Abstract
Fetal and neonatal alloimmune thrombo cytopenia (FNAITP) is a life-threatening bleeding disorder caused by maternal antibodies directed against fetal platelet antigens. The immunoreactive epitopes in FNAITP are primarily located in the extracellular regions of the platelet glycoprotein IIIa (β3 integrin). Here we have established a novel animal model of FNAITP using β3 integrin–deficient (β3-/-) mice. We demonstrated first that these mice are immunoresponsive to β3 integrin; β3-/- mice transfused with wild-type platelets generated specific anti–β3 antibodies which were able to induce thrombocytopenia in wild-type mice. Subsequently, β3-/- female mice (both naive and immunized) were bred with wild-type male mice to recapitulate the features of FNAITP. The titer of generated maternal antibodies correlated with the severity of FNAITP. High titer maternal anti–β3 anti-bodies caused severe fetal thrombocytopenia, intracranial hemorrhage, and even miscarriage. Furthermore, maternal administration of intravenous immunoglobulin G (IgG) ameliorated FNAITP and down-regulated pathogenic antibodies in both the maternal and fetal circulations.
Introduction
Fetal and neonatal alloimmune thrombocytopenia (FNAITP) is an alloimmune disorder which results from maternal antibodies that cross the placenta, bind to fetal platelets, and mediate fetal platelet destruction. The frequency of FNAITP is estimated at 0.5 to 1.5 per 1000 liveborn neonates.1,2 The major risk of FNAITP is intracranial hemorrhage (ICH) with neurologic impairment or death. After birth, ICH occurs in 10% to 20% of neonates with FNAITP, and may be fatal in up to 5% of cases.3
There are at least 16 recognized human platelet antigens (HPAs), and immunoreactivity to the different HPAs can cause FNAITP.4 These antigens result from polymorphisms in the glycoproteins (GPs) on the platelet surface such as GPIaIIa (α2β1 integrin), GPIbα, and GPIIbIIIa (αIIbβ3 integrin). Amino acid sequences inherited from the father that differ from those of the mother may be targeted by the maternal immune system. Most cases of FNAITP are due to incompatibility in the amino acid sequence of the β3 integrin subunit. HPA-1a (polymorphism of residue 33 in the β3 subunit) is the most common antigen causing FNAITP in white newborns, accounting for 75% to 95% of clinical FNAITP cases.5 HPA-4a (polymorphism in residue 143 of the β3 subunit) is the most common antigen causing FNAITP in Asian newborns.6 In addition, incompatibility in residues 62, 140, 407, 489, 611, 633, and 636 of the β3 subunit has also been reported.4 Thus, a variety of alloantigens are located throughout the extracellular β3 integrin subunit and study of the immune response to the entire β3 integrin subunit is of importance to the understanding of FNAITP.
The process of the maternal immune response to fetal platelet antigens is largely unknown. The mechanism by which alloantibodies cross the placenta is also not fully understood, although the neonatal Fc receptor (FcRn) has been implicated as a receptor that mediates placental immunoglobulin G (IgG) transport and controls homeostasis of IgG levels in the circulation.7,8 Furthermore, although it has been hypothesized that the mechanism of platelet destruction may be similar to that of idiopathic thrombocytopenic purpura (ITP),9 the pathogenesis of thrombocytopenia in FNAITP has not yet been clearly established.
Effective therapy for FNAITP is currently limited. Compatible (antigen-negative) platelets for transfusion are often difficult to obtain on short notice. In contrast, intravenous IgG (IVIG) can be readily and quickly made available. IVIG is thus an attractive candidate for the treatment of FNAITP. While IVIG has been reported to alleviate FNAITP, the results from different investigators are conflicting and no randomized trials have been reported.1,10 The mechanism of action of IVIG in the treatment of FNAITP and ITP is under intensive study, but remains incompletely understood.11-13 Given the ethical difficulties in performing basic research on human fetuses and neonates with this life-threatening disorder, an animal model of FNAITP would be very useful to investigate the pathogenesis of the disorder and evaluate the efficacy and mechanism of action of IVIG in FNAITP.
In this study, we established a novel murine model of FNAITP that recapitulates features of the human pathologic condition, and demonstrated that maternal IVIG administration has a systemic effect on the amelioration of this disease.
Materials and methods
Mice
β3-/- mice were previously described14 and have been backcrossed onto a BALB/c background; control wild-type (WT) BALB/c mice (6 to 8 weeks of age) were purchased from Charles River Laboratories (Montreal, QC, Canada). All mice were housed in the St Michael's Hospital Research Vivarium and the experimental procedures were approved by the Animal Care Committee.
Reagents
IVIG and human albumin were obtained from Bayer Inc/Canadian Blood Services (Elkhart, IN). Alkaline phosphatase–conjugated anti–goat and anti–human IgG as well as anti–mouse polyvalent immunoglobulin and FITC-conjugated anti–mouse IgG, were purchased from Sigma (St Louis, MO). FITC-conjugated anti–mouse IgG1 and IgG2a as well as anti–human IgG were purchased from BD Biosciences (Mississauga, ON, Canada). Goat anti–human β3 integrin polyclonal antibody (sc-6627) and donkey anti–goat IgG alkaline phosphatase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rat anti–mouse αIIbβ3 integrin (JON2) and GPIbα (p0p3) monoclonal antibodies were kindly provided by Dr Nieswandt (Wurzburg, Germany). Bovine serum albumin (BSA), Tween-20, and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) were purchased from Sigma.
Induction and treatment of neonatal alloimmune thrombocytopenia
β3-/- female mice were immunized with WT mouse platelets in either 2 or 4 weekly transfusions (108 platelets/transfusion). After immunization, mice were bled via the saphenous vein and sera were collected to test anti–mouse β3 integrin IgG. The immunized female β3-/- mice were then bred with a WT male mouse. After delivery, platelet counts and bleeding disorders in pups, as well as IgG anti–mouse β3 integrin in both the mother and pups were analyzed to determine whether the pups exhibited FNAITP. We also set up breeding cages of WT × WT, β3-/- × β3-/- and naive β3-/- × WT mice as controls. For IVIG treatment, female β3-/- mice which demonstrated FNAITP in their first deliveries were injected intravenously with 300 μL of 10% IVIG (1 g/kg) each week after being bred with WT male mice. Human albumin (1 g/kg) was used as a control. To confirm the effect of IVIG, immunized β3-/- female mice were also treated with IVIG and albumin after breeding with WT male mice during their first pregnancies.
Detection of anti–mouse β3 integrin antibodies in immunized mice by flow cytometry
Blood samples were collected from saphenous veins of β3-/- mice which were immunized with WT platelets. Sera were prepared by centrifuging clotted whole blood at 13 400g for 5 minutes. IgG, IgG1, and IgG2a antibodies were detected using a 1:100 dilution of sera which was allowed to bind WT platelets at room temperature for 1 hour, centrifuged at 600g for 15 minutes, and washed with phosphate-buffered saline (PBS). The bound antibodies were detected with FITC-conjugated goat anti–mouse IgG, rat anti–mouse IgG1, and IgG2a, respectively, and analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The value for anti–platelet IgG level was calculated as a fold increase ratio. Fold = mean fluorescent intensity (MFI) of test serum/MFI of preimmune serum.
Immunoprecipitation
Designated primary antibody (4 μg of either a well-characterized monoclonal antibody to murine β3 integrin [JON2], or GPIbα [p0p3], or antisera from the immunized β3-/- mice) and 20 μL of Protein G Sepharose beads (Amersham Pharmacia Biotech, Baie d'Urfe, QC, Canada) were added to lysates from 108 platelets and mixed end-over-end at 4°C for 1 hour. Beads were pelleted at 2300g and washed 4 times with 800 μL of lysis buffer (0.5% NP-40, 50 mM Tris, 150 mM NaCl, 1 mM PMSF, 5 μg/mL leupeptin, and 1mM EDTA). Beads were then incubated in 60 μL of 2 × protein sample buffer (0.004% bromophenol blue, 50 mM Tris [pH 6.8], 1% SDS, and 5% sucrose) and boiled for 10 minutes. Beads were pelleted at 18 300g for 30 seconds, and the supernatant was loaded on a 7% denaturing SDS–polyacrylamide gel electrophoresis (PAGE). Subsequently, the proteins were transferred and Western blotted in the same manner as described in “Detection of anti–mouse β3 integrin antibodies by Western blotting.”
Detection of anti–mouse β3 integrin antibodies by Western blotting
Mouse platelets (2 × 106) were lysed, and the lysates from either WT or β3-/- mice were separated on a 10% SDS-PAGE gel under nonreducing conditions. After transfer to PVDF membrane (Hybond-P; Amersham Pharmacia Biotech), membrane was immunoblotted with either antisera from the immunized β3-/- female mice or sc-6627 goat anti–human β3 integrin antibody, which cross-reacts with murine β3 integrin, at room temperature overnight, and incubated with alkaline phosphatase–conjugated anti–mouse polyvalent immunoglobulin or anti–goat IgG. Immunoreactive bands were developed by reaction with substrate (BCIP/NBT).
Induction of thrombocytopenia with antisera from immunized β3-/- mice
WT BALB/c mice were bled via the saphenous vein and the initial platelet count was determined for each mouse and 100 μL antisera or their dilutions from immunized β3-/- mice (2- and 4-platelet transfusions) was injected via the tail vein on day 1. Platelet counts for individual mice were enumerated daily up to day 4.
Platelet enumeration
As previously described,11 whole blood (10 μL) was isolated from either adult mice (saphenous bleeding) or pups (carotid bleeding) and diluted into 990 μL of 1% EDTA in PBS. The blood was then further diluted in PBS to a final dilution of 1:12 000. A total of 20 to 30 μL of whole blood from the pups was collected after carotid bleeding for platelet counts and antibody detection. The samples were analyzed for 2 minutes on a flow rate–calibrated FACScan flow cytometer, using forward scatter (FSC) versus side scatter (SSC) to gate platelets. Reference samples were incubated with FITC-conjugated anti–mouse CD61 antibody to identify the platelet population.
Detection of free-circulating IgG against platelets and platelet-associated IgG in heterozygous pups
To detect platelet-associated IgG (PAIgG), platelets were prepared from 5 μL whole blood from heterozygous pups and stained directly with FITC-conjugated anti–mouse IgG for 30 minutes and analyzed with a FACScan flow cytometer. For the determination of free-circulating antiplatelet IgG, sera from heterozygous pups were incubated with 106 WT platelets at a 1:10 dilution for 1 hour, centrifuged at 600g for 15 minutes, and washed with PBS. Binding of IgG anti–mouse platelet β3 integrin was assessed as described in “Detection of anti–mouse β3 antibodies in immunized mice by flow cytometry.”
Detection of IVIG in maternal and neonatal circulations by ELISA
Sera from heterozygous pups or female β3-/- mice were serially diluted in PBS and coated (100 μL/well) on 96-well plates at 4°C overnight. Plates were then washed 3 times with 0.5% Tween-20 in PBS and blocked with 2% BSA in PBS for 3 hours. Plates were then washed with 0.5% Tween-20/PBS and incubated with 1:1000 alkaline phosphatase–conjugated goat anti–human IgG (γ-chain specific) for 30 minutes. The color was developed with p-nitrophenyl phosphate as substrate and the optical density at 405 nm (OD405) values were recorded on a multiwell plate reader.
Anti-idiotype activity of IVIG to the anti–mouse β3 integrin antibody
IVIG (10 μg/mL) was preincubated with antisera from the immunized β3-/- mice in test tubes at a ratio of 1:1 and incubated at 4°C overnight. Samples were diluted to a final dilution of 1:100 and then added to WT mouse platelets (106) for 1 hour at room temperature. Antisera and IVIG alone were used as positive and negative controls, respectively. The samples were centrifuged at 600g for 15 minutes, washed with PBS, incubated with FITC-conjugated goat anti–mouse IgG or FITC-conjugated monoclonal mouse anti–human IgG, and then assayed by a FACScan flow cytometer.
Statistical analysis
Data are presented as means ± SEM. Differences between 2 groups were assessed by Student unpaired t test or χ2 test as indicated.
Results
β3-/- mice are immunoresponsive to platelet β3 integrin
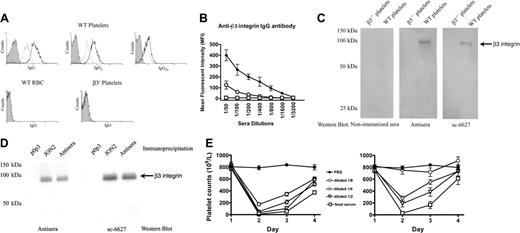
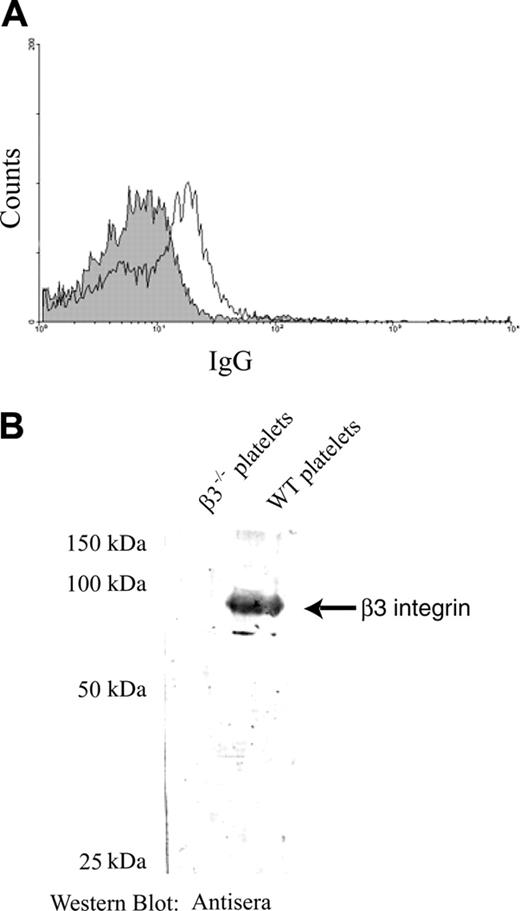
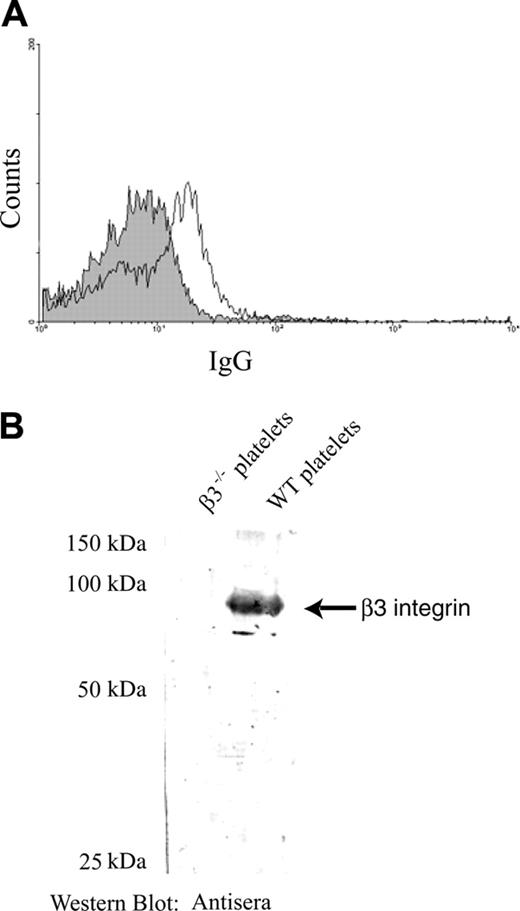
Immune responsiveness of β3-/- mice to platelet β3 integrin antigen is a prerequisite to establish a mouse model of FNAITP. To test this immune responsiveness, we first immunized β3-/- mice by weekly transfusions of 108 WT platelets. The mice were highly responsive to platelet β3 integrin antigen and antiplatelet antibody was detected after 2 weekly transfusions of WT platelets. Both IgG1 and IgG2a antibodies were detected (ie, both T-helper 1 [Th1] and Th2-like immune responses existed in β3-/- mice; Figure 1A). The antibodies were specific to β3 integrin since they did not recognize either platelets from β3-/- mice or WT red blood cells (Figure 1A). An increased titer of anti–β3 integrin IgG was found after 4 weekly transfusions of WT platelets (Figure 1B), and these antibodies were also detected by Western blot (Figure 1C). Immunoprecipitating platelet lysates with either these antibodies, the well-characterized rat monoclonal antibody JON2 (anti-mouse β3 integrin), or p0p3 (anti-mouse GPIbα control), and subsequent immunoblotting further confirmed the specificity of these antibodies (Figure 1D). Notably, the antibodies from the immunized mice induced thrombocytopenia when injected into WT BALB/c mice and a similar extent of thrombocytopenia was observed when high-titer antisera from 4-times-transfused mice was diluted to a titer comparable with that of the twice-transfused mice (Figure 1E).
Murine anti–β3 integrin antibodies were generated after immunizing β3-/- female mice with wild-type mouse platelets. (A) WT mouse platelets were incubated with a 1:100 dilution of sera from β3-/- mice immunized either 2 or 4 times weekly (dotted line indicates 2 times; bold line, 4 times) or preimmune sera (filled area) and stained with FITC-conjugated goat anti–mouse IgG or rat anti–mouse IgG1 or IgG2a. β3-/- platelets or WT red blood cells (RBCs) were used as negative controls. (B) Titration of IgG antibody in preimmune sera of β3-/- mice (□), and sera of mice after 2 (○) or 4 (♦) WT platelet transfusions. (C) Western blot of platelet lysates with both control anti–β3 integrin antibody sc-6627 and antisera of mice after 4 WT platelet transfusions. No β3 integrin band was recognized by sera from nonimmunized β3-/- mice in the negative control. (D) β3 integrin was immunoprecipitated from platelet lysates using either antisera from this study or the monoclonal antibody JON2 (anti-mouse β3 integrin) or p0p3 (anti-mouse GPIbα, negative control). The β3 integrin immunoreactive band was detected by both the antisera (left panel) and the positive control antibody sc-6627 (right panel) in immunoblot analysis. (E) Thrombocytopenia was induced in WT BALB/c mice by 100 μL antisera and their dilutions from the β3-/- mice transfused 2 times (right panel) or 4 times (left panel) with WT platelets. PBS was used as a control. n = 3 in each group. Data are represented as means ± SEM.
Murine anti–β3 integrin antibodies were generated after immunizing β3-/- female mice with wild-type mouse platelets. (A) WT mouse platelets were incubated with a 1:100 dilution of sera from β3-/- mice immunized either 2 or 4 times weekly (dotted line indicates 2 times; bold line, 4 times) or preimmune sera (filled area) and stained with FITC-conjugated goat anti–mouse IgG or rat anti–mouse IgG1 or IgG2a. β3-/- platelets or WT red blood cells (RBCs) were used as negative controls. (B) Titration of IgG antibody in preimmune sera of β3-/- mice (□), and sera of mice after 2 (○) or 4 (♦) WT platelet transfusions. (C) Western blot of platelet lysates with both control anti–β3 integrin antibody sc-6627 and antisera of mice after 4 WT platelet transfusions. No β3 integrin band was recognized by sera from nonimmunized β3-/- mice in the negative control. (D) β3 integrin was immunoprecipitated from platelet lysates using either antisera from this study or the monoclonal antibody JON2 (anti-mouse β3 integrin) or p0p3 (anti-mouse GPIbα, negative control). The β3 integrin immunoreactive band was detected by both the antisera (left panel) and the positive control antibody sc-6627 (right panel) in immunoblot analysis. (E) Thrombocytopenia was induced in WT BALB/c mice by 100 μL antisera and their dilutions from the β3-/- mice transfused 2 times (right panel) or 4 times (left panel) with WT platelets. PBS was used as a control. n = 3 in each group. Data are represented as means ± SEM.
Maternal anti–β3 integrin antibodies caused fetal and neonatal bleeding disorders and promoted fetal miscarriage
To establish a murine model of FNAITP, and to determine if a clinically relevant bleeding disorder could be observed during pregnancy and following delivery, naive female β3-/- mice (preimmunization) were bred with WT male mice. However, anti–β3 integrin IgG was not detectable in these β3-/- mice after the first and the second deliveries. Anti–β3 antibodies were detected in the mothers at low levels by a flow cytometric assay and Western blot in the third and subsequent deliveries (Figure 2A-B). Correspondingly, platelet counts in pups from the first 2 deliveries were not significantly decreased (Figure 3A), and bleeding disorders were not observed (Figure 3Bi). Since the female mice were “old” and generated fewer pups after 3 to 4 deliveries, it was difficult to compare the number of living pups and monitor their bleeding disorders with controls. Therefore, we used immunized female β3-/- mice in order to develop a mouse model for FNAITP in which high-titer anti–β3 antibodies were generated and clinical FNAITP indices were observed. This protocol may mimic those women who have preconceptional exposure to β3 integrin via either a previous pregnancy or exposure to sperm β3 antigen (“Discussion”).
Low-level antibody response in later pregnancies of naive female β3-/- mice after breeding 3 times with wild-type male mice. (A) Detection of antiplatelet IgG by flow cytometry. A 1:100 dilution of sera from the naive β3-/- mice was incubated with WT mouse platelets. Anti–β3 integrin antibodies were detected at very low levels in sera from the mice after 3 pregnancies (thin line) compared with those from mice before breeding (filled area) by a flow cytometric assay. (B) Western blot of platelet antigens with a 1:1000 dilution of sera from the naive β3-/- mice. β3 integrin was recognized by both the control antibody sc-6627 (data not shown) and the antisera from the naive β3-/- mice after 3 pregnancies.
Low-level antibody response in later pregnancies of naive female β3-/- mice after breeding 3 times with wild-type male mice. (A) Detection of antiplatelet IgG by flow cytometry. A 1:100 dilution of sera from the naive β3-/- mice was incubated with WT mouse platelets. Anti–β3 integrin antibodies were detected at very low levels in sera from the mice after 3 pregnancies (thin line) compared with those from mice before breeding (filled area) by a flow cytometric assay. (B) Western blot of platelet antigens with a 1:1000 dilution of sera from the naive β3-/- mice. β3 integrin was recognized by both the control antibody sc-6627 (data not shown) and the antisera from the naive β3-/- mice after 3 pregnancies.
Spontaneous hemorrhage and thrombocytopenia in heterozygous progeny of immunized β3-/- mothers. (A) Thrombocytopenia in heterozygous pups delivered from immunized β3-/- mothers (transfused twice with WT platelets) crossed with WT males. Pups from breeding cages of WT × WT, β3-/- × β3-/-, and naive female β3-/- × WT were used as a control. Data are presented as means ± SEM; n = 7-25 for each group. (B) Spontaneous bleeding in heterozygous pups delivered from immunized β3-/- mothers. (i) A heterozygous pup delivered from a naive β3-/- mother crossed with a WT male as a healthy control. (ii) Bleeding in live pups. (iii) Dead pups with massive ICH or abdominal bleeding. Bleeding is indicated by arrows. (C) Massive ICH and/or abdominal bleeding was found in fetuses in β3-/- mice immunized with 4 weekly transfusions of WT platelets. (i) ICH and abdominal bleeding. (ii) ICH.
Spontaneous hemorrhage and thrombocytopenia in heterozygous progeny of immunized β3-/- mothers. (A) Thrombocytopenia in heterozygous pups delivered from immunized β3-/- mothers (transfused twice with WT platelets) crossed with WT males. Pups from breeding cages of WT × WT, β3-/- × β3-/-, and naive female β3-/- × WT were used as a control. Data are presented as means ± SEM; n = 7-25 for each group. (B) Spontaneous bleeding in heterozygous pups delivered from immunized β3-/- mothers. (i) A heterozygous pup delivered from a naive β3-/- mother crossed with a WT male as a healthy control. (ii) Bleeding in live pups. (iii) Dead pups with massive ICH or abdominal bleeding. Bleeding is indicated by arrows. (C) Massive ICH and/or abdominal bleeding was found in fetuses in β3-/- mice immunized with 4 weekly transfusions of WT platelets. (i) ICH and abdominal bleeding. (ii) ICH.
We set up 8 breeding cages (1 female and 1 male mouse per cage) for WT males crossed with female β3-/- mice that had been immunized twice with WT platelets. The titer of antiplatelet IgG in these female mice was 1:800 (Figure 1B). We observed bleeding in some of the delivered pups (Figure 3Bii-iii); abdominal and skin bleeding as well as ICH were found in 7 of 54 live pups delivered from 6 female β3-/- mice at the first delivery (Table 1). The mortality rate was 24.1% (13 of 54 pups) due to internal organ bleeding, and 2 female β3-/- mice had miscarriages (Table 1). These findings were significantly different from those seen in the naive group (preimmunization) in both mortality rate (χ2 = 8.10, P < .005) and incidence of bleeding (χ2 = 7.89, P < .005).
Because the relationship between antibody titer and the severity of FNAITP is controversial,15 we further studied β3-/- female mice which were immunized 4 times with WT platelet transfusions. As shown in Figure 1B, the titer of antibody in these mice was approximately 4 times higher than that of the twice-immunized mice. After breeding with WT male mice, 1 of the 3 immunized female β3-/- mice died during delivery after a 3-week pregnancy. Immediate autopsy showed that 3 mature-sized and 3 smaller fetuses were present in utero. Severe bleeding including ICH, abdominal hemorrhage, or both, was found in the 3 mature-sized fetuses (Figure 3C). The other 2 pregnant female β3-/- mice had abortions or miscarriages after a 2- to 3-week pregnancy. No live pups were found and cannibalized remains of the neonates were present, which reflected a mortality rate that was significantly more severe than that of the twice-immunized mice (χ2 = 4.96, P < .05). We further examined the reactivity of antiplatelet antibodies (immediately after delivery) from the 4-time-immunized mice, and found that it was 3- to 4-fold higher than that of twice-immunized mice. Thus, the titer of maternal IgG against β3 integrin correlated with the severity of symptoms in this murine model (ie, 4-time-immunized mice > twice-immunized mice > naive mice). Since no living pups were delivered from the β -/- antibody, we used female β3-/- mice with high-titer mice transfused twice with WT platelets for most of the remaining experiments.
Maternal anti–β3 integrin antibodies caused fetal and neonatal thrombocytopenia
To test whether thrombocytopenia indeed occurred in neonates and contributed to the mentioned bleeding disorders in this murine model, we examined platelet counts in live heterozygous pups delivered from female β3-/- mice immunized twice with WT platelet transfusions. Platelet counts in the pups were significantly decreased (132.2 ± 10.5 × 109/L versus 618.3 ± 42.5 × 109/L in control mice; P < .001) (Figure 3A). We were not able to examine the platelet counts in the dead pups, although more severe thrombocytopenia may have been expected. The platelet counts of pups delivered from naive female β3-/- × WT male were similar to those of the pups delivered from WT × WT and β3-/- × β3-/- mice (Figure 3A). Thus, maternal antibodies of the pregnant β3-/- mice, and not the genotype difference, were responsible for the reduction of platelet numbers in the heterozygous pups.
Maternal anti–β3 integrin antibodies crossed the placenta and bound fetal and neonatal platelets
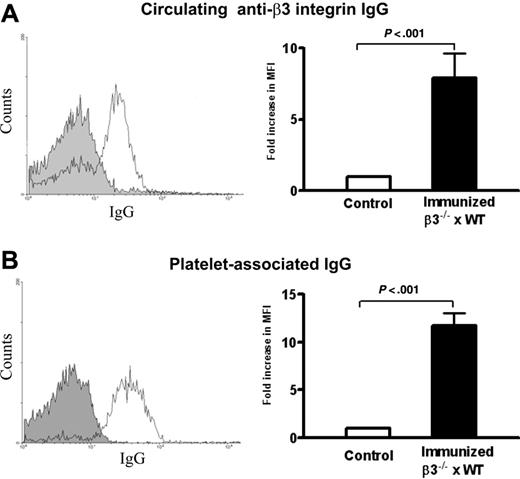
Maternal antiplatelet IgG is the cause of FNAITP in human patients. To test whether neonatal thrombocytopenia was induced by maternal anti–β3 integrin antibody in our mouse model, we examined circulating anti–β3 integrin IgG and PAIgG in heterozygous pups delivered from the immunized β3-/- mice. As expected, antiplatelet IgG (Figure 4A) and IgG2a (MFI = 5.13 ± 0.2 vs 3.77 ± 0.1 in controls; P < .005) were detected in the sera of live heterozygous pups. The level of antibody from pups was approximately 10% of maternal antibody, which is similar to the relative antibody levels reported in human cases.16 Increased PAIgG was found on the surface of platelets from live pups with either low platelet counts and/or bleeding disorders (Figure 4B). There was no antiplatelet antibody detected in the different control groups, including pups delivered from the first 2 naive β3-/- female × WT male litters. These results confirm that, in our murine model, maternal IgG crossed the placenta and bound to fetal platelets, concomitant with the clinical manifestations of FNAITP.
IVIG ameliorated platelet counts and bleeding disorders in the FNAITP murine model
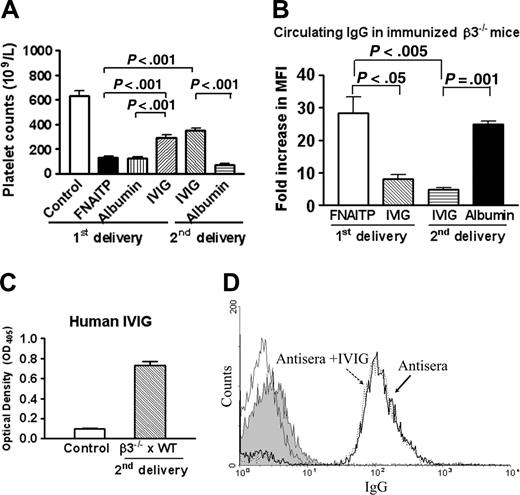
We then evaluated the therapeutic efficacy of IVIG in our murine model of FNAITP using β3-/- female mice that were transfused twice with WT platelets. These mice (which previously delivered FNAITP-affected neonates in the first pregnancy) were injected intravenously with IVIG once a week after being bred with WT male mice. As shown in Table 1, among the 36 pups which were delivered from 5 IVIG-treated female mice, 33 pups did not demonstrate any bleeding disorders, 2 pups had minor bleeding symptoms, and 1 pup was stillborn. The mortality rate was significantly decreased compared with the first litters that did not receive IVIG treatment (P < .05). The platelet counts of these pups were elevated from 132.2 ± 10.5 × 109/L to 353.3 ± 18.0 × 109/L (P < .001; Figure 5A). Notably, when albumin was used in place of IVIG treatment in the same setting (second delivery), neonatal platelet counts remained low (Figure 5A) and bleeding disorders were similar to those of the first litters (Table 1). To exclude the contribution of differing number of pregnancies in the IVIG-mediated amelioration, 2 female β3-/- mice immunized twice with WT platelets were injected intravenously with IVIG once a week during their first pregnancies. The platelet counts of the pups delivered from these female mice were also elevated to 292.5 ± 23.3 × 109/L (P < .001; Figure 5A) and bleeding disorders were attenuated (Table 1). Not surprisingly, bleeding disorders and no significant decrease in circulating antibody (data not shown) and no amelioration in platelet counts (Figure 5A) have been found in the control albumin group. Furthermore, we tested the effect of IVIG on 1 of the female β3-/- mice transfused 4 times with WT platelets that had miscarriages during its first pregnancy. The female mouse delivered 5 live pups without obvious bleeding disorders in its second delivery, although the platelet counts of these live pups were still low (198.6 ± 24.9 × 109/L). These results indicate that, in our mouse model, IVIG is able to: (1) ameliorate the reduction of platelet counts; (2) ameliorate FNAITP bleeding symptoms; and (3) reduce mortality and miscarriage.
Circulating IgG and platelet-associated IgG in heterozygous pups delivered from immunized β3-/- mothers. (A) Circulating IgG against mouse β3 integrin was detected in heterozygous pups (thin line) and pups of WT controls (filled area) by flow cytometry. (B) Platelet-associated IgG was detected in heterozygous pups (thin line) and pups of WT controls (filled area) by flow cytometry. Bar graphs represent means ± SEM fold increase of circulating IgG and PAIgG from heterozygous pups versus WT pups; n = 5-12.
Circulating IgG and platelet-associated IgG in heterozygous pups delivered from immunized β3-/- mothers. (A) Circulating IgG against mouse β3 integrin was detected in heterozygous pups (thin line) and pups of WT controls (filled area) by flow cytometry. (B) Platelet-associated IgG was detected in heterozygous pups (thin line) and pups of WT controls (filled area) by flow cytometry. Bar graphs represent means ± SEM fold increase of circulating IgG and PAIgG from heterozygous pups versus WT pups; n = 5-12.
Effect of IVIG on neonatal platelet counts and maternal IgG levels. (A) Platelet counts in pups from breeding cages of WT × WT and β3-/- × β3-/- mice as a normal control, immunized female β3-/- × WT mice (first delivery), immunized female β3-/- × WT mice (treated with IVIG or albumin in their first delivery), and immunized female β3-/- × WT mice (treated with IVIG or albumin in their second delivery). n = 12-22. (B) IVIG decreased anti–β3 integrin IgG in the maternal circulation. Sera of female β3-/- mice were incubated with 106 WT platelets at a final dilution of 1:100 for 1 hour. IgG anti–mouse β3 integrin was detected by a flow cytometric assay (n = 2-3). There was no significant difference in antibody level between the first delivery (untreated) and the second delivery (albumin-treated), and between the first delivery and second delivery after IVIG treatment. (C) IVIG was detected by enzyme-linked immunosorbent assay (ELISA) in the sera of pups delivered from the IVIG-treated mothers during their second pregnancy. Sera of pups from the first delivery (ie, before IVIG treatment) were used as a negative control. n = 3-5 mice. (D) Anti-idiotype activity of IVIG was not found. Preincubated sera from immunized pregnant β3-/- mice with IVIG (dashed line; MFI = 145.2 in 1:100 dilution) did not decrease antibody-platelet binding activity compared with sera alone (bold line; MFI = 152.0 in a 1:100 dilution). The thin solid line indicates IVIG alone incubated with mouse platelets. The filled area indicates that anti–human IgG-FITC did not bind to WT platelets incubated with IVIG plus antisera. Data are represented as means ± SEM.
Effect of IVIG on neonatal platelet counts and maternal IgG levels. (A) Platelet counts in pups from breeding cages of WT × WT and β3-/- × β3-/- mice as a normal control, immunized female β3-/- × WT mice (first delivery), immunized female β3-/- × WT mice (treated with IVIG or albumin in their first delivery), and immunized female β3-/- × WT mice (treated with IVIG or albumin in their second delivery). n = 12-22. (B) IVIG decreased anti–β3 integrin IgG in the maternal circulation. Sera of female β3-/- mice were incubated with 106 WT platelets at a final dilution of 1:100 for 1 hour. IgG anti–mouse β3 integrin was detected by a flow cytometric assay (n = 2-3). There was no significant difference in antibody level between the first delivery (untreated) and the second delivery (albumin-treated), and between the first delivery and second delivery after IVIG treatment. (C) IVIG was detected by enzyme-linked immunosorbent assay (ELISA) in the sera of pups delivered from the IVIG-treated mothers during their second pregnancy. Sera of pups from the first delivery (ie, before IVIG treatment) were used as a negative control. n = 3-5 mice. (D) Anti-idiotype activity of IVIG was not found. Preincubated sera from immunized pregnant β3-/- mice with IVIG (dashed line; MFI = 145.2 in 1:100 dilution) did not decrease antibody-platelet binding activity compared with sera alone (bold line; MFI = 152.0 in a 1:100 dilution). The thin solid line indicates IVIG alone incubated with mouse platelets. The filled area indicates that anti–human IgG-FITC did not bind to WT platelets incubated with IVIG plus antisera. Data are represented as means ± SEM.
IVIG decreased pathogenic antibodies in both the maternal and neonatal circulations
As shown in Figure 5B, weekly administration of IVIG significantly decreased the level of IgG anti–β3 integrin in the immunized β3-/- mothers during their second pregnancies (P < .005). The level of anti–β3 integrin antibody (MFI) was decreased from 28.3- to 4.6-fold relative to sera from the maternal circulation during their first pregnancies. Furthermore, neither circulating antibody nor PAIgG were detectable in the neonates (data not shown). A similar down-regulatory effect of IVIG on antibody levels was seen in immunized β3-/- mothers during their first pregnancies (P < .05). In control groups, which were treated with albumin, the level of maternal IgG anti–β3 integrin did not significantly decrease in the circulation (Figure 5B). We also found that IVIG administered to pregnant mothers was able to cross the placenta since it was detected in the sera of the heterozygous pups (Figure 5C).
To determine whether anti-idiotypic antibodies were present in IVIG and played a role in decreasing the titers and attenuating immunoreactivity of maternal IgG, sera from β3-/- mothers immunized 4 times were preincubated with IVIG before being incubated with WT platelets. No decrease of MFI was observed for maternal IgG binding to platelets in the presence of IVIG. There was also no IVIG binding to other portions (ie, agretope) of anti–β3 antibodies as determined by our flow-cytometric assay (Figure 5D). We also did not detect IVIG binding to the antibodies generated from β3-/- mothers that were immunized twice (data not shown). These results suggested that anti-idiotypic antibodies were not present in IVIG and may not be responsible for ameliorating FNAITP in this murine model.
Discussion
In the present study, we report the first animal model of FNAITP. Our results showed that murine antibodies against murine β3 integrin were generated in β3-/- β3-/- mice. Breeding immunized female mice with WT male mice reproduced the clinically relevant fetal bleeding disorders exhibited in human cases of FNAITP. This model demonstrates: (1) maternal antiplatelet antibodies correlate with the severity of the observed FNAITP phenotype; (2) maternal IVIG administration has multiple effects on the amelioration of this disorder, including decreased maternal antiplatelet antibody, depleted pathogenic antibody in the neonatal circulation, decreased fetal platelet clearance, reduced bleeding disorders, and increased fetal survival; and (3) the mechanism of action of IVIG is likely not due to an anti-idiotype antibody effect. To our knowledge, this is the first report that IVIG is able to decrease maternal and fetal pathogenic antibody levels during pregnancy.
In human patients, approximately 50% of FNAITP cases occur in the first pregnancy, and are apparent following delivery. It is therefore usually difficult to identify these FNAITP patients and to monitor their maternal immune responses during pregnancy. FNAITP is often complicated with severe bleeding disorders, including ICH and other internal organ hemorrhages. After diagnosis, immediate therapeutic action is required and it is ethically impossible to set an untreated control to investigate this life-threatening disease in the human population. Thus, an animal model is important to study the pathogenesis of FNAITP and to monitor potential therapeutic effects. While several models of other immune thrombocytopenia have been reported,17-19 no previous animal model of FNAITP exists.
We demonstrated that β3-/- mice were immunoresponsive against murine β3 integrin and both Th1- and Th2-associated IgG2a and IgG1 isotypes, respectively, were produced. Th1-like immune responses have been shown to affect the pathogenesis of ITP and its treatment.20,21 Although the relevance of both isotypes being produced in FNAITP is unknown, the presence of complement-fixing IgG2a may be of significance to the pathogenesis of FNAITP in that platelet destruction can occur by at least 2 different mechanisms (Fc-dependent phagocytosis and complement activation).
After demonstrating immune responsiveness to β3 integrin, we bred female naive β3-/- mice with WT male mice. In contrast to human FNAITP, we did not observe significant bleeding disorders following the first delivery, or even the second delivery. This difference between mice and humans may be due to the short period of pregnancy in mice; the time could be too short to generate a significant immune response, in particular during the physiologic immunosuppressive state of pregnancy.22 Another potential explanation is that many women may have been exposed to HPAs (eg, β3 integrin) prior to pregnancy. It has been reported that β3 integrin is expressed in sperm.23 Thus, it is possible that preconceptional intercourse may prime the human female immune system and subsequent exposure to fetal β3 integrin alloantigen during pregnancy may boost the immune response and result in an FNAITP phenotype. Our preliminary data that sera from some pregnant women with FNAITP indeed recognized a sperm antigen at the same molecular mass as β3 integrin (C.M.S. and H.N., unpublished data, October 2004) support this hypothesis. The β3-/- female mice immunized with WT platelet transfusions in this model may mimic such preconceptional exposure (or that of human mothers who have had previous pregnancies) in human FNAITP.
Our murine model was established by breeding β3-/- female mice with WT male mice. This situation, however, may differ from that of human patients. In human FNAITP cases, alloantibodies are mainly formed against the polymorphic structure of the β3 integrin chain, which might recognize different epitopes and therefore might have different properties compared with the antibodies developed in this animal model. In addition, although FcRn has been reported to be responsible for maternofetal IgG transfer in both human and rodents, transfer of IgG in humans shows increased specificity (with respect to murine transfer), and there is preferential transport of some isotypes over others.24 However, the process of immune responsiveness and the pathogenesis of FNAITP between human and mouse may be comparable. In fact, all clinical indices and symptoms were well reproduced in this model. We also found that maternal antibody titer correlated with the severity of bleeding disorders and that a high titer of anti–β3 integrin antibody may even induce miscarriage. The heterogeneity of the experimental picture in heterozygous pups is similar to that seen in humans. The sites of bleeding may depend on the site of trauma during pregnancy and delivery (eg, relative nonprotection of abdominal organs or the large size of the head). Also, we speculate that some anti–β3 antibodies may cross-react with endothelial cells and cause vessel injury, which may enhance the severity of FNAITP and affect the sites of bleeding.
The management of FNAITP is a challenge; currently, the most effective antenatal therapy is weekly in utero platelet transfusions using irradiated maternal/antigen-negative platelets. In addition to technical difficulties associated with this procedure, this invasive procedure may also cause bleeding, fetal trauma, and spontaneous abortion. However, IVIG has been used to treat ITP patients since 198125 and later to treat FNAITP.1,10 The proposed mechanisms of action of IVIG in ITP include: (1) reticuloendothelial system (RES) blockade;11 (2) anti-idiotypic antibody activity;26 (3) induction of T- and B-cell tolerance;27-29 (4) inhibition of dendritic-cell function;30 and (5) inhibition of macrophage phagocytosis via IVIG/Fcγ RIIB interaction.12,31 Recently, the role of FcRn in homeostasis of IgG has been highlighted.32 FcRn may protect IgG from proteolysis during transcytosis in epithelial and endothelial cells.33,34 Thus, IVIG may saturate FcRn and consequently promote the accelerated clearance of pathogenic IgG.35,36 In fact, it has been reported that IVIG may enhance the clearance of pathogenic IgG by this mechanism in both ITP37 and rheumatoid arthritis.7 However, the mechanism of action of IVIG in FNAITP remains to be elucidated. Although significant amelioration of FNAITP was observed in our model, it is unclear whether: (1) T- and B-cell tolerance is induced by IVIG in the maternal immune system, which decreases pathogenic IgG production; (2) enhancement of antibody proteolysis indeed occurs in pregnant mothers after IVIG administration; (3) IVIG saturates FcRn in the placenta and blocks maternal antibody transplacental transportation; (4) RES blockade also occurs in the fetus; (5) the improved prognosis of maternal—compared with in utero—IVIG administration in a previous case report38 was due to the synergistic actions of the described mechanisms; and finally (6) whether variation of the efficacy of IVIG treatment in human FNAITP results from different sources and dosages of IVIG39 used in different therapeutic regimens. Our murine model offers a convenient means of addressing these questions.
In summary, we have established an animal model of FNAITP that reproduced the symptoms of human FNAITP. We demonstrated that anti–β3 integrin antibody may cause abortion and miscarriage, and that maternal IVIG administration has a systemic effect on amelioration of this disease. Our data that IVIG can down-regulate pathogenic antibody in the maternal circulation may have broad implications for other mother–antifetal antigen–related diseases such as hemolytic disease caused by Rh antigen. This model will be important for further investigation of the maternal immune response and pathogenesis of FNAITP.
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-06-2562.
Supported in part by the start-up funds from Canadian Blood Services and St Michael's Hospital (H.N.); Dean's Fund of University of Toronto (H.N.); Bayer/Canadian Blood Services/Hema-Quebec/Canadian Institutes of Health Research Partnership Fund (H.N., principal investigator [PI]; and J.F., co-PI); and Canadian Institutes of Health Research grant no. 129403 (H.N., PI; and J.F., co-PI); National Institutes of Health grant PO1-HL66105 (R.O.H.). E.S. is a fellow of the Keenan Foundation at St Michael's Hospital.
H.N. designed the experiments, analyzed data, and wrote the manuscript; P.C. performed the research, analyzed data, and wrote the manuscript; C.M.S. performed the research, and edited the manuscript; E.S. performed the research, and analyzed data; J.W.S. analyzed data, and edited the manuscript; A.H.L. analyzed data, and edited the manuscript; R.O.H. provided vital reagents (β3-/- mice) and edited the manuscript; and J.F. provided analytic tools (flow cytometer), analyzed data, and edited the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Victor S. Blanchette and Dr Gregory A. Denomme for their advice during the experiments. Dr Bernhard Nieswandt provided anti–mouse GPIbα (p0p3) and anti–mouse αIIbβ3 integrin (JON2) monoclonal antibodies.