Abstract
Although cytomegalovirus (CMV) expresses proteins that interfere with antigen presentation by class I major histocompatibility complex (MHC) molecules, CD8+ cytotoxic T cells (CTLs) are indispensable for controlling infection and maintaining latency. Here, a cytokine flow cytometry assay that employs fibroblasts infected with a mutant strain of CMV (RV798), which is deleted of the 4 viral genes that are responsible for interfering with class I MHC presentation, was used to examine the frequency and specificity of the CD8+ CTLs to CMV in immunocompetent CMV-seropositive individuals. A large fraction of the CD8+ CTL response was found to be specific for viral antigens expressed during the immediate early and early phases of virus replication and presented by fibroblasts infected with RV798 but not wild-type CMV. These results demonstrate that the inhibition of class I antigen presentation observed in CMV-infected cells in vitro is not sufficient to prevent the induction of a broad repertoire of CD8+ CTLs after natural infection in vivo. Thus, reconstitution of T-cell immunity in immunodeficient patients by cell therapy or by vaccination may need to target multiple viral antigens to completely restore immunologic control of CMV.
Introduction
Human cytomegalovirus (CMV) infects one-half of the adult population and persists in the immunocompetent host as an asymptomatic latent infection. CMV causes severe disease in immunocompromised individuals including transplant recipients, individuals with human immunodeficiency virus infection, and congenitally infected infants.1-4 Studies in mice infected with murine CMV (MCMV), which shares biologic properties with human CMV, have identified a critical role for CD8+ T-cell immunity in preventing lethal infection, limiting the viral load in latency, and reestablishing latency after reactivation.5-7 A deficiency of functional CD8+ CMV-specific T cells also correlates with CMV disease in immunosuppressed patients.3,8,9 These observations have led to efforts to restore protective responses by adoptive T-cell immunotherapy.10-13
The design of protocols for reconstituting T-cell immunity in immunodeficient patients and the development of a vaccine to prevent congenital CMV infection would be facilitated by understanding the specificity of CD8+ T cells that contain CMV in the immunocompetent host. CD8+ T cells recognize peptides that are derived from intracellular proteins and displayed at the cell surface bound to class I major histocompatibility complex (MHC) molecules. The human CMV genome contains 230 kb of DNA, and infected cells express more than 160 viral proteins that could provide antigenic peptides for CD8+ T cells.14 However, the viral genome encodes 4 proteins (US2, US3, US6, and US11) that block the generation and/or export of class I MHC peptide complexes and induce a rapid decline in surface class I expression.15-18 Studies using fibroblasts permissively infected with wild-type CMV or with recombinant viruses encoding individual CMV proteins as antigen-presenting cells (APCs) have identified only a small number of CMV antigens recognized by CD8+ CTLs. These include the virion proteins pp65 and pp150, which are presented to CTLs shortly after viral entry without requiring de novo synthesis, and the major immediate early protein (IE-1), which is expressed at high levels in the first hours of infection.19-25 Class I HLA tetramers complexed to pp65 or IE-1 peptides, or staining of intracellular cytokines induced in T cells after peptide stimulation, have been employed to quantitate CD8+ T cells specific in the blood of CMV-seropositive individuals. A significant frequency (0.12% to > 5%) of CD8+ T cells were specific for pp65 and IE-1, which led to the assumption that they were immunodominant targets in humans and to their inclusion in subunit vaccines.20,21,25-29
The recognition of only a few viral antigens by the CTL response to human CMV has been attributed to interference with class I antigen presentation mediated by the viral US proteins.15-18,24 However, a variety of cell lineages may be infected with CMV, and it is unknown if the inhibition of antigen presentation observed in fibroblasts in vitro is equivalent in all infected cells in vivo.30,31 Studies of the specificity of the CD8+ CTL response induced in mice by experimental inoculation with murine CMV identified CTL responses to CMV antigens that are not presented by cells infected with wild-type CMV due to viral interference with class I antigen presentation.32-35 In humans, Elispot assays with synthetic peptides selected from 14 human CMV proteins identified in some donors a significant response to pp50 in addition to pp65 and IE-1, and a low frequency response to peptides from US2, US3, US6, US11, UL16, and UL18, which have not previously been identified as target antigens.36 Therefore, it is conceivable that major CD8+ CTL responses specific for CMV antigens may be present in vivo but have gone undetected using fibroblasts permissively infected with wild-type CMV or with vaccinia recombinant viruses encoding only a few selected CMV proteins as stimulator cells.
The ideal strategy for analyzing and quantitating the CD8+ CTL response to CMV would allow display of all potentially immunogenic viral peptides generated in infected cells. To overcome the limitations imposed by CMV proteins that interfere with antigen presentation, a strain of CMV (RV798) that has a deletion encompassing the 4 US genes but replicates as efficiently as AD16937 was used to infect APCs for analysis of the CD8+ CTLs by cytokine flow cytometry (CFC). The results show that this approach detects a much larger number of CD8+ CMV-specific T cells than stimulation with fibroblasts infected with AD169 or peptides from only a few CMV proteins. Indeed, a significant fraction of CD8+ CMV-specific CTLs in healthy CMV-seropositive donors recognize viral antigens other than pp65 and IE-1, which were perceived to be immunodominant targets for the CTL response. Thus, the CMV proteins that inhibit class I antigen presentation in infected cells in vitro do not prevent a broad CD8+ CTL response in vivo.
Materials and methods
Viruses and cell lines
AD169 CMV was obtained from the American Type Culture Collection, Manassas, VA, and the RV798 virus was generated as described.37 Vaccinia recombinant viruses encoding the CMV major immediate early protein (Vac/IE), pp65 (Vac/pp65), and gB (Vac/gB) under the control of the p7.5 promoter were kindly provided by William Britt, University of Alabama. A vaccinia recombinant virus encoding pp150 (Vac/pp150) was constructed as described.22
Dermal fibroblast lines were generated from skin biopsies and propagated in Waymouth media with 15% fetal calf serum (FCS), penicillin, and streptomycin. Epstein Barr virus (EBV)–transformed B lymphoblastoid cell lines (LCLs) were generated by coculturing peripheral blood mononuclear cells (PBMCs) with EBV supernatant with RPMI containing 10% FCS (Gemini, Woodland, CA) and 200 ng/mL of cyclosporine A. Following transformation, LCLs were propagated in RPMI, 10% FCS.
Approval was obtained from the Fred Hutchinson Cancer Research Center Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Cytokine flow cytometry assay
Blood samples were obtained from CMV-seropositive and CMV-seronegative donors and used in CFC assays either directly or after separation into PBMCs by Ficoll-Hypaque gradient centrifugation.38 Briefly, 1 mL of whole blood or 1 × 106 PBMCs was incubated at 37° C with 1 μg/mL anti-CD49d monoclonal antibody (mAb) (Becton Dickinson, San Jose, CA), 1 μg/mL anti-CD28 mAb (Becton Dickinson), and 5 × 105 autologous fibroblasts that were either mock-infected, infected with AD169 CMV for 24 hours, or infected with RV798 CMV for 48 hours at a multiplicity of infection (MOI) of 1. In some experiments, peptides (5 μg/mL) corresponding to pp65495-503, which binds to HLA A2, or pp65417-426 and pp65265-274, which bind to HLA B7, respectively, were used for stimulation by addition to 1 mL of whole blood or 1 × 106 PBMCs. After 2 hours of incubation at 37° C, brefeldin A (10 μg/mL; Sigma, St Louis, MO) was added to inhibit cytokine secretion, and the incubation continued for an additional 4 hours. Erythrocytes were lysed, and the remaining cells were permeabilized, stained with anti–CD8-Cy5 (Caltag, Burlingame, CA) and anti–interferon (IFN)γ–fluorescein isothiocyanate (FITC) (Becton Dickinson), and analyzed by flow cytometry (Becton Dickinson LSR or Calibur). Lymphocytes were gated by forward and side scatter, and IFNγ-producing cells were enumerated in the CD8+ population.
T-cell cloning
CD8+ T-cell clones were isolated from PBMCs of 5 donors by selecting CD8+ T cells that produced IFNγ after stimulation with CMV-infected APCs using an IFNγ secretion assay (Miltenyi, Bergisch Gladbach, Germany) and plating these cells into cloning wells. Briefly, autologous fibroblasts were infected with AD169 for 24 hours or with RV798 for 48 hours, trypsinized, and cocultured with PBMCs for 12 hours at 37° C. Cells were then washed in buffer (phosphate buffered saline [PBS], 2 mM EDTA [ethylenediaminetetraacetic acid], 0.5% bovine serum albumin [BSA]), resuspended in cold buffer containing IFNγ Catch Reagent (Miltenyi), and incubated for 5 minutes on ice. Warm RPMI supplemented with 10% human AB serum, 4 mM l-glutamine, penicillin, and streptomycin (CTL media) was added, and the cells were incubated for 45 minutes at 37° C under slow continuous rotation. The cells were then washed in cold buffer, resuspended in buffer containing phycoerythrin (PE)–conjugated IFNγ detection reagent (Miltenyi) and anti–CD8-Cy5 (Caltag), and incubated on ice for 10 minutes. Cells were washed twice, resuspended in cold CTL medium, and analyzed by flow cytometry. Double-positive cells (IFN-γ–PE/CD8-Cy5) were sorted and plated in 96-well round-bottom plates at 0.5 cells/well with 30 ng/mL anti-CD3 mAb (Ortho Biotech, Raritan, NJ), 7.5 × 104 γ-irradiated PBMCs, and 1 × 104 γ-irradiated LCL in CTL media with 50 U/mL rhIL-2. After 14 days, a 30-μL aliquot of cells from wells with visible growth were tested in a chromium release assay for recognition of autologous fibroblasts that were mock-infected or infected with RV798 for 48 hours. Clones that lysed RV798-infected fibroblasts (> 10%) but not mock-infected fibroblasts (> 1%) were expanded as described.12
Chromium release assay
CD8+ T-cell clones were assayed for cytotoxic activity in a chromium release assay (CRA) using 51Cr-labeled fibroblasts infected with AD169 for 24 hours or infected with RV798 for 48 hours as target cells.19 Target cells were plated in triplicate at 5 × 103 cells/well in 96-well round-bottom plates, and effector cells were added at various effector-to-target (E/T) ratios. After a 4-hour incubation, supernatant was harvested for gamma counting. In some experiments, the RNA polymerase inhibitor actinomycin D (Sigma) at 10 μg/mL or ganciclovir (Sigma) at 10 μM were added to target cells 30 minutes before infection. The metabolic inhibitor was maintained in the media throughout all washes and the 4-hour CRA.19 The ability of ganciclovir to block viral DNA replication was documented by the complete inhibition of virus production using standard plaque assays on human fibroblasts. The percent specific lysis was calculated using the standard formula. The HLA-restricting allele of individual CTL clones was determined using allogeneic, RV798-infected fibroblasts matched at a single HLA-A or -B allele as target cells.
Clones also were tested for lysis of autologous LCLs infected for 12 hours at an MOI of 10 with vaccinia recombinant viruses encoding individual CMV proteins, or pulsed with synthetic peptides synthesized by standard methods and corresponding to pp50tllncavtk and pp50tvrshcvsk that bind to HLA-A3, pp50yeqhkitsy that binds to HLA-B44, pp28llidptsgl that binds to HLA-A2, pp28arvyeikcr that binds to HLA-B27, and UL18tengsfvagy that binds to HLA-B44.36 A pp65-peptide panel consisting of 108 15-mer peptides that overlap by 5 amino acids also was used to pulse autologous EBV-LCL to screen for reactivity to pp65.
T-cell receptor Vβ gene usage
RNA was isolated from T-cell clones using RNeasy kit (Qiagen, Valencia, CA) and reverse-transcribed into cDNA using oligo (dT)15 primers (Invitrogen, Carlsbad, CA) and M-MLV reverse transcriptase (Invitrogen). The TCR Vβ gene usage of each T-cell clone was determined using a TCR Vβ region specific multiplex reverse transcriptase–polymerase chain reaction (RT-PCR) as described.39
Results
Quantitation of CD8+ CMV-specific T cells in blood by CFC
The frequency of T cells in the blood that are specific for peptides derived from pp65 or IE-1 has been measured by flow cytometry using class I HLA tetramers or staining with a mAb that detects intracellular IFNγ following peptide stimulation with individual peptides or peptide pools.25,26 We wished to develop a method for quantitating CD8+ CMV-specific T cells in healthy CMV-seropositive donors that were specific for any viral antigen displayed by the individual's class I alleles. Therefore, we evaluated whether CD8+ CMV-specific T cells in PBMCs could be quantitated by cytokine flow cytometry (CFC) after stimulation with autologous fibroblasts infected with AD169 CMV or with the RV798 strain that contains a deletion of the US region.37
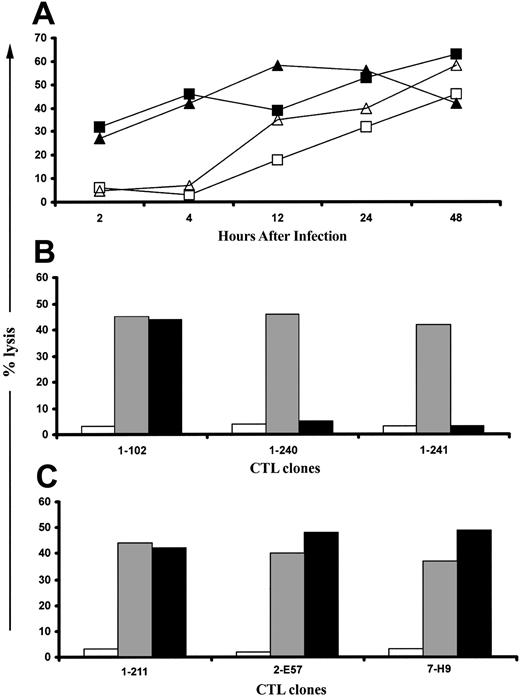
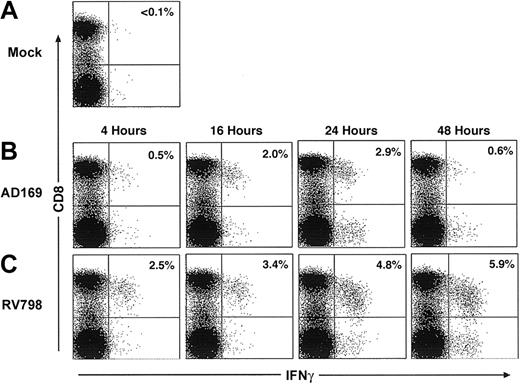
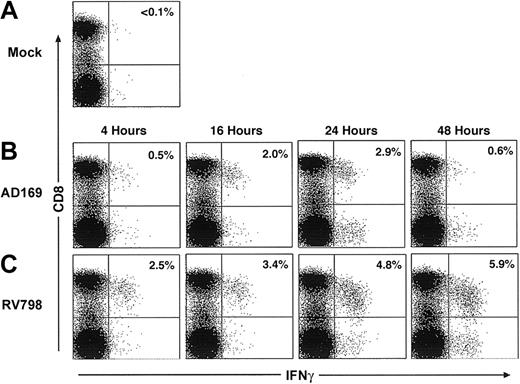
In initial experiments, fibroblasts were infected for various times with AD169 or RV798 for use in CFC. Fibroblasts infected with AD169 showed dramatically reduced levels of cell surface class I MHC expression by 24 hours after infection, whereas fibroblasts infected with RV798 maintained class I MHC expression even after 48 hours (data not shown). The frequency of CD8+ T cells in PBMCs that produced IFNγ after stimulation with autologous AD169-infected or RV798-infected fibroblasts was less than 0.1% in 3 CMV-seronegative donors (data not shown). However, a subset of CD8+ T cells from CMV-seropositive donors produced IFNγ after stimulation with AD169-infected fibroblasts but not mock-infected fibroblasts (Figure 1A), and cells infected with AD169 for 24 hours activated the largest number of CD8+ T cells (Figure 1B). Stimulation of PBMCs from the same CMV-seropositive donors with fibroblasts infected with RV798 induced IFNγ production in a larger fraction of CD8+ T cells, and the greatest response was elicited using fibroblasts infected for 48 hours (Figure 1C).
Autologous fibroblasts infected with AD169 or RV798 can be used as stimulator cells to quantitate CMV-specific CD8+ T cells by cytokine flow cytometry. Fibroblasts were infected with AD169 or RV798 for 4, 16, 24, and 48 hours and then used to stimulate autologous PBMCs or whole blood in a CFC assay. CD8+ T cells that produced IFNγ were identified within the lymphocyte gate (based on forward/side scatter) by 2-color staining with anti-CD8 and anti–IFNγ monoclonal antibodies. The data shown are the results for donor 1 and are representative of the results obtained in 5 donors. (A) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with mock-infected fibroblasts. (B) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with fibroblasts infected with AD169 for various times. (C) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with fibroblasts infected with RV798 for various times.
Autologous fibroblasts infected with AD169 or RV798 can be used as stimulator cells to quantitate CMV-specific CD8+ T cells by cytokine flow cytometry. Fibroblasts were infected with AD169 or RV798 for 4, 16, 24, and 48 hours and then used to stimulate autologous PBMCs or whole blood in a CFC assay. CD8+ T cells that produced IFNγ were identified within the lymphocyte gate (based on forward/side scatter) by 2-color staining with anti-CD8 and anti–IFNγ monoclonal antibodies. The data shown are the results for donor 1 and are representative of the results obtained in 5 donors. (A) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with mock-infected fibroblasts. (B) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with fibroblasts infected with AD169 for various times. (C) Frequency of CD8+ T cells induced to produce IFNγ after stimulation with fibroblasts infected with RV798 for various times.
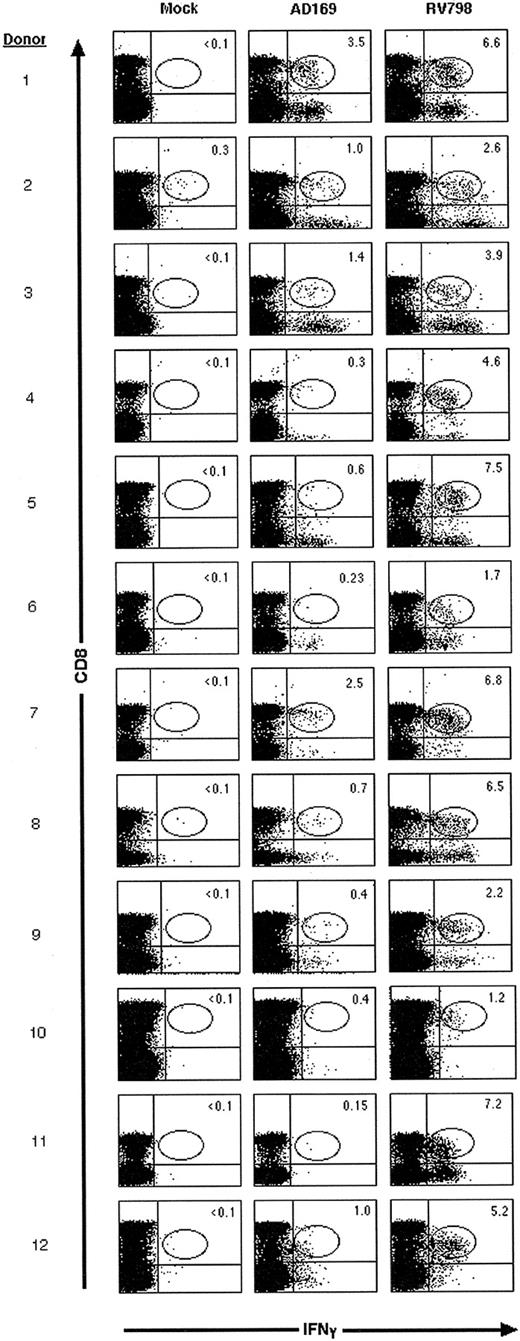
The maximal frequency of CD8+ T cells that responded to RV798- and AD169-infected fibroblasts was compared in 12 CMV-seropositive donors. Stimulation with RV798-infected fibroblasts induced IFNγ production in 1.2% to 7.5% (mean, 4.7%) of CD8+ T cells, whereas stimulation with AD169-infected fibroblasts induced IFNγ production in 0.15% to 3.5% (mean, 1.0%) (Figure 2). In each donor, 1.9- to 48-fold higher numbers of CMV-specific CD8+ T cells were detected using RV798-infected cells as stimulators. These results were consistent in 2 to 3 independent evaluations on blood samples obtained over 1 to 3 months (data not shown).
A higher frequency of CD8+ CMV-specific T cells is detected by CFC using RV798-infected fibroblasts as stimulator cells than with AD169-infected fibroblasts. The frequency of CD8+ T cells that produced IFNγ was determined in 12 donors by CFC after stimulation of PBMC with mock-, AD169-, or RV798-infected fibroblasts. Data shown are representative of at least 2 experiments per donor.
A higher frequency of CD8+ CMV-specific T cells is detected by CFC using RV798-infected fibroblasts as stimulator cells than with AD169-infected fibroblasts. The frequency of CD8+ T cells that produced IFNγ was determined in 12 donors by CFC after stimulation of PBMC with mock-, AD169-, or RV798-infected fibroblasts. Data shown are representative of at least 2 experiments per donor.
Of the 12 CMV seropositive donors, 10 expressed HLA A2 and/or B7, and in these individuals, the frequency of IFNγ-producing CD8+ T cells after stimulation with AD169-infected fibroblasts was compared with the frequency of T cells that responded to pp65 peptides presented by HLA A2 (pp65495-503) and/or HLA B7 (pp65417-426 and pp65265-274). In these experiments, 0.23% to 2.96% (mean = 1.04%) of CD8+ T cells produced IFNγ after stimulation with AD169-infected fibroblasts, and in each donor the response was approximately equal to the total frequency of CD8+ T cells that produced IFNγ after stimulation with the pp65 peptides (Table 1). In one donor (#9), CD8+ T cells that produced IFNγ after stimulation with AD169-infected fibroblasts were selected by flow cytometry and cloned by limiting dilution. All of the T-cell clones recognized autologous AD169-infected but not mock-infected fibroblasts, and more than 90% lysed autologous LCLs infected with vac/pp65 (data not shown). These results demonstrate that stimulation with AD169-infected cells predominantly detects CTLs specific for structural virion proteins such as pp65 and a lower frequency of CD8+ CMV-specific CTLs than stimulation with RV798-infected fibroblasts.
A significant proportion of CD8+ CMV-specific T cells are specific for novel CMV antigens that are not presented by AD169-infected cells
The higher frequency of CMV-specific CD8+ T cells detected after stimulation with RV798-infected fibroblasts could be due to detection of T cells responding to additional epitopes of pp65 or specific for other defined CMV antigens such as IE-1, which are not efficiently presented by AD169-infected fibroblasts.25,28,40,41 To further analyze the antigen specificity of CD8+ T cells activated by stimulation with RV798-infected fibroblasts, these cells were sorted by flow cytometry from 5 of the 12 donors and cloned with αCD3 mAb.24 This approach for cloning T cells directly from PBMCs avoided any potential bias in antigen specificity introduced by prolonged culture with virus-infected APCs. An aliquot of each T-cell clone was screened for recognition of autologous mock-infected and RV798-infected fibroblasts to identify those that were CMV specific. A total of 1505 clones were screened from the 5 donors, and 1072 (71%) exhibited lysis of RV798-infected fibroblasts but not mock-infected fibroblasts.
A subset of at least 50 of the T-cell clones that lysed RV798-infected fibroblasts was randomly selected from each of the 5 donors, expanded, and evaluated for phenotype and for recognition of AD169, RV798, and mock-infected autologous and allogeneic class I MHC–mismatched fibroblasts. All of the clones were CD3+, CD8+, and CD4– and lysed autologous RV798-infected fibroblasts but not mock-infected fibroblasts or allogeneic class I MHC mismatched RV798-infected fibroblasts (Table 2). However, only a fraction (17%-63%) of the clones from each donor lysed AD169-infected fibroblasts (Table 2).
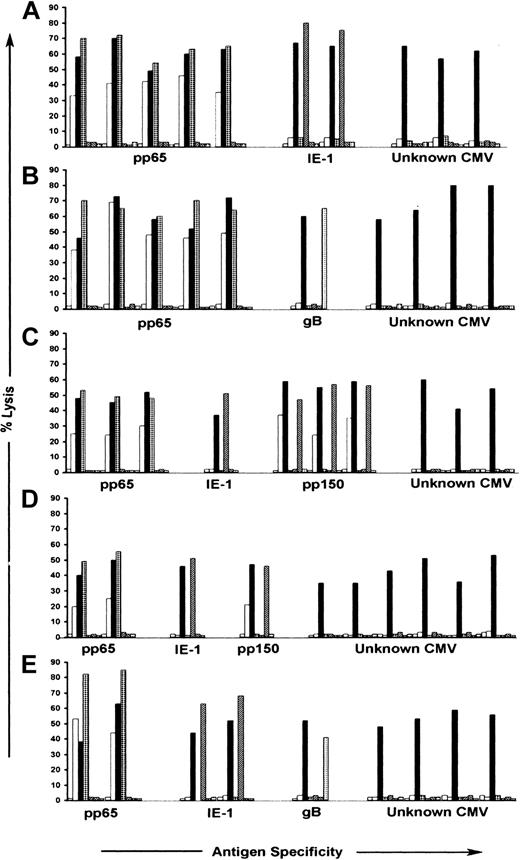
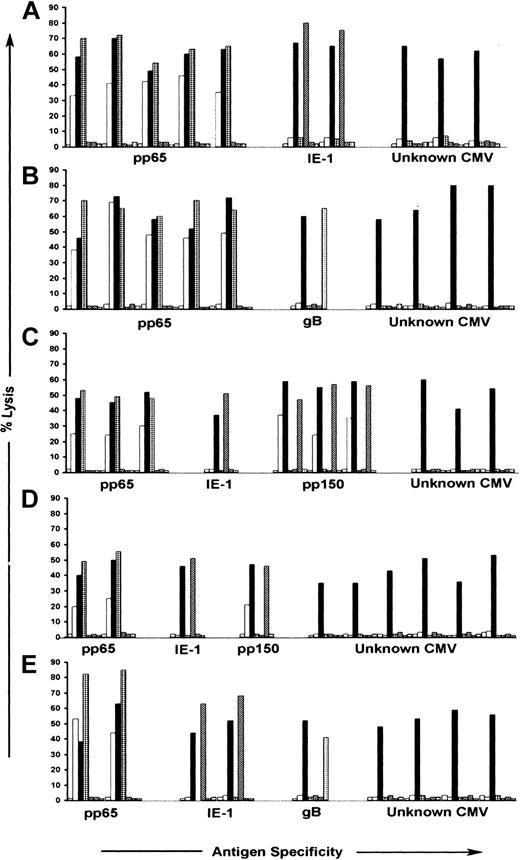
We next evaluated recognition by the CTL clones of autologous B-LCLs infected with vaccinia recombinant viruses encoding CMV IE-1, pp65, gB, and pp150, and B-LCLs pulsed with synthetic peptides derived from pp28, pp50, and UL18.21,25,29,36,40 CTLs specific for pp65 were identified in the panels of clones from all 5 donors and comprised between 14% and 53% of the total number of clones obtained from each donor. CTLs specific for pp150 were identified in the panels of clones from 2 donors and comprised 3%-30% of the clones from these donors (Table 2). The pp65- and pp150-specific CTLs efficiently lysed both AD169- and RV798-infected autologous fibroblasts (Figure 3). CTL clones specific for IE-1 were isolated from 4 donors and comprised between 3% and 28% of the total number of T-cell clones analyzed from each donor, whereas CTL clones specific for gB were isolated from 2 donors and represented fewer than 5% of the response in each donor (Table 2). The IE-1- and gB-specific CTLs failed to lyse AD169-infected fibroblasts, demonstrating that a component of the response detected with RV798-infected fibroblasts in some donors was specific for these previously defined CMV antigens (Figure 3). However, a significant proportion (29% to 80%) of the CD8+ CTL clones isolated from all 5 donors was not specific for any CMV antigens encoded by the panel of recombinant vaccinia viruses, and these CTLs lysed only RV798- and not AD169-infected target cells (Table 2; Figure 3). None of the T-cell clones isolated from donors that expressed HLA A2, A3, B27, and B44 in our study lysed autologous LCLs pulsed with peptides corresponding to sequences of pp50, pp28, or UL18 epitopes previously shown to stimulate interferon release from PBMCs of CMV-seropositive donors with these HLA alleles36 (data not shown). Thus, the repertoire of CMV-specific CTLs in healthy CMV-seropositive individuals with protective immunity includes a large population of CD8+ T lymphocytes specific for novel CMV antigens.
Antigen recognition by CMV-specific CD8+ CTL clones isolated following stimulation with autologous fibroblasts infected with RV798. CMV-specific CD8+ CTL clones were tested for recognition of autologous fibroblasts either mock-infected ( ), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in Table 2. The data are shown at an E/T ratio of 10:1. (A) Donor 1; (B) donor 2; (C) donor 3; (D) donor 5; (E) donor 7.
), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in Table 2. The data are shown at an E/T ratio of 10:1. (A) Donor 1; (B) donor 2; (C) donor 3; (D) donor 5; (E) donor 7.
Antigen recognition by CMV-specific CD8+ CTL clones isolated following stimulation with autologous fibroblasts infected with RV798. CMV-specific CD8+ CTL clones were tested for recognition of autologous fibroblasts either mock-infected ( ), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in Table 2. The data are shown at an E/T ratio of 10:1. (A) Donor 1; (B) donor 2; (C) donor 3; (D) donor 5; (E) donor 7.
), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in Table 2. The data are shown at an E/T ratio of 10:1. (A) Donor 1; (B) donor 2; (C) donor 3; (D) donor 5; (E) donor 7.
The CMV antigens recognized by CD8+ CTLs are encoded by IE and E genes
CMV expresses its genes in 3 overlapping phases termed immediate early (IE; 1-6 hours after infection), early (E; 4-18 hours after infection), and late (L; > 12 hours after infection). Fifty-eight CD8+ CTL clones specific for undefined CMV antigens were evaluated for the ability to lyse fibroblasts infected with RV798 for 2, 4, 8, 12, 24, or 48 hours. Of 58 CTL clones from the 5 donors, 47 efficiently lysed fibroblasts infected with RV798 for only 2 hours, whereas infection of targets for longer than 8 hours was required for recognition by the remaining 11 clones (Figure 4A). CTLs that were specific for IE-1 based on recognition of vac/IE-1–infected target cells were included in these experiments as a control. These CTLs failed to lyse AD169-infected fibroblasts but lysed fibroblasts infected with RV798 for more than 4 hours (Figure 4A).
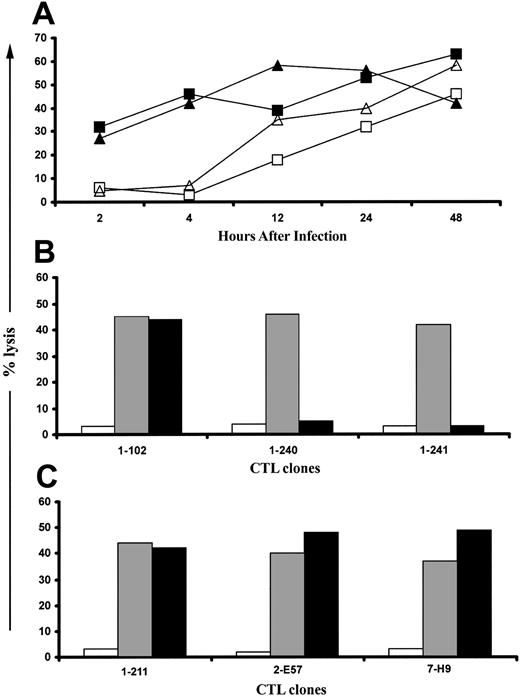
Novel CMV antigens presented to CD8+ CTLs by RV798-infected fibroblasts are predominantly encoded by IE and E genes. (A) Fifty-eight CTL clones that were specific for undefined CMV antigens were tested for their ability to lyse autologous fibroblasts infected for various durations with RV798. Data are shown for 2 CTL clones, clone 1-240 (▪) and clone 1-241 (▴), that exhibited a lytic pattern representative of 47 of the 58 clones and for one CTL clone, clone 2-B23 (▵), that exhibited a lytic pattern representative of the remaining 11 clones. A CTL clone specific for IE-1, clone 1-189 (□), is included as a control. (B) Presentation of novel CMV antigens by RV798-infected fibroblasts requires de novo gene expression. Forty-seven CTL clones that recognized targets as early as 2 hours after infection with RV798 were tested for their ability to lyse autologous fibroblasts either mock-infected (□), infected with RV798 for 4 hours (▦), or infected with RV798 for 4 hours in the presence of actinomycin D (▪). Representative data are shown for 3 CTL clones (clone 1-102 is pp65-specific; clone 1-240 and 1-241 are specific for unknown CMV antigens). (C) Eleven CTL clones that only recognized targets infected for longer than 8 hours were tested for their ability to lyse autologous fibroblasts either mock-infected (□) or infected with RV798 for 48 hours (▦), or infected with RV798 for 48 hours in the presence of ganciclovir (▪) at 10 μM to abrogate L gene expression. Representative data for 3 of 11 CTL clones from 3 different donors is shown at an E/T of 10:1.
Novel CMV antigens presented to CD8+ CTLs by RV798-infected fibroblasts are predominantly encoded by IE and E genes. (A) Fifty-eight CTL clones that were specific for undefined CMV antigens were tested for their ability to lyse autologous fibroblasts infected for various durations with RV798. Data are shown for 2 CTL clones, clone 1-240 (▪) and clone 1-241 (▴), that exhibited a lytic pattern representative of 47 of the 58 clones and for one CTL clone, clone 2-B23 (▵), that exhibited a lytic pattern representative of the remaining 11 clones. A CTL clone specific for IE-1, clone 1-189 (□), is included as a control. (B) Presentation of novel CMV antigens by RV798-infected fibroblasts requires de novo gene expression. Forty-seven CTL clones that recognized targets as early as 2 hours after infection with RV798 were tested for their ability to lyse autologous fibroblasts either mock-infected (□), infected with RV798 for 4 hours (▦), or infected with RV798 for 4 hours in the presence of actinomycin D (▪). Representative data are shown for 3 CTL clones (clone 1-102 is pp65-specific; clone 1-240 and 1-241 are specific for unknown CMV antigens). (C) Eleven CTL clones that only recognized targets infected for longer than 8 hours were tested for their ability to lyse autologous fibroblasts either mock-infected (□) or infected with RV798 for 48 hours (▦), or infected with RV798 for 48 hours in the presence of ganciclovir (▪) at 10 μM to abrogate L gene expression. Representative data for 3 of 11 CTL clones from 3 different donors is shown at an E/T of 10:1.
The lysis of target cells infected for 2 hours could be due to the presentation of input virion proteins other than pp65 or pp150 or to the recognition of newly synthesized IE or E viral proteins. To distinguish these possibilities, fibroblasts were treated with actinomycin D to block de novo viral gene expression prior to infection with RV798 and during the cytotoxicity assay. Actinomycin D treatment of RV798-infected fibroblasts completely abrogated recognition by all of the 47 CTL clones specific for unknown CMV antigens but did not block recognition by pp65-specific CTLs (Figure 4B). These results suggest that proteins encoded by IE genes or by RNA molecules packaged with the virion are targets for these CMV-specific CTLs.42
The viral antigens presented more than 8 hours after infection could be encoded by E or L genes, since the onset of viral DNA replication, which defines the L phase, can begin by 12 hours after infection. Target cells that express only IE and E genes were prepared by pretreatment with ganciclovir, which blocks DNA replication, and tested for recognition by the 11 CTL clones that only lysed fibroblasts infected with RV798 for more than 8 hours. The efficacy of ganciclovir was confirmed by assaying supernatants obtained 48 to 108 hours after infection from ganciclovir-treated and control RV798-infected cultures by plaque assay.37 Ganciclovir completely inhibited virus production (data not shown) but did not interfere with recognition by any of the 11 CTL clones (Figure 4C). Collectively, these data show a significant component of the CD8+ CTL response to CMV is specific for viral antigens encoded by IE or E genes, which are not presented in fibroblasts infected with wild-type CMV due to expression of the US proteins.
Analysis of HLA-restricting alleles and T-cell Vβ receptors reveals a diverse CD8+ CTL response to CMV
The class I HLA–presenting molecule was determined for 74 of the CD8+ CTL clones obtained from 3 of the 5 donors by assessing recognition of RV798-infected fibroblasts from unrelated individuals that were matched at only a single class I allele with the donor. In each of these donors, at least 2 of the 4 class I HLA A and B molecules presented CMV antigens to a subset of the T-cell clones (Table 3). In donors who expressed both HLA A2 and B7 alleles, the frequency of pp65-specific CTLs that were restricted by HLA-B7 was higher than the frequency restricted by HLA-A2, consistent with prior work.43 The Vβ T-cell receptor gene family used by each of the 74 CD8+ CTL clones was assessed by a multiplex polymerase chain reaction that allowed for the determination of Vβ gene usage in 5 separate reactions.39 IE-1 and pp65-specific T-cell clones were highly restricted in their Vβ gene expression. In contrast, the CTLs of unknown CMV antigen specificity employed a number of distinct TCR Vβ receptors (Table 3). For example, the 8 CTL clones from donor 1 that recognized undefined CMV antigens presented by HLA B7 expressed a total of 5 different Vβ T-cell receptors. This analysis of HLA-restricting alleles and T-cell receptor diversity likely underestimates the complexity of the CD8+ T-cell response to CMV since less than 20% of the total number of CMV-specific T-cell clones obtained from each donor were analyzed. These findings indicate that the CMV-specific CTL repertoire is comprised of a large number of individual clones, although this does not necessarily imply an equivalent number of antigen specificities.
Discussion
Individuals with impaired CD8+ CMV-specific T-cell immunity are at increased risk for CMV reactivation and disease, pointing to a crucial contribution of this T-cell subset for limiting viral infection.3,8,9,11 The protective role of CD8+ T cells in CMV is surprising, because the virus expresses 4 glycoproteins that profoundly interfere with the presentation of viral antigens in infected fibroblasts in vitro.37 US3 is expressed rapidly but transiently after infection and retains class I molecules in the endoplasmic reticulum (ER).16 US2 and US11 are expressed in the E phase and cause retrograde translocation of class I molecules from the ER to the cytosol, where they are degraded.15,18 Finally, US6 is expressed in the L phase and blocks transporter associated with antigen processing (TAP)–mediated peptide transport into the ER.17,44 The sequential expression of the US proteins is thought to allow CMV-infected cells to efficiently evade T-cell recognition.
Analysis of the specificity of the CTL response to CMV using APCs infected with AD169 CMV or with recombinant vaccinia viruses encoding individual CMV proteins identified only a few viral target antigens that were less affected by the viral US proteins.19,21,23,25,41 The virion protein pp65, which is presented by infected cells without de novo viral gene expression, and IE-1, which is abundantly synthesized immediately after infection, were identified as major targets for the CTL response.19,25 However, control of persistent CMV infection by a CTL response focused on only a few viral proteins might be tenuous, since the virus could escape immune control by mutation of only a few epitopes.
A recent study by Elkington et al36 used synthetic peptides, selected based on computer prediction algorithms and HLA binding assays from 14 CMV proteins, for stimulation in an interferon gamma Elispot assay and identified T-cell responses specific for CMV antigens other than pp65 and IE-1. Significant responses were detected in some donors to pp50, with additional low-frequency responses to gB, gH, pp28, UL18, and the 4 US proteins.36 Here, the CD8+ CTL response to CMV in the blood of 12 healthy CMV-seropositive donors was evaluated using APCs infected with the RV798 strain of CMV, which is deleted of the US genes and does not down-regulate class I MHC. This approach is not limited to a preselected group of peptides but instead uses infected cells that were capable of presenting peptides derived from any CMV protein except those encoded by the US genes that are deleted in the RV798 strain. The frequency of CD8+ CTLs was measured using CFC to detect T cells that produced IFNγ after stimulation with autologous CMV-infected fibroblasts. In all donors, the frequency of CD8+ T cells that responded to RV798-infected fibroblasts was significantly greater (1.9- to 48-fold) than the frequency that responded to AD169-infected fibroblasts. This was largely due to the presence of CTLs specific for undefined CMV antigens, since a significant fraction (29%-80%) of the CTL response in each donor did not recognize target cells infected with vaccinia recombinant viruses encoding pp65, IE-1, gB, or pp150, or pulsed with immunogenic peptides from pp50, pp28, and UL18. The use of metabolic inhibitors to arrest virus replication in infected cells suggested IE or E genes encoded the unknown CMV antigens. Our results were not obtained due to the selection of donors that lacked HLA alleles known to present pp65 or IE-1 epitopes. Of the 12 donors, including the 5 donors from whom T-cell clones were isolated, 10 expressed the HLA A2 and/or HLA B7 molecules for which immunodominant epitopes in pp65 and IE-1 have been defined. Furthermore, CD8+ CTLs specific for pp65 were detected in these donors at a frequency similar to that observed in prior studies.21,25 Ultimately, the definitive designation of new CTL specificity requires the identification of the peptide epitope and the protein from which it is derived, and these studies are now in progress.
A major question raised by these results is how CD8+ CTLs specific for CMV antigens that are not presented by permissively infected fibroblasts in vitro are activated and maintained at such high frequencies in vivo. Human CMV replicates in a variety of lineages including endothelium, epithelium, smooth muscle, hematopoietic cells, and neuronal cells and can enter latency in a subset of these cells.30,45,46 It is unknown if the US proteins impair antigen presentation in all cells in vivo as efficiently as in fibroblasts in vitro. In mice, the presentation of the MCMV IE peptide pp89 to CD8+ CTLs is blocked in the E phase in fibroblasts but not in macrophages.47 However, we have been unable to definitively demonstrate recognition of macrophages infected with clinical isolates of CMV by human IE-1–specific CTLs or CTLs specific for other IE and E antigens.
The induction of CD8+ CTLs specific for antigens that are not presented by permissively infected cells could also be a consequence of cross-presentation, in which uninfected dendritic cells or macrophages acquire CMV antigens from infected cells and present them to T cells.48,49 Cross-presentation of CMV antigens to CTLs has been demonstrated in vitro and suggested as a mode the virus uses to thwart immune recognition by inducing CTL responses that are unable to recognize cells actively replicating CMV.50 Studies in murine CMV have demonstrated that CD8+ CTLs specific for the M45 antigen, which is not presented by cells infected with wild-type virus in vitro due to the m152 immune evasion protein, are elicited in vivo after infection with wild-type virus, and this has been suggested to occur by cross-presentation.32 In preliminary studies, we found the human CMV-specific CTL clones isolated here able to recognize uninfected dendritic cells (DCs) that were cocultured with allogeneic AD169-infected fibroblasts (T.J.M. and S.R.R., unpublished data, February 2002). However, the relevance of in vitro experiments is unclear, and there are arguments against cross-presentation being the sole mechanism for eliciting such CTLs in vivo. First, our data show the CTL response recognizes predominantly IE and E viral antigens, and at the present time there is no reason to believe L proteins would be precluded from cross-presentation. Indeed, it might be anticipated that infected cells would be more efficiently phagocytosed at L stages of replication, potentially favoring activation of T cells specific for L antigens. Second, the CTL response specific for antigens presented only by RV798 CMV is quantitatively large, representing up to 7.4% of CD8+ T-cell numbers in some individuals. The maintenance of a CTL response at this level by cross-presentation would require that reactivation provides sufficient antigen to maintain these CTLs. Such a mechanism is difficult to reconcile with the infrequent detection of replicating virus in immunocompetent CMV-seropositive individuals.
There are other possibilities to explain the presence of a large CTL response to antigens that are not efficiently presented by permissively infected cells in vitro. Perhaps the kinetics of the expression of virus immune evasion genes differ in cell types in vivo or in cells reactivating from latency, allowing additional antigens to be presented and recognized by the CTL response. Our data suggest that the sequential expression of CMV immune evasion proteins in fibroblasts is important for subversion of the CD8+ CTL response. The presentation of IE-1 to CD8+ CTLs was shown to be inhibited in infected fibroblasts by virion pp65.41 The studies here using infection with RV798, which contains pp65 but lacks the US genes that inhibit class I, demonstrate that the inhibition by pp65 must be transient, since IE-1–specific CTLs lyse RV798-infected targets if the infection proceeds beyond 4 hours. The failure of IE-1–specific CTLs to recognize AD169-infected fibroblasts at any stage of infection suggests that the expression of the US genes cooperates with pp65 to completely prevent IE-1 presentation. Thus, alteration in the kinetics of US gene expression in some cell types could permit presentation of additional CMV antigens.
The discovery of a population of CD8+ CTLs specific for as-yet-undefined CMV antigens has implications for immunotherapy to control CMV in immunodeficient individuals and for vaccination to prevent infection. The adoptive transfer of CD8+ T cells specific primarily for pp65 has safely restored T-cell responses to CMV in hematopoietic stem cell transplant recipients.12,13 Preliminary studies suggest that this approach prevents CMV disease in susceptible patients, but reactivation of virus measured by detection of CMV antigen in neutrophils or by culture may still occur.12 This suggests that the immunologic control of CMV exhibited in healthy individuals may not be completely restored by adoptive transfer of a limited repertoire of virus-specific CTLs.
Immunization of women prior to childbearing could potentially reduce morbidity from congenital CMV infection.51 Subunit vaccines incorporating only pp65, IE-1, and gB have been developed and elicit both T-cell and antibody responses.52,53 Studies to identify the viral genes that encode additional CMV antigens recognized by CD8+ CTLs and to elucidate their role in protective immunity should provide insights into the necessity to incorporate additional determinants in a CMV vaccine.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2003-06-1937.
Supported by National Institutes of Health grants CA18029 (S.R.R.), AI053193 (S.R.R.), and AI41754 (S.R.R.), and by the Westlund Supporting Organization (T.J.M.) and the Child Health Research Center (T.J.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



 ), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in
), infected with AD169 (□), or infected with RV798 (▪), in a chromium release assay. Autologous LCL infected with Vac/pp65 (▦), Vac/IE-1 (▨), Vac/pp150 (▧), and Vac/gB (▦) were used as target cells in the same assay to confirm specificity. Data are shown for 10 representative CTL clones from each of the 5 donors in proportions selected based on the relative frequency of antigen specificities shown in