Abstract
Gain-of-function mutations of the receptor tyrosine kinase KIT play a key role in the pathogenesis of systemic mastocytosis (SM), gastrointestinal stromal tumors (GISTs), and some cases of acute myeloid leukemia (AML). Whereas KIT juxtamembrane domain mutations seen in most patients with GIST are highly sensitive to imatinib, the kinase activation loop mutant D816V, frequently encountered in SM, hampers the binding ability of imatinib. We investigated the inhibitory activity of the novel tyrosine kinase inhibitor EXEL-0862 against 2 subclones of human mast cell line-1 (HMC-1)—HMC-1.1, harboring the juxtamembrane domain mutation V560G, and HMC-1.2, carrying V560G and the activation loop mutation D816V, found in more than 80% of patients with SM. EXEL-0862 inhibited the phosphorylation of KIT in a dose-dependent manner and decreased cell proliferation in both mast cell lines with higher activity against HMC-1.2 cells. The phosphorylation of KIT-dependent signal transducer and activator of transcription-3 (STAT3) and STAT5 was abrogated upon exposure to nanomolar concentrations of EXEL-0862. In addition, EXEL-0862 induced a time- and dose-dependent proapoptotic effect in both mast cell lines and caused a significant reduction in mast-cell content in bone marrow samples from patients with SM harboring D816V and from those without the D816V mutation. We conclude that EXEL-0862 is active against KIT activation loop mutants and is a promising candidate for the treatment of patients with SM and other KIT-driven malignancies harboring active site mutations.
Introduction
Systemic mastocytosis (SM) is characterized by clonal proliferation of mast cells in the bone marrow, spleen, and other extracutaneous organs.1 Indolent and aggressive disease variants have been described.2 Clinically, SM can manifest with mediator-related symptoms or organomegaly. In patients with aggressive disease variants, signs of organ dysfunction caused by mast-cell infiltration are present. Patients with indolent SM (ISM) can be treated successfully with antimediator drugs. By contrast, patients with aggressive SM (ASM) or mast-cell leukemia (MCL) are candidates for cytoreductive or targeted drugs. Current therapy for ASM and MCL includes interferon-α and cladribine, but their efficacy is limited and the prognosis for patients remains poor.3 Nearly all patients with SM harbor the activating oncogenic mutation KIT D816V, which involves the substitution of an aspartic residue at codon 816 of the activation loop with a valine residue. This mutation promotes receptor autophosphorylation without the requirement of stem-cell factor (SCF) stimulation.4-7
KIT is a 145-kDa transmembrane receptor tyrosine kinase of the type III subgroup characterized by 5 extracellular immunoglobulinlike domains and a split tyrosine kinase domain.8 KIT-dependent cell types include mast cells, hematopoietic stem cells, germ cells, melanocytes, and interstitial cells of Cajal, among others.8-10 Upon binding of SCF to the extracellular immunoglobulinlike domains, KIT undergoes homodimerization and autophosphorylation at the Y568 and Y570 tyrosine residues of the juxtamembrane domain.11 This leads to the phosphorylation and activation of multiple signaling pathways such as Janus kinase/signal transducer and activator of transcription (Jak-STAT), Src kinases, mitogen-activated protein (MAP) kinases, and phosphatidylinositol-3 (PI3) kinase.11 Gain-of-function point mutations in the KIT kinase domain result in ligand-independent constitutive activation of KIT signaling, which leads to uncontrolled cell proliferation and resistance to apoptosis.12 Activation of the KIT tyrosine kinase by somatic mutation has been documented in a variety of human malignancies, including SM, acute myeloid leukemia (AML), and gastrointestinal stromal tumors (GISTs).9
Several small molecule tyrosine kinase inhibitors such as imatinib and SU5614 have shown activity against the tyrosine kinase activity of wild-type and some KIT mutants.13-15 However, the inhibitory effect of these agents depends greatly upon the nature of the KIT mutant isoform. For instance, imatinib has been associated with sustained objective responses in more than 50% of patients with metastatic GIST bearing the juxtamembrane KIT mutation V560G.15,16 However, mutations mapping to the KIT kinase domain17 render imatinib completely ineffective.18 The latter are best exemplified by the D816V mutation, which involves the substitution of aspartate to valine in codon 816 in the activation loop17 lying at the entrance to the KIT enzymatic pocket, thus interfering with imatinib binding.18 Neither imatinib18 nor the chemically related tyrosine kinase inhibitor AMN10713 has significant in vitro activity against the D816V KIT mutants. Thus, drugs specifically targeting the D816V KIT mutant hold great promise for the treatment of neoplastic disorders harboring this mutation.
We report herein that the novel tyrosine kinase inhibitor EXEL-0862 kills mast cell lines harboring the juxtamembrane V560G KIT mutant and, significantly more, those bearing the imatinib-resistant kinase domain D816V KIT mutation. It also kills bone marrow mast cells in patients with SM. Furthermore, EXEL-0862 exerts a strong inhibitory effect on the KIT-dependent downstream signaling pathways STAT3 and STAT5 and induces apoptosis in human mast cells harboring the D816V KIT mutant isoform.
Materials and methods
Reagents and antibodies
EXEL-0862 (WO2004050681 A2) was obtained from Exelixis (South San Francisco, CA) and was stored as a 10-mM stock solution in dimethyl sulfoxide (DMSO). Drug stock dilutions were stored at −20°C. Working dilutions were prepared in 10% media with freshly thawed aliquots for immediate use in experiments. Recombinant human SCF was purchased from Peprotech (Rocky Hill, NJ). Antibodies and their sources were as follows: antibodies against Mcl-1 (S-19), antiphosphotyrosine (py99), and protein-A/G agarose, were from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) and anti-CD117 conjugated with phycoerythrin were from Becton-Dickson Biosciences PharMingen (San Diego, CA); rabbit polyclonal antibodies against caspase-3, caspase-9, XIAP, and Bax were from Cell Signaling Technology (Beverly, MA); mouse monoclonal antibody specific against phosphotyrosine 705 of Stat3 (clone 9E12), rabbit polyclonal anti-Stat3, mouse monoclonal anti–phospho-Stat5A/B (Y694/Y699; clone 8-5-2), and rabbit polyclonal anti-Stat5A were from Upstate Technology (Lake Placid, NY); mouse anti–human-KIT (CD117) monoclonal antibody was from R&D Systems (Minneapolis, MN); mouse monoclonal antibody against actin was from Sigma (St Louis, MO); and anti–mouse immunoglobulin G and anti–rabbit immunoglobulin G horseradish peroxidase–conjugated antibodies were from Amersham Biosciences (Arlington Heights, IL).
Kinase IC50 determinations
Recombinant human kinase domains from KIT, VEGFR2, FGFR1, FLT3, PDGFRβ, and IRK were obtained from ProQuinase (Freiberg, Germany). Recombinant human KIT kinase domain containing the D816V mutation was purified from baculovirus-infected Sf9 cells. Kinase activity was monitored by measuring ATP consumption using luciferase as a readout (VEGFR2, FLT3, IRK, PDGFRβ) or by detecting peptide phosphorylation using Alphascreen (Perkin Elmer, Wellesley, MA) (KIT, KIT D816V, FGFR1). All IC50 determinations were performed at an ATP concentration equal to the Michaelis-Menten dissociation constant (Km) for each kinase.
Cell culture
Two subclones of the human mast cell line-1 (HMC-1) were used: HMC-1.1, which harbors the mutation V560G in the juxtamembrane domain of KIT, and HMC-1.2, which is a subclone of the HMC-1.1 cell line with the SM-associated D816V kinase domain mutation on the same allele as the original V560G mutation.19 Both HMC-1 subclones were kindly provided by Dr Joseph Butterfield (Mayo Clinic, Rochester, MN) and were maintained in Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT) and 1.2 mM α-thioglycerol (Sigma).
Cell proliferation inhibition assay
The MTS assay (CellTiter 96Aqueous One Solution reagent; Promega, Madison, WI) was used to evaluate the effect of EXEL-0862 on mast-cell viability. The assay was performed according to the manufacturer's recommendations. Briefly, cells were seeded in triplicate in 96-well microtiter plates (Falcon, Franklin Lakes, NJ), incubated in the presence of different EXEL-0862 concentrations for 72 hours, and proliferation was measured as a percentage of the proliferation of untreated cells. Four hours before culture termination, 20 μL MTS solution was added to the culture. During the incubation period, the MTS solution was reduced only by viable cells into an insoluble colored formazan. Absorbance or optical density was read on a 96-well plate reader at a single wavelength of 595 nm. Drug concentrations resulting in 20%, 50%, and 80% inhibition of cell proliferation (IC) were determined.
Cell-cycle analysis by flow cytometry
After drug treatment, cells were collected, washed in Ca2+-free phosphate-buffered saline (PBS), and fixed overnight in 70% cold ethanol at −20°C. The cells were then washed twice in cold PBS and labeled with propidium iodide (PI) for 1 hour in the dark. Cell-cycle distribution, including the percentage of cells in sub-G1 phase, was determined using a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer equipped with CellQuest (BD Biosciences) software.
Measurement of mitochondrial transmembrane potential (ΔΨm)
After treatment with EXEL-0862, changes in inner mitochondrial transmembrane potential in HMC-1.1 and HMC-1.2 cells were examined by flow cytometry following incubation with submicromolar concentrations of MitoTracker (Molecular Probes, Eugene, OR) probes. Briefly, cells were stained with 2 probes: MitoTracker Red (chloromethyl-X-rosamine [CMXRos]; Molecular Probes, Eugene, OR) and MitoTracker Green FM (MTGreen; Molecular Probes). Cells were washed in Ca2+-free PBS, stained with MitoTracker dyes, and incubated at 37°C for 1 hour in the dark. MitoTracker probes passively diffuse across the plasma membrane and accumulate in mitochondria. CMXRos is taken up by mitochondria as a result of the ΔΨm and reacts with thiol residues to form covalent thiol ester bonds. MTGreen FM preferentially accumulates in mitochondria regardless of the mitochondrial membrane potential, which makes it a useful tool for determining mitochondrial mass. MTGreen FM is a mitochondrion-selective probe that contains a thiol-reactive chloromethyl moiety and becomes fluorescent in the lipid environment of mitochondria. Samples were analyzed in a flow cytometer and analyzed using CellQuest (BD Biosciences) software.
Apoptosis assay
To determine the proportion of apoptotic HMC-1.1 and HMC-1.2 cells after incubation in the presence of EXEL-0862, cells were pelleted, washed in Ca2+-free PBS, and resuspended in 100 μL annexin V binding buffer (10 mM 4-[2-hydroxyethyl]-1-piperazineethane-sulfonic acid, [pH 7.4]; 0.15 M NaCl; 5 mM KC1; 1 mM MgCl2; 1.8 mM CaCl2) before the fluorogenic substrate annexin V–fluorescein isothiocyanate (Sigma) was added. Next, cells were incubated for 15 minutes at room temperature in the dark. After incubation, cells were washed in 2 mL Ca2+-free PBS and resuspended in 0.5 mL binding buffer. PI was added to permit identification and exclusion of cells that had lost membrane integrity during analysis. Binding of annexin V to apoptotic cells was determined with a flow cytometer, and the resultant data were analyzed with CellQuest (BD Biosciences) software.
Preparation of cell lysates, SDS-PAGE, and immunoblotting
Preparation of total cell lysates.
Control cells and cells treated with EXEL-0862 were rinsed with PBS and then lysed with RIPA buffer (1 α PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with freshly added 10 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 1 × Roche complete Mini protease inhibitor cocktail (Roche, Indianapolis, IN). The DNA in the lysates was sheared by rapidly passing the lysate 10 times through a 23-gauge needle or by sonication with eight 1-second bursts at medium power.
Cytosolic fraction extraction.
Control cells and cells treated with EXEL-0862 were washed twice with ice-cold PBS. Cell pellets were mildly resuspended with digitonin extraction buffer (10 mM PIPES [pH 6.8], 0.015% [wt/vol] digitonin, 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 5 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride supplemented with freshly added phosphatase inhibitors and protease inhibitors, as described in the preceding paragraph. After incubation on ice for 10 minutes, samples were centrifuged at 20 000g (14 000 rpm) for 10 minutes. Supernatants containing cytosolic protein were transferred to a clean tube. Protein concentration was determined in the final supernatant using the Bio-Rad protein assay dye reagent, following the manufacturer's instructions (Bio-Rad, Hercules, CA). Samples were then stored in aliquots at −80°C.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were described previously.20
Immunoprecipitation
Immunoprecipitation was carried out according to the methods described previously.21 In brief, total cell lysates obtained from control or treated cells were mixed with 3 μg mouse anti–human KIT (CD117) monoclonal antibody overnight at 4°C, followed by the addition of 50 μL protein-A/G agarose slurry, and incubated for 3 hours. The immunoprecipitated complex was washed 3 times and then subjected to Western blotting.
Immunofluorescence staining
HMC-1.2 cells were first starved in serum-free medium for 24 hours and then stimulated with SCF (100 ng/mL, final concentration) in 20% serum for 1 hour. EXEL-0862 (0.1 or 1.0 μM) was added to the culture 20 minutes before the addition of SCF. Next, cells were harvested, washed twice with PBS, and cytospun onto glass slides. After fixation with 3% paraformaldehyde for 20 minutes, cells were permeabilized with 1% Triton X-100 and 0.5% NP-40 for 10 minutes. Immunofluorescence staining was performed as described previously.20 Cells were mounted onto slides with Pro-Long Gold Antifade reagent with DAPI (P36935; Molecular Probes).
Patient samples and evaluation of mast cells
Bone marrow cells were obtained by aspiration from 6 patients undergoing evaluation for SM. Four patients (3 men, 1 woman; age range, 50-70 years) were diagnosed with ISM according to the WHO classification, and 2 others had no diagnostic evidence of SM. Three patients with SM carried the D816V KIT mutation, and 1 carried wild-type KIT. No end-organ involvement was apparent in any of these patients. All had 10% or less bone marrow cellularity involvement by neoplastic mast cells, and mast cells coexpressed CD117, CD2, and CD25 in all. The research protocol was approved by the M. D. Anderson Cancer Center institutional review board (Houston, TX). Patients provided written informed consent in accordance with the Declaration of Helsinki.
Bone marrow mononuclear cells were separated by Histopaque (density 1.077) gradient centrifugation. Contaminating red cells were lysed in 0.8% ammonium chloride solution (StemCell Technologies, Vancouver, BC, Canada) for 10 minutes. The presence of the D816V KIT point mutation in bone marrow samples was detected by reverse transcriptase–polymerase chain reaction and restriction fragment length polymorphism analysis, as described previously,21,22 and was confirmed by direct sequencing in all patients. Mononuclear cells were cultured in Stem-Pro serum-free medium (Invitrogen) supplemented with recombinant human SCF (100 ng/mL) for 7 days in the presence or absence of EXEL-0862 and then stained with anti–CD117-phycoerythrin (PE) for 30 minutes and washed. Detection of mast cells was performed by detection of surface CD117 expression by flow cytometry, as previously described.21
Statistical analysis
GraphPad Prism software (GraphPad Software, San Diego, CA) was used to conduct the statistical analysis. P values of less than .05 were considered statistically significant.
Results
EXEL-0862 inhibits cell proliferation and KIT phosphorylation at nanomolar concentrations
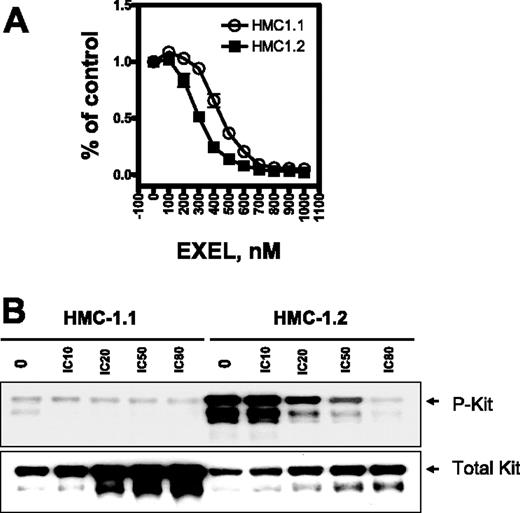
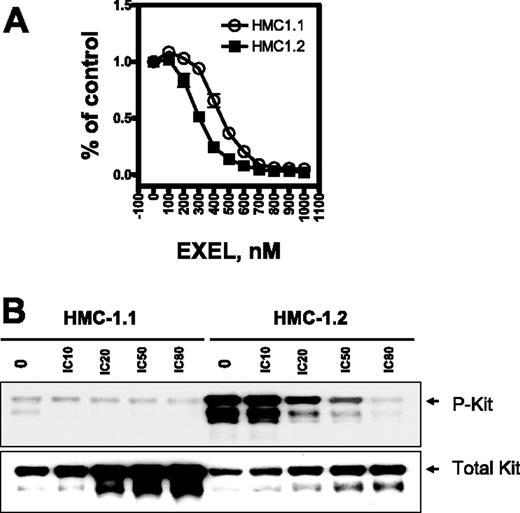
Mutations within the activation loop of the KIT kinase lead to ligand-independent activation and increased catalytic activity of KIT.8-10 EXEL-0862 is a novel kinase inhibitor optimized for activity against fibroblast growth factor receptors (FGFRs), vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and FLT3 (Table 1)EXEL-0862 is also a potent inhibitor of wild-type KIT (IC50, 8.5 nM) and retains significant activity against KIT bearing the D816V mutation (IC50, 42 nM). We examined the effect of EXEL-0862 on KIT phosphorylation in HMC-1.2 cells harboring the KIT loop activation mutant D816V, which confers resistance to imatinib. The inability of imatinib to bind this mutant isoform was attributed to allosteric conflict between the imatinib structure and the open conformation of the KIT activation loop. HMC-1.1 cells harboring the juxtamembrane KIT V560G mutant are sensitive to imatinib and were used as a control.
We first investigated HMC-1.1 and HMC-1.2 cell viability on exposure to EXEL-0862 by the MTS assay. Three independent samples were measured for each data point. The viability of HMC-1.1 and HMC-1.2 cells was markedly reduced after 72 hours of exposure to increasing concentrations of EXEL-0862 up to 1 μM (Figure 1A), indicating that EXEL-0862 inhibited cell viability at nanomolar concentrations. The IC50 for EXEL-0862 was significantly higher in HMC-1.1 cells (approximately 510 nM) than in HMC-1.2 cells (approximately 350 nM) (F test; P < .001; Table 2)Interestingly, the IC50 of imatinib for cellular proliferation of HMC-1.2 cells (greater than 10 000 nM)18 was approximately 30-fold higher than that of EXEL-0862. Overall, our results indicated that EXEL-0862 might be more active against D816V HMC-1.2 cells than against HMC-1.1 cells.
EXEL-0862 inhibits proliferation of human mast cells bearing D816V mutation and KIT phosphorylation. (A) Dose-response curves of HMC-1.1 and HMC-1.2 cells treated with EXEL-0862. Cells were exposed in vitro for 72 hours to increasing concentrations of EXEL-0862, and the MTS assay was used to evaluate growth inhibition. Graphs show data from a representative experiment performed in triplicate; error bars represent standard deviation. (B) KIT phosphorylation was inhibited by EXEL-0862 in a dose-dependent manner in HMC-1.1 and HMC-1.2 cells when exposed at the indicated concentrations for 3 hours. EXEL-0862 ICs were based on the MTS assay, as shown in panel A. Cell lysates were immunoprecipitated with antibody against KIT. Western blot analysis was performed with antiphosphotyrosine antibody.
EXEL-0862 inhibits proliferation of human mast cells bearing D816V mutation and KIT phosphorylation. (A) Dose-response curves of HMC-1.1 and HMC-1.2 cells treated with EXEL-0862. Cells were exposed in vitro for 72 hours to increasing concentrations of EXEL-0862, and the MTS assay was used to evaluate growth inhibition. Graphs show data from a representative experiment performed in triplicate; error bars represent standard deviation. (B) KIT phosphorylation was inhibited by EXEL-0862 in a dose-dependent manner in HMC-1.1 and HMC-1.2 cells when exposed at the indicated concentrations for 3 hours. EXEL-0862 ICs were based on the MTS assay, as shown in panel A. Cell lysates were immunoprecipitated with antibody against KIT. Western blot analysis was performed with antiphosphotyrosine antibody.
After treatment of HMC-1.2 cells with escalating concentrations of EXEL-0862, cell lysates were immunoprecipitated with mouse anti–human KIT monoclonal antibody, and KIT phosphorylation was detected with specific antibodies against phosphotyrosine. The level of phosphorylated KIT was significantly higher in untreated HMC-1.2 cells than in untreated HMC-1.1 cells (Figure 1C), which is consistent with previous reports demonstrating that the mutation D816V elicits KIT activation.12 Treatment with EXEL-0862 decreased the phosphorylation of KIT in a concentration-dependent manner, indicating that EXEL-0862 substantially inhibited the activation of KIT in D816V-expressing cells. When the membrane was stripped and reprobed with anti-KIT antibody, we observed that the amount of total KIT was unchanged, suggesting that EXEL-0862 abolished KIT phosphorylation without altering KIT expression.
EXEL-0862 inhibits the activation of STAT3 and STAT5
Because EXEL-0862 inhibited KIT phosphorylation, we reasoned that downstream signal transduction events critical in promoting KIT-mediated cell survival, such as STAT3 and STAT5 activation,23,24 might also be inhibited by EXEL-0862. HMC-1.2 cells were exposed to various concentrations of EXEL-0862 for 24 hours. Phosphorylation of STAT3 and STAT5 was evaluated with their respective phosphospecific antibodies by Western blot. Phosphorylated STAT3 and STAT5 were detectable in untreated HMC-1.1 and HMC-1.2 cells (Figure 2A). With the addition of EXEL-0862 at concentrations ranging between 0.35 μM and 1.0 μM, STAT3 and STAT5 were completely dephosphorylated in HMC-1.2 cells. In HMC-1.1 cells, treatment with EXEL-0862 at the same dose range rendered complete inhibition of phosphorylation of STAT5. However, complete dephosphorylation of STAT3 was observed only on treatment with EXEL-0862 at 1.0 μM (Figure 2A). Time-course studies revealed that the inhibition of STAT5 phosphorylation occurred rapidly and was detectable as early as 4 hours after treatment with EXEL-0862 at 0.35 μM (Figure 2B).
Activation of STAT3 and STAT5 is blocked by EXEL-0862. (A) HMC-1.1 and HMC-1.2 cells were treated with EXEL-0862 at concentrations ranging from 0.35 μM to 1.0 μM for 24 hours. Cell lysates were then analyzed by Western blot with phosphospecific antibodies, as indicated. (B) Time-course study of STAT3 and STAT5 phosphorylation inhibition. Cells were exposed to EXEL-0862 at 0.35 μM for 24 hours. Phosphorylation of STAT3 and STAT5 was analyzed by Western blot. (C-D) EXEL inhibited the phosphorylation of STAT3 and STAT5 and their nuclear translocation on SCF stimulation. HMC-1.2 and HMC-1.1 cells were starved in serum-free medium for 24 hours. SCF (100 ng/mL, final concentration) in 20% serum was added for 1 hour. Cells were harvested to prepare cell lysates for Western blot (C) or to be fixed in 3% paraformaldehyde for immunofluorescence staining with FITC and/or DAPI (D; arrows indicate cytoplasmic [CONTL and EXEL+SCF] or nuclear [SCF] STAT3 localization). EXEL-0862 (1.0 μM) was added to the culture 20 minutes before the addition of SCF. Cells were visualized with a 40 ×/0.9 objective lens mounted on an Olympus BX60 epifluorescence microscope (Olympus, Melville, NY), and the images were recorded with an Optronics CCD camera and Fluoview software version 4.3 (Olympus, Center Valley, PA).
Activation of STAT3 and STAT5 is blocked by EXEL-0862. (A) HMC-1.1 and HMC-1.2 cells were treated with EXEL-0862 at concentrations ranging from 0.35 μM to 1.0 μM for 24 hours. Cell lysates were then analyzed by Western blot with phosphospecific antibodies, as indicated. (B) Time-course study of STAT3 and STAT5 phosphorylation inhibition. Cells were exposed to EXEL-0862 at 0.35 μM for 24 hours. Phosphorylation of STAT3 and STAT5 was analyzed by Western blot. (C-D) EXEL inhibited the phosphorylation of STAT3 and STAT5 and their nuclear translocation on SCF stimulation. HMC-1.2 and HMC-1.1 cells were starved in serum-free medium for 24 hours. SCF (100 ng/mL, final concentration) in 20% serum was added for 1 hour. Cells were harvested to prepare cell lysates for Western blot (C) or to be fixed in 3% paraformaldehyde for immunofluorescence staining with FITC and/or DAPI (D; arrows indicate cytoplasmic [CONTL and EXEL+SCF] or nuclear [SCF] STAT3 localization). EXEL-0862 (1.0 μM) was added to the culture 20 minutes before the addition of SCF. Cells were visualized with a 40 ×/0.9 objective lens mounted on an Olympus BX60 epifluorescence microscope (Olympus, Melville, NY), and the images were recorded with an Optronics CCD camera and Fluoview software version 4.3 (Olympus, Center Valley, PA).
Given that phosphorylation is critical for STAT3 and STAT5 dimerization, nuclear translocation, and DNA binding, we investigated whether EXEL-0862 affected the phosphorylation and nuclear translocation of STAT3 and STAT5 on cytokine stimulation. Western blot analysis (Figure 2C) revealed the presence of phosphorylated STAT3 and STAT5 in starved HMC-1.2 and HMC-1.1 cells, suggesting that the phosphorylation of STATs was independent of exogenous cytokines and was likely mediated by the gain-of-function D816V KIT and V560G mutants, resulting in constitutively activated STAT3 and STAT5 in a ligand-independent manner. Of note, STAT3 and STAT5 phosphorylation was further enhanced on SCF stimulation (Figure 2C). The level of phosphorylated STAT3 and STAT5 in the presence of EXEL-0862 was lower than at baseline (lanes 3-4 vs lane 1; Figure 2C). Indirect immunofluorescence staining with anti–total STAT3 antibody (Figure 2D) revealed that STAT3 was localized in cytoplasm and nucleus in control (starved) HMC-1.2 and HMC-1.1 cells, albeit dominantly in the cytoplasm. After stimulation with SCF and serum, STAT3 was localized mainly within the cell nuclei, as shown by its colocalization with the nuclear DAPI (4′,6-diamidino-2-phenylindole) stain (Figure 2D). In contrast, after treatment with EXEL-0862, most STAT3 remained localized to the cytoplasm, even after stimulation with SCF, suggesting that EXEL-0862 abrogated STAT3 nuclear translocation. In aggregate, these data suggest that EXEL-0862 exerts a potent inhibitory effect on STAT3 and STAT5 phosphorylation that results in the inhibition of nuclear translocation.
EXEL-0862 induces apoptosis without significantly affecting cell-cycle distribution
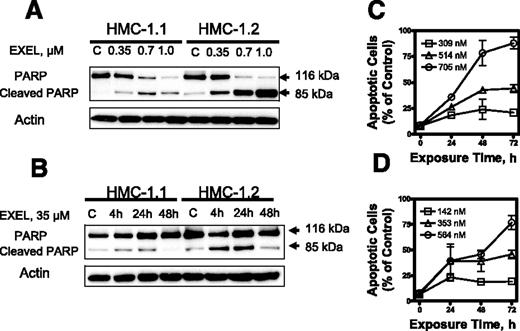
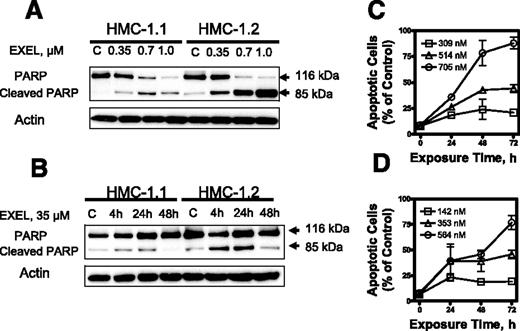
We next analyzed the capacity of EXEL-0862 to induce apoptosis in KIT mutant cell lines. When HMC-1.1 and HMC-1.2 cells were exposed to escalating doses of EXEL-0862, a dose-dependent specific cleavage of PARP, which is widely accepted as a specific marker of apoptosis, was observed (Figure 3A)Further, a time-dependent cleavage of PARP was demonstrated in both human mast cell lines treated at a fixed EXEL-0862 concentration (0.35 μM) and harvested at various time points (Figure 3B). Flow cytometry analysis of cells subjected to annexin V/PI double staining revealed that the percentage of annexin V–positive cells increased after treatment with EXEL-0862, further supporting that EXEL-0862 induced cell death by apoptosis (Figure 3C-D).
EXEL-0862 induces apoptosis in human mast cells expressing D816V KIT. (A) EXEL-0862 induced PARP cleavage in a dose-dependent manner. HMC-1.1 and HMC-1.2 cells were exposed to EXEL-0862 at the indicated concentrations for 24 hours. Cell lysates were analyzed by Western blot with antibody against PARP. (B) EXEL-0862 induced PARP cleavage in a time-dependent manner. Cells were treated with 0.35 μM for 4, 24, and 48 hours, respectively. PARP was analyzed by Western blot. (C-D) Apoptosis analysis by flow cytometry of HMC-1.1 and HMC-1.2 cells exposed to escalating concentrations of EXEL-0862 for 24 hours and subjected to annexin V-PI double staining. Cells stained with annexin V were defined as apoptotic. Ratios between apoptotic cells and total cells are plotted and represent the mean ± SEM of experiments performed in duplicate.
EXEL-0862 induces apoptosis in human mast cells expressing D816V KIT. (A) EXEL-0862 induced PARP cleavage in a dose-dependent manner. HMC-1.1 and HMC-1.2 cells were exposed to EXEL-0862 at the indicated concentrations for 24 hours. Cell lysates were analyzed by Western blot with antibody against PARP. (B) EXEL-0862 induced PARP cleavage in a time-dependent manner. Cells were treated with 0.35 μM for 4, 24, and 48 hours, respectively. PARP was analyzed by Western blot. (C-D) Apoptosis analysis by flow cytometry of HMC-1.1 and HMC-1.2 cells exposed to escalating concentrations of EXEL-0862 for 24 hours and subjected to annexin V-PI double staining. Cells stained with annexin V were defined as apoptotic. Ratios between apoptotic cells and total cells are plotted and represent the mean ± SEM of experiments performed in duplicate.
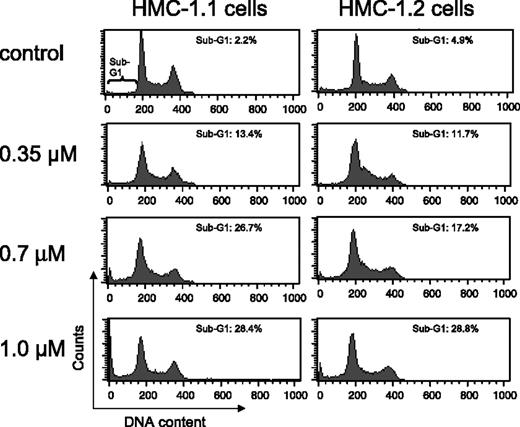
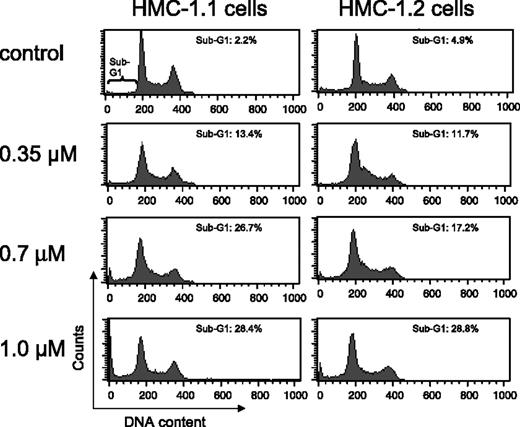
Exposure of HMC-1.1 and HMC-1.2 cells to increasing concentrations of EXEL-0862 for 24 hours did not result in significant cell-cycle disturbance, as evidenced by flow cytometry analysis (Figure 4). More important, increasing doses of EXEL-0862 enhanced the accumulation of HMC-1.1 and HMC-1.2 cells in the sub-G1 cell-cycle phase, indicating apoptotic cells.
Cell-cycle distribution in human mast cells after treatment with EXEL-0862. HMC-1.1 and HMC-1.2 cells were exposed to increasing concentrations of EXEL-0862 for 24 hours. Then cells were fixed in ethanol and analyzed by flow cytometry. Accumulation of HMC-1.1 and HMC-1.2 cells in sub-G1 phase was observed with increasing doses of EXEL-0862.
Cell-cycle distribution in human mast cells after treatment with EXEL-0862. HMC-1.1 and HMC-1.2 cells were exposed to increasing concentrations of EXEL-0862 for 24 hours. Then cells were fixed in ethanol and analyzed by flow cytometry. Accumulation of HMC-1.1 and HMC-1.2 cells in sub-G1 phase was observed with increasing doses of EXEL-0862.
EXEL-0862 induces mitochondrial damage, cytochrome c release, and activation of caspase-3 and caspase-9
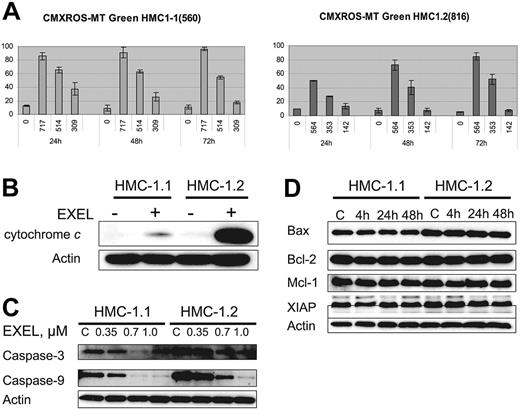
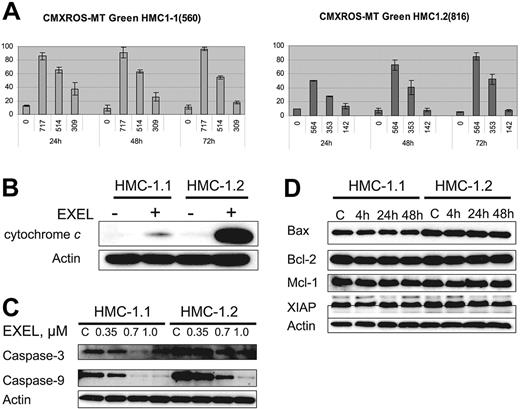
Modifications in mitochondrial transmembrane potential (ΔΨm) and cytochrome c translocation from mitochondria to the cytoplasm have been correlated with the induction of cell death by apoptosis. Thus, to gain further insight into the mechanism of apoptosis induced by EXEL-0862, we first analyzed whether EXEL-0862 induces mitochondrial changes in HMC-1.1 and HMC-1.2 cells. We measured by flow cytometry the mitochondrial uptake of CMXRos and MTGreen double staining. Figure 5A illustrates the graphs in which fluorescence of CMXRos and MTGreen is plotted against the percentage of positive cells over time. Treatment of HMC-1.1 and HMC-1.2 with EXEL-0862 at IC80 concentrations produced a time-dependent increase in the proportion of cells with altered ΔΨm in HMC-1.1 and HMC-1.2 cells. Paradoxically, when IC50 concentrations were used, a marked alteration in ΔΨm over time was seen in HMC-1.2, whereas the opposite effect occurred in HMC-1.1 cells. Next, we compared the release of cytochrome c in untreated cells and cells treated with EXEL-0862 at 0.35 μM for 24 hours. To this end, cells were harvested, washed with PBS, and resuspended with digitonin extraction buffer to obtain the cytosolic fraction. Cytochrome c could not be detected in untreated cells, as measured by immunoblotting with anti–cytochrome c antibody (Figure 5B). Treatment with EXEL-0862 significantly enhanced cytochrome c release. Of note, this effect was markedly higher in HMC-1.2 cells than in HMC-1.1 cells.
EXEL-0862 leads to mitochondrial damage, cytochrome c release, and activation of caspase-3 and -9. (A) Mitochondrial potential damage was elicited by EXEL-0862. Cells were exposed to EXEL-0862, stained with CMXRos and MTGreen, and immediately analyzed by flow cytometry. Histograms represent the population of cells with damaged mitochondrial potential. Values represent the mean ± SEM values from duplicate experiments. (B) EXEL-0862 treatment induced cytochrome c release into the cytosol. Cells were treated with 0.35 μM EXEL for 24 hours, and the cytosolic fraction was extracted with digitonin buffer. Cytochrome c was detected with immunoblots. (C) EXEL-0862 activated caspase-3 and -9. Cells were exposed to EXEL-0862 at the indicated concentrations for 24 hours. Cell lysates were analyzed by Western blot with antibodies against caspase-9 and caspase-3. (D) Cells were treated with 0.35 μM EXEL-0862 for 24 hours. Expression of apoptosis-related proteins was analyzed by Western blot.
EXEL-0862 leads to mitochondrial damage, cytochrome c release, and activation of caspase-3 and -9. (A) Mitochondrial potential damage was elicited by EXEL-0862. Cells were exposed to EXEL-0862, stained with CMXRos and MTGreen, and immediately analyzed by flow cytometry. Histograms represent the population of cells with damaged mitochondrial potential. Values represent the mean ± SEM values from duplicate experiments. (B) EXEL-0862 treatment induced cytochrome c release into the cytosol. Cells were treated with 0.35 μM EXEL for 24 hours, and the cytosolic fraction was extracted with digitonin buffer. Cytochrome c was detected with immunoblots. (C) EXEL-0862 activated caspase-3 and -9. Cells were exposed to EXEL-0862 at the indicated concentrations for 24 hours. Cell lysates were analyzed by Western blot with antibodies against caspase-9 and caspase-3. (D) Cells were treated with 0.35 μM EXEL-0862 for 24 hours. Expression of apoptosis-related proteins was analyzed by Western blot.
Western blot analysis was performed on HMC-1.1 and HMC-1.2 cell lysates obtained after treatment with EXEL-0862 at 0.35, 0.7, and 1.0 μM. Decreasing levels of caspase-3 and caspase-9 were observed with higher concentrations of EXEL-0862, reflecting increased cleavage of caspase-3 and caspase-9 (Figure 5C), which occurred in parallel with increased cytochrome c release. Additionally, we explored the effect of EXEL-0862 at 0.35 μM for 4 hours, 24 hours, and 48 hours on the expression of other apoptosis-related proteins. Cell lysates were subjected to SDS-PAGE, and immunoblots revealed no significant changes in the expression of Bcl-2, Bax, Mcl-1, and X-linked inhibitor of apoptosis protein (XIAP) (Figure 5D). Overall, these data suggest that EXEL-0862 may trigger mast-cell apoptosis through direct mitochondrial damage with the release of cytochrome c and caspase activation.
Ex vivo effect of EXEL-0862 on primary mast cells from patients with SM
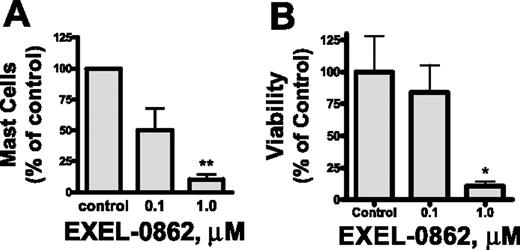
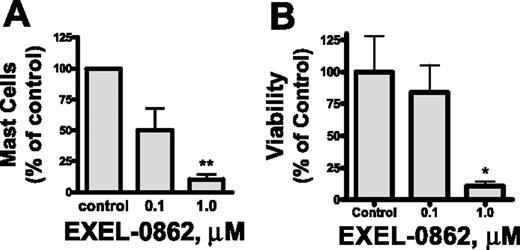
Our in vitro results prompted us to assess the efficacy of EXEL-0862 on neoplastic mast cells of patients with SM. We evaluated the ex vivo antineoplastic effect of EXEL-0862 in bone marrow mast cells obtained from patients with SM bearing the D816V KIT mutant in short-term cultures. Human mast cells express a high level of surface CD117 (KIT) and exhibit marked granularity,25 which make this cell population readily identifiable by flow cytometry. Treatment with EXEL-0862 at doses ranging from 0.1 to 1.0 μM for 7 days in the presence of SCF resulted in a significant reduction in the mast-cell percentage in bone marrow from patients with SM. Figure 6A illustrates the percentage of mast-cell viability normalized to the results obtained in cells incubated in the absence of EXEL-0862. Treatment with EXEL-0862 at 1.0 μM for 7 days led to almost 90% reduction in bone marrow mast cells compared with bone marrow incubated without EXEL-0862 (P < .005). As a control and comparison for the ex vivo studies, we tested the effect of the drug on CD34+-derived mast cells without D816V KIT mutation in samples from 3 donors (2 healthy adults and 1 patient with ISM who carried wild-type KIT). EXEL-0862 significantly reduced the viability of mast cells at 1 μM (P < .05), as evidenced by annexin V binding (Figure 6B), suggesting that EXEL-0862 also kills mast cells that depend on wild-type KIT for survival. This result is consistent with the in vitro inhibitory effect of EXEL-0862 on wild-type KIT (Table 1).
Ex vivo EXEL-0862 treatment inhibits the viability of primary bone marrow mast cells. (A) Bone marrow mononuclear cells from 3 patients with ISM who carried D816V KIT were incubated for 7 days with EXEL-0862 at 0.1 μM and 1 μM. Then, the mast-cell content in cultures was determined by flow cytometry. (B) Mast cells without D816V KIT mutation in samples from 3 donors were derived from bone marrow CD34+ cells by culture in Stem-Pro and SCF (100 ng/mL) and IL-3 (30 ng/mL, only for the first week) and weekly hemirepletion of the culture medium for approximately 9 to 14 weeks. Mast cells were then incubated with or without EXEL-0862 for 48 hours at the indicated concentrations. Viable cells, defined as Annexin-V-FITC–negative cells, were measured by flow cytometry. Data are expressed as percentage of mast-cell survival normalized to results obtained in cells incubated in the absence of drug (control). Each point represents the mean ± SEM values from 3 patients with ISM. *P < .05; **P < .005; Student t test.
Ex vivo EXEL-0862 treatment inhibits the viability of primary bone marrow mast cells. (A) Bone marrow mononuclear cells from 3 patients with ISM who carried D816V KIT were incubated for 7 days with EXEL-0862 at 0.1 μM and 1 μM. Then, the mast-cell content in cultures was determined by flow cytometry. (B) Mast cells without D816V KIT mutation in samples from 3 donors were derived from bone marrow CD34+ cells by culture in Stem-Pro and SCF (100 ng/mL) and IL-3 (30 ng/mL, only for the first week) and weekly hemirepletion of the culture medium for approximately 9 to 14 weeks. Mast cells were then incubated with or without EXEL-0862 for 48 hours at the indicated concentrations. Viable cells, defined as Annexin-V-FITC–negative cells, were measured by flow cytometry. Data are expressed as percentage of mast-cell survival normalized to results obtained in cells incubated in the absence of drug (control). Each point represents the mean ± SEM values from 3 patients with ISM. *P < .05; **P < .005; Student t test.
Discussion
KIT mutations at codon 816 have been reported in a variety of malignancies, including SM. More than 80% of patients with SM harbor the KIT D816V mutant isoform.4,5,18 This mutation constitutively activates KIT tyrosine kinase and stabilizes the activation loop in the active conformation, thus precluding the binding of kinase inhibitors such as imatinib and AMN107, with selective affinity for the open configuration of the kinase domain. Hence, KIT D816V has been proposed as a key therapeutic target for the treatment of SM. We investigated the activity of the novel tyrosine kinase inhibitor EXEL-0862 against the mast cell lines HMC-1.1 and HMC-1.219 and against bone marrow cells from patients with SM harboring KIT D816V to predict whether this compound might have clinical activity in patients with SM. That HMC-1.1 and HMC-1.2 differ only by the presence of the D816V mutation makes them ideal models for the study of the activity of novel agents against this kinase domain mutation. We documented that EXEL-0862 potently inhibited cell proliferation and KIT phosphorylation of HMC-1.1 and HMC-1.2 with IC50 values of 514 and 353 nM, respectively. In addition, EXEL-0862 inhibited phosphorylation of the KIT-dependent downstream signaling molecules STAT3 and STAT5, and, consistent with findings on proliferation assays, EXEL-0862 promoted apoptosis in vitro and ex vivo in cells carrying the D816V KIT mutant isoform.
Imatinib, which is approved for the treatment of patients with chronic myelogenous leukemia (CML) and metastatic GIST, exhibits a significant inhibitory effect on HMC-1.1 cells harboring KIT V560G but fails to inhibit the growth of HMC-1.2 cells carrying KIT D816V, translating into a lack of significant clinical response in patients with SM harboring KIT loop activation mutations.1,4,13,14 Like imatinib, AMN107 exhibited a weak inhibitory effect on HMC-1.2 cell growth and phosphorylation (IC50, approximately 1-5 μM) after exposure to this agent for 48 hours in the MTS assay. To overcome this problem, several small molecule tyrosine kinase inhibitors have been tested against D816V KIT, including MLN518,26 PD-180970,26 PKC412,27 BMS-354825,28 AP-23464,29 and AP-23848.29 MLN518 and PD180970 demonstrated activity against cell lines expressing juxtamembrane mutant KIT, but only MLN518, at nanomolar concentrations, targeted active-site mutant cell lines. PKC412, AP-23464, and AP-23848 have in vitro inhibition effects on activation-loop mutations of KIT in nanomolar ranges. Recently, it was reported that the dual Abl/Src kinase inhibitor dasatinib (formerly BMS-354825) induces apoptosis in mast cells expressing KIT activation loop mutations, including D816V. PKC412 has produced a transient clinical response in a patient with MCL harboring this mutation. The potential clinical benefit and the toxicity profile of these compounds remain to be determined in clinical trials.
In the study reported here, exposure of HMC-1.2 cells harboring KIT D816V to nanomolar concentrations of EXEL-0862 caused a significant inhibition of KIT phosphorylation. In fact, the IC50 values in a 72-hour MTS assay were significantly lower in HMC-1.2 (353 nM) than in HMC-1.1 (514 nM) cells, indicating that EXEL-0862 might be more potent against imatinib-resistant cells bearing KIT activation loop mutations involving codon D816.18 Furthermore, ex vivo exposure of bone marrow mast cells from patients with SM bearing KIT D816V to EXEL-0862 resulted in a dramatic reduction of bone marrow mast-cell content, suggesting that therapeutic inhibition of KIT kinase by compounds such as EXEL-0862 could be effective for patients with SM associated with the gain-of-function KIT D816V mutation.
Both the juxtamembrane V560G and the kinase domain D816V mutations give rise to constitutively activated KIT isoforms that preferentially phosphorylate STAT3 and STAT5, thus leading to cell proliferation. STAT proteins act as transcription factors capable of delivering a rapid and direct signal from extracellular stimuli to the nucleus in normal and malignant cells.30-32 STAT5 knockout mice exhibit mast-cell deficiency,33 indicating that STAT5 might be a key regulator of mast-cell development, proliferation, and survival. Activation of STAT3/STAT5 is a key event in KIT-mediated cell proliferation and inhibition of apoptosis. Our data demonstrate the presence of constitutive STAT3 and STAT5 phosphorylation in untreated starved HMC-1.2 and HMC-1.1 mast cells (Figure 2A), corroborating previous reports suggesting that STAT3 and STAT5 are aberrantly phosphorylated by KIT D816V, V560G, and other KIT-activating mutations.34-37 This has been further supported by the observation that 10 of 14 KIT mutations (including D816V and V560G) conferred interleukin 3–independent growth and were associated with constitutive phosphorylation of tyrosine residues in STAT3 and STAT5.36 This might explain our observation regarding the phosphorylation of STAT3 and STAT5 (Figure 2C), which is consistent with the existence of a limited amount of STAT3 in the nucleus of starved HMC-1.1 and HMC-1.2 cells (Figure 2D). The cytoplasmic STAT3 in starved cells might reflect incomplete phosphorylation in the absence of growth factor (SCF), even though there is constitutive activation by the mutant KIT. Upon SCF stimulation, STAT3 is fully phosphorylated, and more STAT3 molecules are translocated into the nucleus, a process blocked by EXEL-0862. Because EXEL-0862 inhibits STAT3/STAT5 phosphorylation before the occurrence of apoptosis, the former process may contribute to the proapoptotic effect of EXEL-0862 on HMC-1.2 and HMC-1.1 cells.
EXEL-0862–induced apoptosis was more pronounced in HMC-1.2 D816V-bearing cells than in HMC-1.1 cells expressing wild-type KIT and appeared to be mediated by activation of downstream elements of the intrinsic apoptotic pathway (eg, cytochrome c/caspase-9/caspase-3). In addition, the alteration in ΔΨm and the release of cytochrome c into the cytoplasm appear to indicate that EXEL-0862 may promote mitochondrial damage through an as yet unknown mechanism. Notably, EXEL-0862–induced apoptosis was not associated with a change in the expression of other apoptosis-related proteins that have been associated with resistance to chemotherapy, such as Bcl-2, Mcl-1, XIAP, and Bax.
In summary, our in vitro and ex vivo data demonstrate that EXEL-0862 can effectively target juxtamembrane and, more remarkably, activation loop mutants of KIT, including the imatinib-resistant KIT D816V mutation. This suggests that EXEL-0862 may have clinical efficacy against human neoplasms driven by gain-of-function KIT mutations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: P.L. is an employee of Exelixis, which is the owner of EXEL-0862, the compound studied and reported in this work.
Acknowledgments
This work was supported in part by the Joe W. and Dorothy Dorsett Brown Foundation (Metairie, LA). The flow cytometry core facility at M. D. Anderson Cancer Center is supported by National Cancer Institute Cancer Center Core grant CA16672.

![Figure. 2. Activation of STAT3 and STAT5 is blocked by EXEL-0862. (A) HMC-1.1 and HMC-1.2 cells were treated with EXEL-0862 at concentrations ranging from 0.35 μM to 1.0 μM for 24 hours. Cell lysates were then analyzed by Western blot with phosphospecific antibodies, as indicated. (B) Time-course study of STAT3 and STAT5 phosphorylation inhibition. Cells were exposed to EXEL-0862 at 0.35 μM for 24 hours. Phosphorylation of STAT3 and STAT5 was analyzed by Western blot. (C-D) EXEL inhibited the phosphorylation of STAT3 and STAT5 and their nuclear translocation on SCF stimulation. HMC-1.2 and HMC-1.1 cells were starved in serum-free medium for 24 hours. SCF (100 ng/mL, final concentration) in 20% serum was added for 1 hour. Cells were harvested to prepare cell lysates for Western blot (C) or to be fixed in 3% paraformaldehyde for immunofluorescence staining with FITC and/or DAPI (D; arrows indicate cytoplasmic [CONTL and EXEL+SCF] or nuclear [SCF] STAT3 localization). EXEL-0862 (1.0 μM) was added to the culture 20 minutes before the addition of SCF. Cells were visualized with a 40 ×/0.9 objective lens mounted on an Olympus BX60 epifluorescence microscope (Olympus, Melville, NY), and the images were recorded with an Optronics CCD camera and Fluoview software version 4.3 (Olympus, Center Valley, PA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-04-013805/4/m_zh80010705950002.jpeg?Expires=1766040742&Signature=oG96GSnyYdjL3O3S71vQmLDbdOt3eflStHlNHYoF61nV7mSFlT01ExdWrfu8fhOOqqxDkgTnr1HtA0kSbgzln-aT3Tyw8v~anKJIuwt9UsL27~vhAkXM6ey7O2iBAa2CYeGJ-hvoWKH0atHONIQHVv17zV-987KRR958YmXGyLeQGxNkaLmz3A73Qe5pXpu5vuGk5dj-z037qGl6XzDzxqnR9kdbzVRT-5-21CJjT5qbhdK1ekydNl-O~Ch47xKh9gLd16fia6vcf4DK1fvgoo3rP1aPeRsf07rI1SujN4SQypo2luF79SGAypsbRpUmbAZF-3QCArFyAJQB8zmTgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure. 2. Activation of STAT3 and STAT5 is blocked by EXEL-0862. (A) HMC-1.1 and HMC-1.2 cells were treated with EXEL-0862 at concentrations ranging from 0.35 μM to 1.0 μM for 24 hours. Cell lysates were then analyzed by Western blot with phosphospecific antibodies, as indicated. (B) Time-course study of STAT3 and STAT5 phosphorylation inhibition. Cells were exposed to EXEL-0862 at 0.35 μM for 24 hours. Phosphorylation of STAT3 and STAT5 was analyzed by Western blot. (C-D) EXEL inhibited the phosphorylation of STAT3 and STAT5 and their nuclear translocation on SCF stimulation. HMC-1.2 and HMC-1.1 cells were starved in serum-free medium for 24 hours. SCF (100 ng/mL, final concentration) in 20% serum was added for 1 hour. Cells were harvested to prepare cell lysates for Western blot (C) or to be fixed in 3% paraformaldehyde for immunofluorescence staining with FITC and/or DAPI (D; arrows indicate cytoplasmic [CONTL and EXEL+SCF] or nuclear [SCF] STAT3 localization). EXEL-0862 (1.0 μM) was added to the culture 20 minutes before the addition of SCF. Cells were visualized with a 40 ×/0.9 objective lens mounted on an Olympus BX60 epifluorescence microscope (Olympus, Melville, NY), and the images were recorded with an Optronics CCD camera and Fluoview software version 4.3 (Olympus, Center Valley, PA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-04-013805/4/m_zh80010705950002.jpeg?Expires=1766012489&Signature=MCPm7gfXW7bcuRDXHch5kuN9rPrDybl~arbSXqne-LpUAPuuptHjsslorIsI4Z~j2~S~o~CBqqRS6vep0k~42Imc9RcbkHE9Hz-Z~yuwyax96sRzvrDUCzaWfCKp~izhBot4jwhOtr04zkpU0rPYnEBQyAPaxG1VZ83JN9xLf8QSm-1xyAK66BdaHFsbhBwUR2qAMBt6o9IBX15ll7oPTCnv7MhcrqODmr7VUcKvscj87RaP898PVrJP7FXlBls24UvCdTtVUThowB~3eHxesMZxBr-aNB4UuqDCnX~XP53COdv2wuZ73ZXEgAWBly~9qCkNWzudnxYYf4X1eB4Ahg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)