Abstract
Central nervous system (CNS) relapse accompanying the prolonged administration of imatinib mesylate has recently become apparent as an impediment to the therapy of Philadelphia chromosome–positive (Ph+) leukemia. CNS relapse may be explained by limited penetration of imatinib mesylate into the cerebrospinal fluid because of the presence of P-glycoprotein at the blood-brain barrier. To overcome imatinib mesylate–resistance mechanisms such as bcr-abl amplification, mutations within the ABL kinase domain, and activation of Lyn, we developed a dual BCR-ABL/Lyn inhibitor, INNO-406 (formerly NS-187), which is 25 to 55 times more potent than imatinib mesylate in vitro and at least 10 times more potent in vivo. The aim of this study was to investigate the efficacy of INNO-406 in treating CNS Ph+ leukemia. We found that INNO-406, like imatinib mesylate, is a substrate for P-glycoprotein. The concentrations of INNO-406 in the CNS were about 10% of those in the plasma. However, this residual concentration was enough to inhibit the growth of Ph+ leukemic cells which expressed not only wild-type but also mutated BCR-ABL in the murine CNS. Furthermore, cyclosporine A, a P-glycoprotein inhibitor, augmented the in vivo activity of INNO-406 against CNS Ph+ leukemia. These findings indicate that INNO-406 is a promising agent for the treatment of CNS Ph+ leukemia.
Introduction
Imatinib mesylate (Gleevec; imatinib [Glivec]; formerly STI571), a specific inhibitor of ABL tyrosine kinase, is efficacious in treating Philadelphia chromosome–positive (Ph+) leukemias such as chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL).1,2 Within a few years of its introduction to the clinic, imatinib mesylate had dramatically altered the first-line therapy for CML, because it was found that most patients with newly diagnosed CML in the chronic phase (CP) achieved durable responses when treated with imatinib mesylate.3 However, a small percentage of these patients, as well as most patients with advanced-phase CML and Ph+ ALL, relapse on imatinib mesylate therapy.2,4 Several mechanisms of refractoriness and relapse have been reported, including point mutations within the ABL kinase domain, amplification of the bcr-abl gene, overexpression of bcr-abl mRNA,5-8 increased drug efflux via a process mediated by P-glycoprotein (P-gp),9 and activation of the Src-family protein Lyn.10-12
There has recently been an increase in the numbers of reported cases of isolated central nervous system (CNS) relapse in which mainly patients with CML-blast crisis (BC) and Ph+ ALL who continued to have complete cytogenetic responses (CCgR) developed an extramedullary BC in the CNS.13-22 Leis et al23 reported that isolated CNS relapse occurred in 5 (20.8%) of 24 patients whose protocols included imatinib mesylate treatment, whereas Pfeifer et al24 reported that CNS leukemia developed in 13 (12.1%) of 107 patients with Ph+ ALL. Isolated CNS relapse may be due to a limited penetration of imatinib mesylate into the cerebrospinal fluid (CSF), because it has been shown that concentrations of imatinib mesylate in the CSF are 1 to 2 orders of magnitude lower than the corresponding plasma levels.24-27 Brain endothelial cells are characterized by their barrier properties, including tight junctions and various selective transporters. One of the transporters in the BBB, P-gp, which is expressed at the luminal side of the endothelial cells of the capillaries in the brain, plays an important role in drug efflux from the brain. Preclinical in vitro and in vivo studies have shown that imatinib mesylate is a substrate for P-gp, so that P-gp limits the distribution of imatinib mesylate to the brain.28,29 The CNS can thereby become a sanctuary site of relapse in patients who are on prolonged imatinib mesylate therapy.
To overcome resistance to imatinib mesylate, we recently developed a specific dual BCR-ABL/Lyn inhibitor, INNO-406 (formerly NS-187), which is 25 to 55 times more potent than imatinib mesylate in vitro and at least 10 times more potent than imatinib mesylate in vivo.30,31 The aim of the present study was to investigate the efficacy of INNO-406 in the treatment of CNS Ph+ leukemia. We found that INNO-406 inhibited the growth of Ph+ leukemic cell lines in the murine CNS despite the fact that INNO-406, like imatinib mesylate, is a substrate for P-gp. Furthermore, cyclosporine A (CsA), a P-gp inhibitor, augmented the in vivo activity of INNO-406 against CNS Ph+ leukemia.
Materials and methods
Reagents and cell lines
INNO-406 and imatinib mesylate were synthesized and purified at Nippon Shinyaku (Kyoto, Japan). For in vitro experiments, both compounds were dissolved as 10 mM aliquots in dimethyl sulfoxide (Sigma Aldrich, St Louis, MO) and stored at −20°C until use. Verapamil and CsA were purchased from Sigma Aldrich and Novartis Pharma (Basel, Switzerland), respectively. The human leukemic cell line K562, which was established from the blastic phase of Ph+ CML cells, was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The P-gp–overexpressing multidrug-resistant (MDR) cell line K562/D1-9 was established previously and maintained in suspension culture in RPMI-1640 medium (Gibco, Paisley, Scotland) with 10% heat-inactivated fetal calf serum (FCS) (Hyclone, Logan, UT) and 0.1 μM daunorubicin.32 K562 cells expressing green fluorescent protein (GFP) (K562GFP), Ba/F3 cells expressing both wild-type (wt) BCR-ABLp185 and GFP (Ba/F3/wt bcr-ablGFP), and Ba/F3 cells expressing BCR-ABL/Q252H (Ba/F3/Q252H) or BCR-ABL/M351T (Ba/F3/M351T) were generated as previously described.30 Cells were maintained at 37°C in a fully humidified atmosphere of 5% CO2 in air as suspension cultures in RPMI-1640 medium supplemented with 10% heat-inactivated FCS. For the transport study, renal porcine epithelial (LLC-PK1) cells were obtained from the ATCC and LLC-GA5-COL150 cells were established in our laboratory.33 LLC-PK1 and LLC-GA5-COL150 cells were maintained by serial passage in plastic culture dishes as described elsewhere.34 Cells undergoing exponential growth were used in the experiments.
In vitro growth inhibitory effects
Cell proliferation was determined by a modified assay with MTT (3-(4,5-dimethylthiazol -2-yl)-2,5-diphenyltetrazolium bromide; Nacalai Tesque, Kyoto, Japan). K562, K562GFP, K562/D1-9, and Ba/F3/wt bcr-ablGFP cells were seeded in a flat-bottomed 96-well plate (Greiner Labortechnik, Kremsmuenster, Germany) to give 1 × 104 cells in 100 μL medium in each well and incubated with various concentrations of imatinib mesylate, INNO-406, verapamil, or CsA for 72 hours. The mean of 5 determinations at each concentration was calculated. IC50 values were obtained using the nonlinear regression program CalcuSyn (Biosoft, Cambridge, United Kingdom).
Cellular accumulation of INNO-406
We confirmed the presence of a small amount of native P-gp in LLC-PK1 cells and overexpressed P-gp in LLC-GA5-COL150 cells by Western blotting as previously described.34,35 The accumulation of [14C]INNO-406 (51.2 mCi/mmol [1894.4 MBq/mmol]; Nippon Shinyaku, Kyoto, Japan) was measured in cells grown on 24-well plates. [3H]D-Mannitol (17 Ci/mmol [62.9 × 1010 Bq/mmol]; PerkinElmer, Boston, MA) was used to calculate the extracellular trapping of [14C]INNO-406. After removal of the culture medium, cells were washed once with Dulbecco phosphate-buffered saline (PBS buffer; 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, and 0.5 mM MgCl2, pH 7.4), and preincubated for 10 minutes in PBS supplemented with 5 mM D-glucose and 3% bovine serum albumin (Sigma-Aldrich). After preincubation, the cells were incubated with [14C]INNO-406 (6.92 μM, 13.1 kBq/mL) and [3H]D-Mannitol (1 μM, 74 kBq/mL) in the presence or absence of 10 μM CsA for a specified period at 37°C. After incubation, the drug solution was removed by aspiration, and the cells were washed once with ice-cold PBS containing 3% bovine serum albumin and twice with ice-cold PBS. The cells were solubilized in 0.5 N NaOH, and the cell-associated radioactivity was determined in ACSII scintillation cocktail (Amersham, Piscataway, NJ) by liquid scintillation counting. The protein content of the cells was determined by the method of Bradford, using a Bio-Rad protein assay kit (Bio-Rad, Tokyo, Japan) with bovine γ-globulin as the standard according to the manufacturer's instructions. The cellular accumulation of [14C]INNO-406 was determined by subtracting the nonspecific association as determined by the [3H]D-mannitol space.
Calculation of brain penetration of imatinib mesylate and INNO-406
INNO-406 was labeled with 4-methyl-3-(trifluoromethyl)[carbonyl-14C]benzoic acid (BlyChem, Billingham, United Kingdom). The radiochemical purity of [14C]INNO-406 was greater than 98% and the specific activity was 3.28 MBq (88.6 μCi)/mg. Male Sprague-Dawley (SD) rats (Japan SLC, Shizuoka, Japan) and male BALB/cA Jcl-nu mice (Clea Japan, Osaka, Japan) were used at 7 and 6 weeks of age, respectively. Approval for all in vivo experiments was obtained from the institutional review board at Kyoto University Hospital. SD rats or BALB/cA Jcl-nu mice were administered 50 mg CsA/kg, a dose that is reported to be sufficient to inhibit P-gp in the BBB36 or vehicle by oral gavage. Because the blood and brain concentrations of CsA remain approximately equal from 30 to 270 minutes after oral administration,37 the mice were administered 10 or 30 mg [14C]INNO-406/kg 2 hours after administration of CsA. At 0.5, 2, and 4 hours after the administration of [14C]INNO-406, blood samples were collected via the orbital plexus under anesthesia, and whole brains were removed immediately after killing by cervical dislocation. Blood samples were centrifuged to obtain plasma. Plasma and brain [14C]INNO-406 concentrations were determined by thin-layer chromatography (TLC) and scintillation counting in a Tri-Carb 3100TR scintillation-counter (PerkinElmer).
Murine systemic and CNS Ph+ leukemia model
A murine systemic Ph+ leukemia model was established by intravenous injection of 1 × 106 Ba/F3/M351T cells into the tail vein of male BALB/cA Jcl-nu mice as previously described.30,31
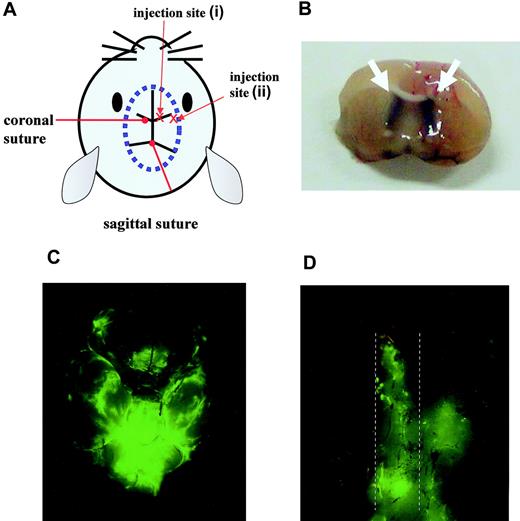
To establish the CNS leukemia model, male BALB/cA Jcl-nu mice and nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice 6 to 7 weeks of age (Clea Japan) were inoculated in the right cerebral ventricle with 5 × 104 Ba/F3/wt bcr-ablGFP, Ba/F3/Q252H, or Ba/F3/M351T cells or with 1 × 106 K562GFP cells, respectively, in a total volume of 5 μL. Leukemic cells were injected through the right coronal suture at a point 1 mm to the right of the center line defined by the sagittal sutures with a microsyringe equipped with a polyethylene stopper tube designed to inject cells to a depth of exactly 3 mm. Without any treatment, mice had lost weight and displayed abnormal neurologic behavior such as gait disturbance 11 days after inoculation of Ba/F3/wt bcr-ablGFP cells.
In vivo effects of INNO-406 on leukemic cells in the CNS
BALB/cA Jcl-nu mice that had received Ba/F3/wt bcr-ablGFP cells and NOD/SCID mice that had received K562GFP cells were randomized into control and treated groups. Five days after inoculation of leukemic cells into the right cerebral ventricle, mice were administered vehicle, imatinib mesylate, or INNO-406 by oral gavage twice a day for 14 consecutive days. The volume of each dose was 200 μL. For in vivo treatment, imatinib mesylate was dissolved in sterile distilled water, and INNO-406/DMSO stock solution was dissolved in 5 g/L methylcellulose before administration. Mice were divided into 6 groups as follows: (1) untreated mice (vehicle only); (2) mice treated with 400 mg imatinib mesylate/kg per day; (3) mice treated with 6 mg INNO-406/kg per day; (4) mice treated with 20 mg INNO-406/kg per day; (5) mice treated with 60 mg INNO-406/kg per day; and (6) mice treated with 120 mg INNO-406/kg per day. When 120 mg INNO-406/kg per day is administrated orally to the mice, the concentration of INNO-406 in the CNS is estimated to be 0.24 μM according to pharmacokinetic (PK) studies described previously30 and tCOPY CNS performed in the present study. Because IC50 of INNO-406 for Ba/F3-expressing wt or mutated BCR-ABL except T315I is less than 0.25 μM,31 mice were treated with up to 120 mg INNO-406/kg per day. Treatment of mice with 200 mg imatinib mesylate/kg per day, a dose that on the basis of earlier studies was expected to produce blood concentrations comparable to those seen in humans38-40 did not show any effects for leukemic cells in the CNS (data not shown). Thus, we treated mice with up to 400 mg imatinib mesylate/kg per day which was nearly the maximal tolerant dose of imatinib mesylate in this mouse model. Each group of BALB/cA Jcl-nu mice receiving Ba/F3/wt bcr-ablGFP cells consisted of 10 animals, and each group of NOD/SCID mice receiving K562GFP cells consisted of 5 animals. On day 16, 3 BALB/cA Jcl-nu mice from each group which had received Ba/F3/wt bcr-ablGFP cells were killed, and their brains were examined under a fluorescence stereoscopic microscope (Leica, Heerbrugg, Switzerland). Images of brains were taken with an AxioCam digital camera (Carl Zeiss Vision GmbH, Hallbergmoos, Germany). The original magnifications of these images were ×8 (10× ocular lens; 0.8×/65 mm NA objective lens). The weights of all mice engrafted with Ba/F3/wt bcr-ablGFP or K562GFP cells were measured every week. The survival of the mice was determined as the number of days from inoculation to death (or to killing of moribund mice) and analyzed by the method of Kaplan-Meier with statistical significance assessed by the log-rank test. To examine the cause of death in mice treated with INNO-406, mRNA was extracted from the brains of mice treated with 120 mg INNO-406/kg per day. Direct sequencings of ABL kinase domain were performed by Bio Medical Laboratories (Tokyo, Japan).
Combined effects of INNO-406 and CsA in systemic and CNS Ph+ leukemia models
We examined the combined effect of 60 mg INNO-406/kg per day with 50 mg CsA/kg in a systemic Ph+ leukemia model. BALB/cA Jcl-nu mice that had been intravenously injected with Ba/F3/M351T cells were randomized into groups of 4 (day 0) and orally administered twice a day with vehicle, INNO-406 at 60 mg/kg per day, CsA at 50 mg/kg, or a combination of both drugs, for 10 consecutive days from day 1 to day 10. Each group consisted of 7 mice. CsA (0.1 g/mL) was diluted to 5 mg/mL with sterile distilled water on the day of administration. CsA was administered 2 hours before every administration of INNO-406.
We next examined the combined effect of INNO-406 at 60 mg/kg per day and CsA at 50 mg/kg in a CNS Ph+ leukemia model. BALB/cA Jcl-nu mice that had received Ba/F3/wt bcr-ablGFP, Ba/F3/Q252H, or Ba/F3/M351T cells were randomized into groups of 4 (day 0) and orally administered twice a day with vehicle, 60 mg INNO-406/kg per day, 50 mg CsA/kg, or a combination of both drugs, for 10 consecutive days from day 5 to day 14. Each group consisted of 7 mice. CsA was administered 2 hours before every administration of INNO-406. Blood samples from mice treated with INNO-406, CsA, or both were also analyzed at the last administration of these agents for GPT, blood creatinine (Cre), and total protein (TP) by colorimetric methods with diagnostic kits from Wako (Osaka, Japan).
Statistical analysis
P values were derived from 2-sided tests, and values of less than .05 were considered statistically significant.
Results
INNO-406 is a substrate for P-gp
Both INNO-406 and imatinib mesylate suppressed the growth of the BCR-ABL–positive cell line K562 in a dose-dependent manner. The corresponding IC50 values were 0.026 μM for INNO-406 and 0.687 μM for imatinib mesylate, indicating that INNO-406 was 25 times more potent than imatinib mesylate against this cell line (Figure 1A)It is well known that imatinib mesylate is a substrate for P-gp, and accordingly imatinib mesylate was less effective against the P-gp–overexpressing leukemic cell line K562/D1-9 than against the parent cell line, displaying an IC50 value of 5.169 μM against K562/D1-9 cells (Figure 1B). Although no interaction between P-gp and INNO-406 has been reported, INNO-406 was also less effective against K562/D1-9 cells than against K562 cells, displaying an IC50 value against K562/D1-9 cells of 0.626 μM (Figure 1B). We next examined the growth-inhibitory effect on K562/D1-9 cells of a combination of INNO-406 and one of the P-gp inhibitors CsA and verapamil. Both CsA (Figure 1C; also see Figure S1A, available on the Blood website by clicking on the Supplemental Materials link at the top of the online article) and verapamil (Figure 1D; Figure S1B) synergistically augmented the growth-inhibitory effect of INNO-406 against K562/D1-9 cells in a dose-dependent manner.
INNO-406 is a substrate for P-gp. (A) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562 cells, (B) growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on P-gp–overexpressing K562/D1-9 cells. (C) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) CsA. (D) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) verapamil. Leukemic cell lines were treated with serial dilutions of either drug for 3 days, and the growth-inhibitory effect of the drugs was assessed by MTT assay. Intracellular concentrations of [14C]INNO-406 in LLC-PK1 cells (•) and P-gp–overexpressing LLC-GA5-COL150 cells (○) in the absence of CsA (E), or in the presence of 10 μM CsA (F). Error bars indicate standard deviation.
INNO-406 is a substrate for P-gp. (A) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562 cells, (B) growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on P-gp–overexpressing K562/D1-9 cells. (C) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) CsA. (D) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) verapamil. Leukemic cell lines were treated with serial dilutions of either drug for 3 days, and the growth-inhibitory effect of the drugs was assessed by MTT assay. Intracellular concentrations of [14C]INNO-406 in LLC-PK1 cells (•) and P-gp–overexpressing LLC-GA5-COL150 cells (○) in the absence of CsA (E), or in the presence of 10 μM CsA (F). Error bars indicate standard deviation.
To clarify whether INNO-406 is in fact a substrate for P-gp, we examined the cellular accumulation of [14C]INNO-406 in monolayers of LLC-PK1 cells or P-gp–overexpressing LLC-GA5-COL150 cells. The intracellular accumulation of [14C]INNO-406 in LLC-GA5-COL150 cells was much less than that in LLC-PK1 cells (Figure 1E), indicating that INNO-406 was only poorly retained by cells overexpressing P-gp. The addition of 10 μM CsA increased the intracellular accumulation of [14C]INNO-406 in both LLC-PK1 cells and LLC-GA5-COL150 cells (Figure 1F). Taken together, these findings indicate that INNO-406, as well as imatinib mesylate, is a substrate for P-gp.
Concentration of INNO-406 in the brain
We have previously reported that, when BALB/c mice were given INNO-406 orally at a dose of 30 mg/kg, the PK parameters were as follows: Tmax, 2 hours; Cmax, 661 ng/mL; AUC0-∞, 2294 ng[mdot]h/mL; T1/2, 1.0 hour; and bioavailability, 32%.30 In SD rats, radioactivity levels in the plasma 2 hours after oral administration of 10 mg/kg [14C]INNO-406 or [14C]imatinib mesylate were 0.29 ± 0.08 μg·eq/g and 0.60 ± 0.26 μg·eq/g (n = 3), respectively. Radioactivity levels in the brain 2 hours after oral administration of 10 mg/kg [14C]INNO-406 or [14C]imatinib mesylate were 0.03 ± 0.01 μg·eq/g or 0.06 ± 0.03 μg·eq/g (n = 3), respectively. The concentrations of both imatinib mesylate and INNO-406 in the brain 4 hours after oral administration were almost undetectable (Figure 2A)These findings indicate that the concentration of both INNO-406 and imatinib mesylate in the brain was approximately 10% of the corresponding plasma concentration.
Concentrations of INNO-406 in the brain. (A) Concentrations of [14C]INNO-406 and [14C]imatinib mesylate in the plasma (□) and brain (▪) in SD rats were measured 2 and 4 hours after administration of 10 mg/kg of either drug (n = 3 for both). (B) Concentrations of INNO-406 in the BALB/cA Jcl-nu mice were measured 0.5, 2, 4, 6, and 8 hours after administration of 30 mg INNO-406/kg. The data shown are representative of 3 independent experiments. (C) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on Ba/F3/wt bcr-ablGFP cells. (D) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562GFP cells. Error bars indicate standard deviation.
Concentrations of INNO-406 in the brain. (A) Concentrations of [14C]INNO-406 and [14C]imatinib mesylate in the plasma (□) and brain (▪) in SD rats were measured 2 and 4 hours after administration of 10 mg/kg of either drug (n = 3 for both). (B) Concentrations of INNO-406 in the BALB/cA Jcl-nu mice were measured 0.5, 2, 4, 6, and 8 hours after administration of 30 mg INNO-406/kg. The data shown are representative of 3 independent experiments. (C) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on Ba/F3/wt bcr-ablGFP cells. (D) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562GFP cells. Error bars indicate standard deviation.
We next examined the concentration of INNO-406 from 0.5 to 8 hours after oral administration of 30 mg INNO-406/kg to the BALB/cA Jcl-nu mice. The peak concentration of INNO-406 in the brain was 50 ng/g (0.087 μM) at 2 hours (Figure 2B), which is 2- to 3-fold higher than the level required to achieve 50% inhibition of growth of Ba/F3/wt bcr-ablGFP cells (Figure 2C) or K562GFP cells (Figure 2D) (the IC50 values of INNO-406 for Ba/F3/wt bcr-ablGFP cells and K562GFP cells, which were used for the mouse experiments in this study, were 0.027 μM and 0.035 μM, respectively). By contrast, when 30 mg imatinib mesylate/kg was administered, the estimated concentration of imatinib mesylate in the brain at 2 hours (0.174 μM) was much lower than the level required to achieve 50% inhibition of growth of Ba/F3/wt bcr-ablGFP cells or K562GFP cells (Figure 2A,C-D). These findings led us to expect that even if most of the INNO-406 is effluxed by P-gp at the BBB, the residual concentration of INNO-406 in the CNS may be sufficient to inhibit leukemia growth in vivo.
Murine CNS Ph+ leukemia model
We believe that, to generate a reliable murine CNS leukemia model, precise injection into the cerebral ventricle is crucial. When injection was performed at the correct site (injection site (i), Figure 3A-B), injected leukemic cells were detected not only in the brain (Figure 3C) but also in the spinal cord (Figure 3D) on day 16. In contrast, when injection was performed at an incorrect site (injection site (ii), Figure 3A), leukemic cells were not detected along with the spinal cord on day 16 (data not shown). Clinically, in CNS leukemia cases, leukemic cells usually distribute to the CSF in the absence of any obvious solid tumor. Therefore, compared with random injection into the brain, the injection procedure that we adopted in this study is more physiologically relevant and may allow us to obtain more reliable data to evaluate therapies for CNS leukemia.
Mouse model of CNS Ph+ leukemia. (A) Diagram showing the injection sites. (B) Section from the brain of a mouse that had received trypan blue at the correct injection site. The white arrow indicates the trypan blue distributed in the bilateral cerebral ventricles. Brain (C) and spinal cord (D) taken from a mouse on day 16 after inoculation of 5 × 104 Ba/F3/wt bcr-ablGFP cells at the correct injection site. The dotted lines indicate the outline of the spinal cord. The data shown are representative of 3 independent experiments.
Mouse model of CNS Ph+ leukemia. (A) Diagram showing the injection sites. (B) Section from the brain of a mouse that had received trypan blue at the correct injection site. The white arrow indicates the trypan blue distributed in the bilateral cerebral ventricles. Brain (C) and spinal cord (D) taken from a mouse on day 16 after inoculation of 5 × 104 Ba/F3/wt bcr-ablGFP cells at the correct injection site. The dotted lines indicate the outline of the spinal cord. The data shown are representative of 3 independent experiments.
In vivo effect of INNO-406 in the murine CNS leukemia model
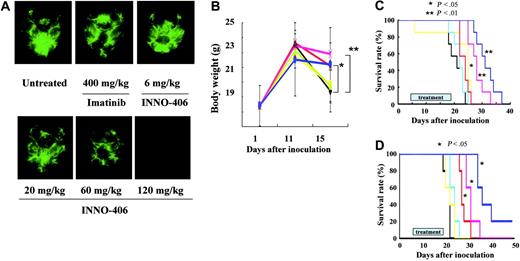
Murine pro-B Ba/F3/wt bcr-ablGFP cells are easily transplanted into BALB/cA Jcl-nu mice, whereas human leukemic K562GFP cells cannot be transplanted into BALB/cA Jcl-nu mice but can be transplanted into NOD/SCID mice. Therefore, we used 2 CNS leukemia models: one in which BALB/cA Jcl-nu mice received Ba/F3/wt bcr-ablGFP cells and one in which NOD/SCID mice received K562GFP cells. On day 16, 3 BALB/cA Jcl-nu mice from each group that had received Ba/F3/wt bcr-ablGFP cells were killed, and their brains were examined under a fluorescence stereoscopic microscope. INNO-406 inhibited the proliferation of leukemic cells in the brain in a dose-dependent manner, whereas, even at a dosage of 400 mg/kg per day, imatinib mesylate did not significantly slow the growth of the leukemia (Figure 4A). Untreated mice and mice treated with 400 mg imatinib mesylate/kg per day or 6 mg INNO-406/kg per day all started to lose significant body weight from day 11. In contrast, mice treated with more than 20 mg INNO-406/kg per day did not lose significant body weight until day 15 (Figure 4B). We next analyzed the activity of INNO-406 against CNS Ph+ leukemia in the BALB/cA Jcl-nu mice by the Kaplan-Meier method. Imatinib mesylate at a dosage of 400 mg/kg per day slightly prolonged the survival of the mice. At more than 60 mg/kg per day, INNO-406 significantly prolonged the survival of the mice in a dose-dependent manner compared with untreated mice or mice treated with 400 mg imatinib mesylate/kg per day (Figure 4C). Although INNO-406 prolonged significantly the survival of mice that received Ba/F3/wt bcr-ablGFP, it could not completely suppress the growth of leukemic cells in the brain, and the cause of death in the mice treated with 120 mg INNO-406/kg per day was also the progression of leukemic cells. mRNA extracted just before death from the brain of mice treated with 120 mg INNO-406/kg per day revealed wt bcr-abl without any point mutations in ABL kinase domain (Figure S2).
Effects of INNO-406 in an in vivo CNS Ph+ leukemia model. (A) Brains taken at day 16 from untreated, imatinib mesylate–treated or INNO-406–treated mice that had received 5 × 104 Ba/F3/wt bcr-ablGFP cells. The data shown are representative of 3 independent experiments. (B) The body weights of mice that had received 5 × 104 Ba/F3/wt bcr-ablGFP cells and that were then not treated (black) or treated with imatinib mesylate (green, 400 mg/kg per day) or INNO-406 (yellow, 6 mg/kg per day; blue, 20 mg/kg per day; red, 60 mg/kg per day; pink, 120 mg/kg per day). BALB/cA Jcl-nu mice (C) and NOD/SCID (D) mice were inoculated into the right cerebral ventricle with 5 × 104 BaF3/wt bcr-ablGFP cells or 1 × 106 K562GFP cells, respectively, on day 0. Mice were given vehicle (black), imatinib mesylate (yellow, 120 mg/kg per day), or INNO-406 (light blue, 6 mg/kg per day; red, 20 mg/kg per day; pink, 60 mg/kg per day; dark blue, 120 mg/kg per day) for 14 consecutive days from day 5. *P < .05; **P < .01.
Effects of INNO-406 in an in vivo CNS Ph+ leukemia model. (A) Brains taken at day 16 from untreated, imatinib mesylate–treated or INNO-406–treated mice that had received 5 × 104 Ba/F3/wt bcr-ablGFP cells. The data shown are representative of 3 independent experiments. (B) The body weights of mice that had received 5 × 104 Ba/F3/wt bcr-ablGFP cells and that were then not treated (black) or treated with imatinib mesylate (green, 400 mg/kg per day) or INNO-406 (yellow, 6 mg/kg per day; blue, 20 mg/kg per day; red, 60 mg/kg per day; pink, 120 mg/kg per day). BALB/cA Jcl-nu mice (C) and NOD/SCID (D) mice were inoculated into the right cerebral ventricle with 5 × 104 BaF3/wt bcr-ablGFP cells or 1 × 106 K562GFP cells, respectively, on day 0. Mice were given vehicle (black), imatinib mesylate (yellow, 120 mg/kg per day), or INNO-406 (light blue, 6 mg/kg per day; red, 20 mg/kg per day; pink, 60 mg/kg per day; dark blue, 120 mg/kg per day) for 14 consecutive days from day 5. *P < .05; **P < .01.
Ba/F3/wt bcr-ablGFP cells are derived from mice and artificially transfected with the bcr-abl gene. Therefore, we next examined the effects of INNO-406 on CNS leukemia in the NOD/SCID mice using the human leukemic cell line K562. INNO-406 at more than 20 mg/kg per day significantly prolonged the survival of the mice in a dose-dependent manner in the murine models compared with imatinib mesylate (Figure 4D). This finding suggests that INNO-406 is effective against both murine and human leukemic cells in the CNS.
Inhibition of P-glycoprotein promotes INNO-406 entry into the CNS
To test whether CsA-mediated inhibition of P-gp would promote INNO-406 entry into the CNS, the radioactivity of 14 C and the concentrations of INNO-406 as determined by TLC were measured when 30 mg [14C]INNO-406/kg was orally administered followed by 50 mg CsA/kg. In the brain, the levels of both the radioactivity and the concentrations of INNO-406 were increased by prior administration of 50 mg CsA/kg (Figure 5A-B)However, in the plasma, there was a difference between the behavior of the radioactivity and that of INNO-406 on prior administration of 50 mg CsA/kg. Administration of CsA caused an increase in plasma radioactivity levels (Figure 5C) but a decrease in the plasma concentrations of INNO-406 itself (Figure 5D). This was an unexpected result. We speculate that the elevated radioactivity levels in the plasma might be explained by increased net absorption of INNO-406 from the gastrointestinal (GI) tract caused by inhibition of P-gp by CsA in the GI tract with consequent suppression of the efflux of INNO-406 to the GI tract. The decreased concentrations of INNO-406 in the plasma, meanwhile, could be explained by increased influx of INNO-406 into the CNS in the presence of CsA. To investigate whether lowering the plasma levels would adversely affect the systemic activity of INNO-406, we examined the combined effects of CsA with INNO-406 in the systemic Ph+ leukemia model. In this model, combination with CsA did not reduce but in fact augmented the effect of INNO-406 (Figure S3).
CsA increases the concentrations of INNO-406 in the CNS. Radioactivity (A) and concentration (B) of INNO-406 in the brains of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) prior administration of 50 mg CsA/kg. Radioactivity (C) and concentration (D) of INNO-406 in the plasma of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) 50 mg CsA/kg. Error bars indicate standard deviation.
CsA increases the concentrations of INNO-406 in the CNS. Radioactivity (A) and concentration (B) of INNO-406 in the brains of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) prior administration of 50 mg CsA/kg. Radioactivity (C) and concentration (D) of INNO-406 in the plasma of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) 50 mg CsA/kg. Error bars indicate standard deviation.
In vivo combined effects of INNO-406 and CsA
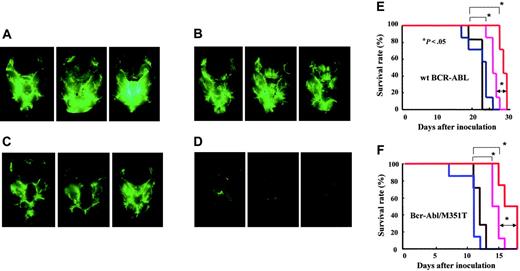
We examined the combined effect of 60 mg INNO-406/kg per day and 50 mg CsA/kg in a CNS leukemia model in which the mice received Ba/F3/wt bcr-ablGFP (Figure 6A), Ba/F3/Q252H, or Ba/F3/M351T cells. CsA alone did not show any inhibition of leukemia growth in the brain (Figure 6B), and INNO-406 alone showed some inhibition of leukemia growth in the brain (Figure 6C). Combined therapy with INNO-406 and CsA, however, revealed striking inhibition of leukemia growth in the brain (Figure 6D). The log-rank test for overall survival in a CNS leukemia model in which the mice received Ba/F3/wt bcr-ablGFP cells showed statistically significant differences between the groups that were not treated with INNO-406 and those that were treated with INNO-406 (P < .05). Moreover, there was also a significant difference between the group treated with INNO-406 alone and that treated with a combination of INNO-406 and CsA (P < .05) (Figure 6E). There were no significant differences in GPT, TP, or Cre among the groups after administration of INNO-406, CsA, or both. In a CNS mouse model in which the mice received either Ba/F3/M351T cells (Figure 6F) or Q252H cells (data not shown), INNO-406 alone prolonged the survival of the mice, and CsA also significantly augmented the effects of INNO-406. These data clearly indicate that CsA significantly enhances the anti-CNS leukemia effects of INNO-406 in vivo not only against cells expressing wt but also mutated BCR-ABL.
Combined effect of CsA and INNO-406 in an in vivo CNS leukemia model. BALB/cA Jcl-nu mice were inoculated into the right cerebral ventricle with 5 × 104 BaF3/wt bcr-ablGFP cells on day 0. Brains from each group were taken on day 17 from mice given vehicle (A), 50 mg CsA/kg (B), 60 mg INNO-406/kg per day (C), or a combination of 60 mg INNO-406/kg per day and 50 mg CsA/kg (D). On day 17, 3 mice from each group were killed, and their brains were examined under a fluorescence stereoscopic microscope. Kaplan-Meier analysis of the in vivo effects of INNO-406, CsA, or both on wt BCR-ABL (E) and BCR-ABL/M351T (F). Mice were orally administered vehicle (black), 50 mg CsA/kg (blue), 60 mg INNO-406/kg per day (pink), or a combination of CsA and INNO-406 (red) from day 5 to day 15.
Combined effect of CsA and INNO-406 in an in vivo CNS leukemia model. BALB/cA Jcl-nu mice were inoculated into the right cerebral ventricle with 5 × 104 BaF3/wt bcr-ablGFP cells on day 0. Brains from each group were taken on day 17 from mice given vehicle (A), 50 mg CsA/kg (B), 60 mg INNO-406/kg per day (C), or a combination of 60 mg INNO-406/kg per day and 50 mg CsA/kg (D). On day 17, 3 mice from each group were killed, and their brains were examined under a fluorescence stereoscopic microscope. Kaplan-Meier analysis of the in vivo effects of INNO-406, CsA, or both on wt BCR-ABL (E) and BCR-ABL/M351T (F). Mice were orally administered vehicle (black), 50 mg CsA/kg (blue), 60 mg INNO-406/kg per day (pink), or a combination of CsA and INNO-406 (red) from day 5 to day 15.
Discussion
CNS relapse may be an unavoidable result of the prolonged administration of imatinib mesylate in many patients. Reported cases of CNS relapse under imatinib mesylate treatment are summarized in Table S1. To date, CNS relapse has been reported in 33 patients (23 men and 10 women) within 0.9 to 20.5 months (median, 6.8 months) since the start of imatinib mesylate therapy. The initial diagnosis of these patients was either Ph+ ALL (15 patients) or CML (18 patients: CP, 5; lymphoid BC, 4; myeloid BC, 2; bilineage BC, 4; phase not described, 3). Eight of 33 patients achieved a CCgR. Fourteen of 18 cases of CNS relapse in which the blast type was reported occurred with leukemias displaying a lymphoid or bilineage phenotype, and 3 cases of CNS relapse occurred with leukemias displaying a myeloid phenotype. Although most patients who developed CNS leukemia received intrathecal chemotherapy (IT), cranial radiation therapy (CRT), or both, the prognosis of these patients was not favorable.
The penetration of imatinib mesylate into the cerebrospinal fluid (CSF) of humans and nonhuman primates has been reported,24-27 and imatinib mesylate concentrations in the CSF have been found to be 1 to 2 orders of magnitude lower than the corresponding plasma levels.25 Thus, the limited ability of imatinib mesylate to cross the BBB allows the CNS to become a sanctuary site of relapse for BCR-ABL–induced leukemia.41 It may be necessary to offer CNS prophylaxis to patients with CML on imatinib mesylate. Although IT chemotherapy and CRT have been used for CNS prophylaxis and are highly effective, they sometimes induce critical adverse effects such as infection or myeloencephalopathy. Especially in children and young adults, there are late effects of IT chemotherapy and CRT on cognition and growth.42,43 Therefore, an orally available drug that can inhibit leukemia cell growth in the CNS without adverse effects is desirable.
Because of its structural similarity to imatinib mesylate, which is a substrate for P-gp,30 we first investigated whether INNO-406 was also a substrate for P-gp. The present study clearly demonstrated that INNO-406, like imatinib mesylate, is a substrate for P-gp (Figure 1B-F). We have not determined the concentration of INNO-406 in the cerebrospinal fluid (CCSF). However, the CCSF of INNO-406, which can be estimated to be approximately 1.75 times higher than the concentration of INNO-406 in the brain (Cbrain),44 is sufficient to suppress the growth of Ph+ leukemic cells. The penetration of INNO-406 into the CNS is limited because of its efflux by P-gp. Although a rapid decline in INNO-406 levels after 4 hours was observed (Figure 2A, 2B), the concentration of INNO-406 in the brain 2 hours after administration was sufficient to suppress the growth of leukemia. We confirmed that in vitro, only 2 hours of exposure to 0.12 μM INNO-406, a concentration that could be maintained for 2 hours after oral administration of more than 42 mg INNO-406/kg according to PK studies (Figure 2B), suppressed the growth of leukemia cells (Figure S4).
Imatinib mesylate and INNO-406, both which are derived from 2-(phenylamino)pyrimidine, are therefore both substrates for P-gp. Meanwhile, dasatinib (formerly BMS-354825), another novel ABL tyrosine kinase inhibitor, was originally developed as an Src kinase inhibitor.45 Dasatinib is reported not to be a substrate for P-gp, and it inhibits leukemia growth in the brain.46 No detailed PK studies of dasatinib in the CNS have been reported, and the reason why dasatinib is not a substrate for P-gp has not been fully elucidated. Dasatinib has very high affinity not only for ABL but also for all known Src-family proteins in the CNS,45 and it is expected to be useful in inhibiting tumor growth. However, Src-family proteins play diverse pivotal roles such as Ca2+ signaling,47 vascular adaptations,48 and signaling through a variety of cell-surface receptors.49 INNO-406 inhibits Lyn and ABL while inhibiting as few other kinases as possible.30 With this unique profile of kinase inhibition, INNO-406, which achieves only the minimum concentration (Figure 2B-D) required to inhibit tumor growth (Figure 4), may be safer than a drug which easily penetrates into the CNS.
The penetration of INNO-406 into the CNS is limited because of its efflux by P-gp. Therefore, we next examined the combined effects of CsA, a potent inhibitor of P-gp, and INNO-406, to test whether CsA would augment the in vivo antitumor effects of INNO-406 in the CNS. Coadministration of P-gp inhibitors improves the delivery of imatinib mesylate to the CNS in mice.50 As we expected, both the radioactivity of [14C]INNO-406 and the concentration of INNO-406 in the brain increased 2-fold through administration of 50 mg CsA/kg (Figure 5A-B), and combined treatment with CsA and INNO-406 prolonged survival in a mouse model of CNS leukemia expressing not only wt but also mutated BCR-ABL (Figure 6E-F). However, lowering the plasma levels to raise the CNS levels through the use of CsA might possibly have a detrimental effect on the efficacy of INNO-406. The plasma levels of INNO-406 were reduced from 690 ng/mL to 440 ng/mL (1.31 μM) by the combined administration of 50 mg CsA/kg (Figure 5D). However, this reduction may not be critical because the reduced concentration (1.31 μM) is still much higher than the IC50 value of INNO-406 for most leukemic cells, including all mutation-bearing cells tested except T315I.31 In fact, combination with CsA did not reduce but augmented the effect of INNO-406 in a systemic Ph+ leukemia mouse model (Figure S3). The direct combined effects of CsA and INNO-406 against leukemic cells (Figure 1C) might have overcome any possible detrimental effect of lowering the plasma levels through the use of CsA.
This combination therapy could be easily introduced in clinical settings. A randomized controlled trial was performed to test the benefit of adding CsA to the chemotherapy protocol of patients with high-risk acute myeloid leukemia (AML), because overexpression of P-gp is often observed in patients with AML with advanced disease or poor prognosis.51-53 In the clinical study, CsA was administered by 72-hour continuous intravenous infusion at a dosage of 16 mg/kg per day,54 which is not very different from the dose used in the present study.
In conclusion, this study showed that the residual concentration of INNO-406 in the CNS is sufficient for inhibition of both wt and mutated ABL kinase in the CNS. Furthermore, CsA augmented the antitumor effects of INNO-406 against leukemic cells in CNS. A multicenter phase 1 clinical study on INNO-406 has been initiated in the United States in July 2006. The efficacy and safety of INNO-406 in the treatment of CNS Ph+ leukemias is expected to be verified by early-phase clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: A.Y. and S.M. performed the research and analyzed the data; S.K. designed and performed the research and wrote the paper; E.A., J.K., K.S., Y.K., E.K., Y.D., Y.U., Y.T., M.R., T.U., K.H., and K.I. performed research; and T.M. designed the research and wrote the paper.
We thank Gerald E. Smyth for advice on the preparation of this manuscript and Yoko Nakagawa, Tatsuya Oyama, and Asumi Jinno for their excellent technical support.
This work was supported by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Yasuda Medical Research Foundation; Japan Leukemia Research Fund; the Uehara Memorial Foundation; Grant-in-Aid of the Japan Medical Association; and the Sagawa Foundation for Cancer Research.

![Figure 1. INNO-406 is a substrate for P-gp. (A) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562 cells, (B) growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on P-gp–overexpressing K562/D1-9 cells. (C) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) CsA. (D) K562/D1-9 cells were treated with a combination of INNO-406 and 0 μM (•), 1 μM (▴), 2 μM (▪), 5 μM (○), or 10 μM (△) verapamil. Leukemic cell lines were treated with serial dilutions of either drug for 3 days, and the growth-inhibitory effect of the drugs was assessed by MTT assay. Intracellular concentrations of [14C]INNO-406 in LLC-PK1 cells (•) and P-gp–overexpressing LLC-GA5-COL150 cells (○) in the absence of CsA (E), or in the presence of 10 μM CsA (F). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-03-013250/4/m_zh80010706040001.jpeg?Expires=1770892137&Signature=q4DfA89znbZydO-2S2vHCiYrNhrytXj-V0F52iQJjRGawqba5u9b4p6cM8T80CQrvRP4zl8nXoS9EdrxXMSZbIdqsbj8h1uczU2JYnsPjPLzlA4G1zILgDsp3A5GN4GmBa3b8HowZjc8omQy30uAk2zKuHi4mh6X1ZI8jyMtOxHAYM0OhhF31jCNBee1XjisPYScEOvFNal5Z9PLHGNm83At7eLAuJ5bP768WTPQ6wk5pImgvuC-BsxCftBRDtllAk-U-jrCvAq8WOBXshJBocaCyt63-Z6kMEwEV4-ILU1PDVsi0ukGupuiu9jYHWVibCyFioVWZ03dkjuFwwWj4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Concentrations of INNO-406 in the brain. (A) Concentrations of [14C]INNO-406 and [14C]imatinib mesylate in the plasma (□) and brain (▪) in SD rats were measured 2 and 4 hours after administration of 10 mg/kg of either drug (n = 3 for both). (B) Concentrations of INNO-406 in the BALB/cA Jcl-nu mice were measured 0.5, 2, 4, 6, and 8 hours after administration of 30 mg INNO-406/kg. The data shown are representative of 3 independent experiments. (C) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on Ba/F3/wt bcr-ablGFP cells. (D) Growth-inhibitory effects of INNO-406 (○) and imatinib mesylate (•) on K562GFP cells. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-03-013250/4/m_zh80010706040002.jpeg?Expires=1770892137&Signature=toQX6HFPAWi23aPH-Q07gFc0~1H4GDlSLrWnvMe19FxfayQwIG1pFBnremFh3vtxQbRJSm4YQ~uF31qSeihPI4UVip5oVDIBmHjgYZ3brLbBkq8fhsOnGC4BUoaUznB1QqUDyIKxfrQ9SuuzT6u8XjrrJjfdTvrehe4MVVQk8f9BJYDc9V215sXrSeV0PJOyhv5vOfQTWQFzdbrnjLgnAdCvWbLEjtSBW~0qMWGyAgzY~DJb9cBfGQhOsDqllL~f2xqvVeE52JPWF0Gl1cREWWTWE39wXEgGiJfjR5Ghze4FIde~L7dRqf5~FN5xYBH1uz09jInKSO3RJuGuv0RRRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CsA increases the concentrations of INNO-406 in the CNS. Radioactivity (A) and concentration (B) of INNO-406 in the brains of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) prior administration of 50 mg CsA/kg. Radioactivity (C) and concentration (D) of INNO-406 in the plasma of mice that had been orally administered 30 mg [14C]INNO-406/kg with (○) or without (•) 50 mg CsA/kg. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/1/10.1182_blood-2006-03-013250/4/m_zh80010706040005.jpeg?Expires=1770892137&Signature=tKC3ZKh0f9U7DsRqHiN61TzbbXzdXXYObSQWUB8nNQuQ73rtEbqSHa8FhEUyfv6E6hq9YnhMSI4FxJiSMsQ1Kv2~fPWQH9xNy70RVVRhHCbgQZ1f6G4YqXHMlTr9PTpgUGizbMwZQ6an0UiDeu6Eu~XV9EXThZIWvvklnptUAOIFW0RY4prTdFy3iVAHMDPbVwhDQetqTvNONIteELZDoA-HskWi2HD6Wtxn8AubO~MKKWTD7LdgRuA4hU-ANeyhePJRMuj~PhRZ~w9nRhyS77G3YOWbQ7uHMJCsMFdggM2iMr9RsbrixGX2uk8vh20By6DmPCBcVgSefkiap8RPxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal