients were considered nonresponders (NRs) in the absence of CR, PR, or PD 7 days after the initiation of corticosteroids for skin GVHD or 72 hours after its initiation for GI and liver GVHD. All responses had a minimal duration of 14 days. Overall response integrated the responses at all sites (skin, GI, and liver). An overall CR was defined as the resolution of GVHD in all evaluable organs. PR was any improvement in at least one evaluable organ without deterioration of others; PD was deterioration in at least one evaluable organ without improvement of the others. NR was the absence of any change, or any situation other than CR, PR, or PD.
Statistical methods
Analysis was performed on the basis of outcomes documented by July 2004. Patients who had a primary graft failure were not eligible for inclusion in the study. Patients who experienced secondary graft failure after neutrophil engraftment were censored at the time of the secondary graft failure. Distribution of organ involvement was compared among patients with grade II-IV hyperacute and other acute GVHD using the χ2 and Fisher exact tests. Prognostic factors for the occurrence of grade II-IV hyperacute GVHD and other grade II-IV acute GVHD and nonrelapse mortality among patients diagnosed with grade II-IV acute GVHD were evaluated using the Cox regression analysis.11 Observation time was split at the day 14 cut-off point to evaluate risk factors for hyperacute and other acute GVHD. Consequently, evaluation of acute GVHD after day 14 was performed as a landmark analysis starting at day 15 after HSCT. Landmark analysis was also used to compare rates of nonrelapse mortality after the diagnosis of grade II-IV acute GVHD. Predictors of response to first-line therapy among patients diagnosed with grade II-IV acute GVHD were evaluated by logistic regression analysis. The cumulative incidence of nonrelapse mortality was estimated considering death due to persistence or recurrence of underlying malignancy as competing risk.12 Factors significant at the 0.1 level on univariate analysis were considered for multivariate analyses using backward elimination. Two-sided P values less than .05 were considered significant. Analysis was performed using STATA 7.0 (Stata, College Station, TX).
Results
Patient characteristics
A total of 809 consecutive patients met the inclusion criteria. Of these, 265 (33%) developed grade II-IV acute GVHD. Patient characteristics are listed in Table 1. The median age was 47 years (range, 19-70 years). Ninety-six percent of patients received an allogeneic stem cell transplant for hematologic malignancies. Most patients received an HLA-matched sibling donor transplant (n = 488, 60%), and the remainder received HLA-MUD grafts (n = 253, 31%) and HLA-mismatched related (MMrel) donor grafts (n = 68, 8%). The cell source was PB in 53% of patients and BM in 47%. The main source of stem cells was peripheral blood from Mrel donors (82%) and BM from MUDs (98%) and MMrel donors (72%). Donor-recipient sex was mismatched (female-to-male, respectively) in 24% of cases. A third of the patients were in remission of their malignancy at the time of transplantation. The preparative regimen was myeloablative for 477 patients (59%), and 102 (21%) of these patients received TBI-based conditioning; 332 (41%) patients had reduced-intensity conditioning regimens.
Incidence of acute GVHD
Twenty-eight percent (n = 73) of the 265 cases of grade II-IV acute GVHD occurred by day 14 after HSCT and are described as hyperacute GVHD. The majority of hyperacute GVHD cases (58 of 73, 79%) were diagnosed before neutrophil engraftment, and 87% of these cases (49 of 56) were diagnosed within 7 days prior to engraftment. Two of the 58 patients who were diagnosed with acute GVHD before engraftment died without neutrophil recovery. In contrast, 5% (10 of 192) of cases of acute GVHD diagnosed after day 14 occurred before neutrophil engraftment. Twelve cases of grade II-IV acute GVHD occurred after disease progression and withdrawal of immunosuppression (3 of 73 hyperacute cases and 9 of 192 other acute GVHD cases). Results of subsequent analyses were unchanged when progression of the underlying malignancy was considered as a competing risk and observation time was censored at the time of disease progression before the occurrence of these 12 cases of acute GVHD (data not shown).
Severity and organ distribution of acute GVHD are summarized in Table 2. Severe, grade III-IV acute GVHD was seen in 29 of 73 (40%) patients with hyperacute GVHD and in 84 of 192 (44%) of those with other acute GVHD (P = .5). The frequency and severity of liver and GI involvement were comparable in both groups, whereas severe skin involvement was more common among patients with hyperacute GVHD (stage III-IV skin GVHD, 71% versus 50%, P = .001).
Risk factors for the development of grade II-IV hyperacute GVHD
Risk factors for hyperacute GVHD are summarized in Table 3. On univariate analysis, significant factors included a MUD (HR = 2.2, P = .002) or MMrel donor (HR = 4.1, P < .001); a myeloablative conditioning regimen, irrespective of whether it included TBI (HR = 3.4, P = .002) or not (HR = 3.3, P < .001); and more than 5 lines of chemotherapy prior to transplantation (HR = 2.1, P = .04). There was a trend toward higher rates of hyperacute GVHD in female-to-male transplants (HR = 1.5, P = .09). Age, sex, disease status, prior autologous transplantation, PB (evaluable only among recipients of Mrel donor grafts), and ATG in the conditioning regimen of MUD transplants (data not shown) did not affect the rate of hyperacute GVHD.
We evaluated the independent effects of histocompatibility, conditioning regimen, number of lines of prior chemotherapy, and sex mismatch in a multivariate model. The results were consistent with those obtained on univariate analysis, with the exception of female-to-male transplants, which became statistically significant after adjustment for confounding variables (HR = 1.7, P = .03).
The impact of T-cell subset cell dose on the incidence of hyperacute GVHD was evaluable only in patients who received a PB graft from a Mrel donor and had complete cell dose data. A quartile analysis showed a trend toward a lower incidence of hyperacute GVHD when either the CD3+ or CD4+ cell doses were within the first quartile (HR
Risk factors for the development of other grade II-IV acute GVHD
Histocompatibility was the only significant predictor of both hyperacute and other acute GVHD. Myeloablative regimens showed a trend toward a higher incidence of other acute GVHD (P = .05) when TBI- and non–TBI-based regimens were combined. Prior autologous transplantation had a protective effect for other acute GVHD. However, this effect was only present in patients who received reduced-intensity conditioning (HR = 0.4; P = .008) but not in those who received high-dose conditioning (HR = 1.1; P = .8) regimens. On multivariate analysis adjusting for donor type, prior autologous transplantation, conditioning regimen, and the interaction effect between conditioning regimen and prior autologous transplantation, only donor type (HR for MUD = 1.6, P = .004; HR for MMrel donor = 1.9, P = .011) and prior autologous transplantation among recipients of reduced-intensity conditioning (HR = 0.4; P = .01) remained significant predictors for other acute GVHD (Table 3).
Response to therapy in patients with grade II-IV acute GVHD
Response to first-line therapy was assessed among 259 of the 265 patients who developed grade II-IV GVHD. Six patients diagnosed with other acute GVHD were excluded from the response evaluation because their responses were not documented or were unknown. Of the 259 patients, 109 (42%) achieved a CR, and 7 (3%) had a PR, resulting in an overall response rate (CR or PR) of 45%. The overall response rate was significantly lower in the hyperacute GVHD group compared with the other acute GVHD group (34% versus 49%; P = .03). Forty-seven (64%) of the 73 patients with hyperacute GVHD and 100 (52%) of the 192 patients with other acute GVHD required second-line therapy (P = .07). This included 4 and 9 patients in both groups, respectively, who had responded to first-line therapy.
Predictors of response to first-line therapy in patients with grade II-IV acute GVHD
Type of donor and hyperacute GVHD were the only significant predictors of overall response to first-line GVHD therapy (Table 4). Because of the strong association between these 2 factors (based on the risk factor analysis described in “Risk factors for the development of grades II-IV hyperacute GVHD), we evaluated their independent effects by grouping patients according to donor histocompatibility and time of onset of acute GVHD. This analysis showed that patients with a MUD or MMrel donor graft who developed hyperacute GVHD had a significantly lower response rate to first-line therapy (OR = 0.3; P = .002). Patients who had active disease at transplantation showed a trend toward a better response rate, yet this effect was not significant after adjusting for donor type and hyperacute GVHD in multivariate analysis. Patient age, patient sex, donor-recipient sex mismatch, type of conditioning regimen, and cancer diagnosis had no significant impact on the response rate.
Survival and incidence of chronic GVHD among patients with grade II-IV acute GVHD
Fifty-seven patients (21%) diagnosed with grade II-IV acute GVHD were alive at the time of this analysis, with a median follow-up of 42 months (range, 5-73 months) since diagnosis of GVHD. The actuarial survival rate since the diagnosis of grade II-IV GVHD was 21% (95% CI, 16%-26%) at the median follow-up time. Overall survival was comparable for patients diagnosed with hyperacute (20%; 95% CI, 14%-27%) and other acute GVHD (22%; 95% CI, 14%-33%; P = .9). Causes of death are detailed in Table 5. There was no significant difference in nonrelapse mortality for patients with hyperacute (cumulative incidence 51%) and other acute GVHD (cumulative incidence 50%; P = .6). Similarly, there was no significant difference in the incidence of chronic GVHD between the 2 groups (cumulative incidence, 54% and 59%, respectively; P = .2).
Prognostic factors for nonrelapse mortality among patients with grade II-IV acute GVHD
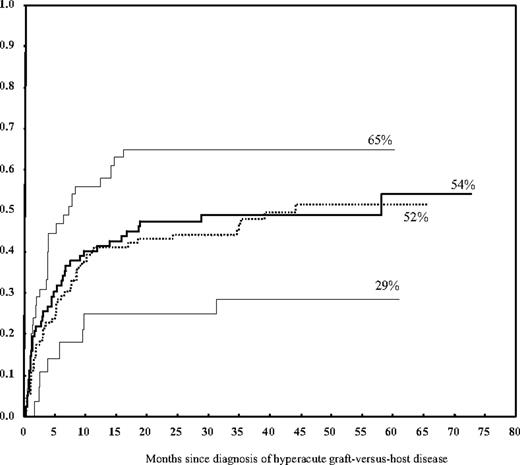
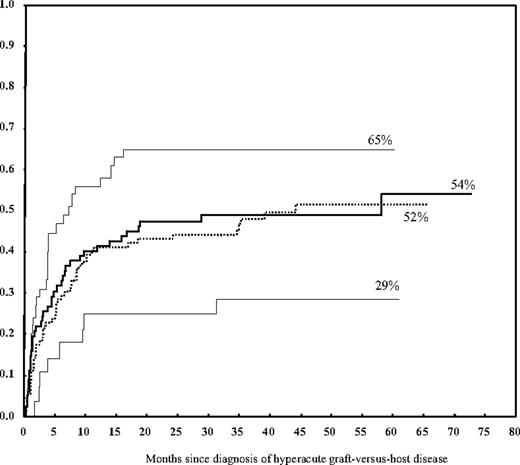
Failure to respond to first-line therapy for treatment of grade II-IV acute GVHD (HR = 1.96; P < .001) and TBI-based conditioning (HR = 1.7; P = .02) were the only 2 statistically significant predictors on multivariate analysis of nonrelapse mortality at 48 months after the diagnosis of acute GVHD. The cumulative incidence of nonrelapse mortality was 60% in nonresponders to first-line therapy versus 39% in responders and 67% in patients who received a TBI-based conditioning regimen versus 47% in those whose conditioning regimen was not TBI-based. Hyperacute GVHD did not have a significant impact on nonrelapse mortality overall but was associated with a higher mortality rate when it occurred among patients with MMrel or MUD transplants (cumulative incidence 65%). This rate was significantly higher when compared with the rate in MMrel or MUD transplants without hyperacute GVHD or Mrel transplants (cumulative incidence 47%; HR = 1.7, P = .01; Table 6; Figure 1), but it did not reach statistical significance on multivariate analysis. This increased risk persisted when evaluated among patients with grade II and III-IV separately, yet it was more pronounced in the latter group (Table 7). Similarly, the overall nonrelapse mortality was higher for grade III-IV acute GVHD (62%) than for grade II (35%; HR = 2.8, P < .001). Our data showed that patient sex, donor-patient sex mismatch, patient age, cancer diagnosis, disease status at transplantation, number of prior chemotherapy regimens, cell source, and prior autologous transplantation had no significant impact on the rate of nonrelapse mortality.
Cumulative incidence of nonrelapse mortality according to donor type and the occurrence of grade II-IV hyperacute GVHD. Hyperacute GVHD was associated with a higher rate of nonrelapse mortality when it occurred in patients who received a MMrel or a MUD graft (top line) but not in patients who received a Mrel graft (lower line). In the absence of hyperacute GVHD, the rate of nonrelapse mortality was similar for related and unrelated grafts (2 middle lines).
Cumulative incidence of nonrelapse mortality according to donor type and the occurrence of grade II-IV hyperacute GVHD. Hyperacute GVHD was associated with a higher rate of nonrelapse mortality when it occurred in patients who received a MMrel or a MUD graft (top line) but not in patients who received a Mrel graft (lower line). In the absence of hyperacute GVHD, the rate of nonrelapse mortality was similar for related and unrelated grafts (2 middle lines).
Discussion
To date, this is the largest study evaluating the incidence of hyperacute GVHD using standard criteria for the diagnosis of acute GVHD. The large sample size and the heterogeneity of the study population allowed us to evaluate risk factors for the occurrence of hyperacute GVHD, which is a relatively rare event. Overall, hyperacute GVHD accounted for 27% of all cases of grade II-IV acute GVHD diagnosed in our series. A comparison between the incidence of hyperacute GVHD in the current study and in the few published reports is not possible because the definitions vary substantially in terms of both time of onset and clinical manifestations.7,13-16 In our study we used the same diagnostic criteria for hyperacute GVHD and acute GVHD occurring after HSCT.
Although GVHD is classically related to reactivity of donor T cells against host tissues, the severity of acute GVHD is affected by the toxicity of the preparative regimen,4,5 the breakdown of mucosal barriers, and inflammatory cytokines, in addition to the immune graft-versus-host reaction. The effects of inflammatory cytokines produced early after the preparative regimen are likely to contribute to the manifestations of hyperacute GVHD. Our data support the hypothesis that preparative regimen toxicity and associated cytokine release would have the most impact on the early manifestations of acute GVHD showing that conditioning regimens and multiple chemotherapy regimens prior to transplantation were significant risk factors for hyperacute GVHD but not for other acute GVHD. The frequency of hyperacute GVHD is expected to vary according to the intensity of the conditioning regimen, thus within and across transplantation centers. On the other hand, HLA disparity was a significant risk factor irrespective of the timing of GVHD onset. The impact of HLA disparity on the incidence of early-onset acute GVHD has been reported by Kim et al13 and by Powles and colleagues.14,15 Powles et al described the most severe form of hyperacute GVHD, which occurred following haploidentical transplantation and consisted of an abrupt and often fatal clinical syndrome of fever, rash, massive noncardiogenic pulmonary edema, often with renal failure, and seizures. In the study of Kim et al,13 an alternative donor source was the only significant predictor of the incidence of hyperacute GVHD, which was defined as unexplained fever in addition to skin rash, hepatic dysfunction, or diarrhea, all occurring before neutrophil engraftment. The authors reported that conditioning regimen, stem cell source, mononuclear cell dose, or CD34+ cell dose did not affect the incidence of hyperacute GVHD among 90 patients, 71 of whom received a graft from a Mrel donor and 19 from an alternative donor. Donor-recipient matching based on high-resolution HLA typing is likely to lower the incidence of acute GVHD in this context.
In the current study, 2 risk factors that have been traditionally associated with acute GVHD—age (even when evaluated per decade, data not shown) and the infused cell dose of CD3+, CD4+, CD8+, or CD34+ with PBSC grafts—did not significantly increase the incidence of GVHD. Our results show that a prior autologous transplantation had a significant protective effect for the occurrence of acute GVHD after day 14 but not for hyperacute GVHD. Most of these patients received an allogeneic transplant with fludarabine-based reduced-intensity conditioning after the failure of a prior autologous transplant for the treatment of non-Hodgkin lymphoma (n = 35), multiple myeloma (n = 18), or Hodgkin disease (n = 12). The significant protective effect of a prior autologous transplant was seen only among patients who received reduced-intensity conditioning and persisted when we restricted the comparison to patients who received a Mrel or to each one of the diagnoses just listed (data not shown), suggesting that the impact of a prior autologous transplant among patients who received reduced-intensity conditioning is independent of donor type or diagnosis. This lower incidence of grade II-IV acute GVHD had been noted recently by our group in a subset of the patients with non-Hodgkin lymphoma17 and multiple myeloma18 included in our current analysis. It is in contrast to prior reports from our institution19,20 and to reports in the literature of reduced-intensity allogeneic transplantation as salvage therapy after receiving an autologous transplant.21-29 Differences in patient characteristics, intensity of the conditioning regimen, and the use of donor lymphocyte infusions could account for this discrepancy. Although the significance of the protective effect is unclear, one could hypothesize that a prior autologous transplant could induce immunologic changes, leading to reduced allogeneic reactivity in recipients of a reduced-intensity conditioning regimen.
In addition to having a negative impact on response, hyperacute GVHD was associated with a significantly higher rate of nonrelapse mortality on univariate analysis in patients who received MUD or MMrel transplants. This effect could be mainly attributed to resistance to first-line therapy in this group of patients, which itself is a strong independent predictor of nonrelapse mortality and would explain the lack of significant effect for hyperacute GVHD after adjusting for response to first-line therapy.
The heterogeneity in diagnosis and stage of malignancy of this study population limits our ability to assess the graft-versus-malignancy effect. On univariate analysis, patients diagnosed with grade II-IV hyperacute GVHD had a lower rate of disease progression compared with patients with other grade II-IV GVHD, but this trend did not reach statistical significance (HR = 0.6; 95% CI, 0.3-1.1). Comprehensive evaluation of the impact of GVHD on disease progression requires adjustment for confounding variables, which was beyond the scope of this study.
In conclusion, our data show that acute GVHD comprises a clinical spectrum of GVHD that can occur early after transplantation, even before neutrophil engraftment. The timing of acute GVHD within this clinical spectrum has a definite impact on the outcomes of patients. Patients at risk for hyperacute GVHD should be considered for GVHD prophylaxis within a clinical trial whenever possible.
Authorship
Contribution: R.M.S. designed and performed data analysis and wrote the paper; M.d.L., S.G., and R.E.C. helped to write the paper; B.A. and I.F.K. contributed analytical tools; C.H. and M.H.Q. provided vital comments; S.G. performed data management; J.N. and Y.S. provided patient care in the GVHD clinic; J.D.J. was study coordinator; and D.R.C. designed and performed research and wrote paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel R. Couriel, Department of Stem Cell Transplantation and Cellular Therapy, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0423, Houston, TX 77030; e-mail: dcouriel@mdanderson.org.
Presented at the 46th annual meeting of the American Society of Hematology, San Diego, CA, December 7, 2004.30
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge all research nurses, data managers, nurses, physicians, and physician assistants for their dedication and hard work in the day-to-day care of our patients.