Abstract
High-dose therapy has become a common treatment for myeloma. The objective of this study (Intergroupe Francophone du Myélome [IFM] 9502 trial) was to compare in a prospective and randomized trial the 2 most widely used conditioning regimens before autologous stem cell transplantation in newly diagnosed symptomatic patients younger than 65 years old: 8 Gy total body irradiation plus 140 mg/m2 melphalan (arm A) versus 200 mg/m2melphalan (arm B). A total of 282 evaluable patients were compared—140 in arm A and 142 in arm B. Baseline characteristics and disease response to 4 cycles of the VAD regimen performed before randomization and autologous stem cell transplantation were identical in the 2 treatment arms. In arm B, hematologic recovery was significantly faster for both the duration of neutropenia and thrombocytopenia, transfusion requirements were also significantly lower, and the median duration of hospitalization was significantly shorter. In arm A, the incidence of severe mucositis was significantly increased. The median duration of event-free survival was similar in both arms (21 vs 20.5 months, P = .6), but the 45-month survival was 65.8% in arm B versus 45.5% in arm A (P = .05). This difference might be attributed in part to better salvage regimens after relapse in arm B compared with arm A. We conclude that 200 mg/m2 melphalan is a less toxic and at least as effective conditioning regimen when compared with 8 Gy total body irradiation with 140 mg/m2 melphalan. This regimen should be considered as the standard of care before autologous stem cell transplantation in multiple myeloma.
Introduction
The outcome of autografted patients for newly diagnosed multiple myeloma (MM) is superior to that of patients receiving conventional chemotherapy.1-3 The Intergroupe Francophone du Myélome (IFM) 90 trial, a French trial that has prospectively compared conventional chemotherapy with high-dose therapy (HDT), has shown that patients treated in the intensive (HDT) arm had significantly improved response rates, event-free survival (EFS), and overall survival (OS).1 In this trial, the conditioning regimen consisted of 8 Gy total body irradiation (TBI) plus 140 mg/m2 intravenous melphalan (HDM140). The complete response (CR) rate after intensive therapy was 22%, and the probability of EFS for 5 years after the diagnosis was only 28%. No plateau of the survival curves was observed. However, the presence of a response to HDT was related to survival, and the 5-year probability of survival after diagnosis was 72% among patients who had complete or very good partial responses (VGPRs). To improve survival, the objective is to increase the CR rates.
A total of 200 mg/m2 melphalan (HDM200) without TBI is a valuable alternative and is usually the highest dose of melphalan infused before autologous stem cell transplantation (ASCT).2-4 The London team has shown impressive results with HDM200 in an open series of 53 patients, with 40 patients (75%) achieving a CR following HDT, and an estimated probability of survival at 54 months of 63%.4 In the tandem transplantation program developed by the Little Rock group, the CR rate after the first ASCT prepared by HDM200 was 26%.5
Other studies have tried to compare conditioning regimens, but most of them are from international6 or national7 data registries and not from prospective randomized trials. Until now, no specifically designed prospective study has evaluated the impact of 2 conditioning regimens, and the optimal one is still unknown. Thus, in 1995 we initiated a multicenter randomized clinical trial comparing 8 Gy TBI + HDM140 versus HDM200 (IFM 9502 trial) followed by ASCT. We present the results of this trial in 399 previously untreated patients.
Patients, materials, and methods
Patients
Eligibility criteria included age between 60 and 65 years and symptomatic MM. Patients were excluded if they had the following: (1) stable stage I MM (Durie-Salmon classification); (2) previous cytotoxic chemotherapy or radiotherapy; (3) severe abnormalities of cardiac, pulmonary, and hepatic functions; or (4) serum creatinine level above 500 μM. All patients gave informed consent, and the study was approved by the institutional ethics committee of the University Hospital of Nantes. After the completion of the IFM 94 trial,8 patients under the age of 60 were also included once the protocol had been amended in May 1997. Because there was no significant difference between the patients under 60 years of age and the patients 60 to 65 years of age regarding the CR rate, the EFS, and OS after HDT, they were pooled for the comparison of conditioning regimens.
Study design
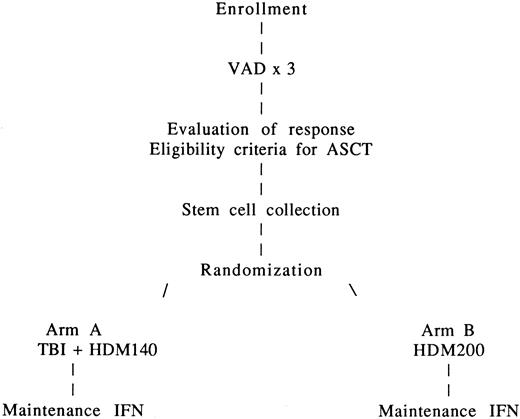
The treatment plan is outlined in Figure1. After enrollment, patients received 3 cycles of the vincristine-adriamycin-dexamethasone (VAD) regimen because of its marked and speedy tumor cell reduction without induction of hematopoietic stem cell compromise.
In the absence of tumor progression (> 25% increase in tumor mass) and if cardiopulmonary, hepatic, and renal functions remained adequate after the third course of VAD, peripheral blood stem cells (PBSCs) were collected. Serum creatinine had to improve to less than 150 μM after the third course of VAD to undergo stem cell collection. Stem cells were collected after granulocyte colony-stimulating factor (G-CSF) priming (10 μg/kg/d for 6 days) in steady-state or during recovery from short-term aplasia induced by cyclophosphamide 4 g/m2followed by G-CSF or G-CSF plus stem cell factor.9 Daily apheresis was continued until at least 2 × 106 CD34 cells per kilogram were collected. No CD34+ selection was performed. Because there was no significant difference between the patients mobilized with cyclophosphamide plus G-CSF before ASCT and those mobilized with G-CSF alone regarding the CR rate, EFS, and OS after HDT, they were pooled for the comparison of conditioning regimens.
After PBSC collection, patients received a fourth course of VAD and were then randomly assigned to one of the 2 HDT groups. Randomization was stratified according to the center and was carried out by telephone and fax.
In arm A, patients received 8 Gy TBI delivered in 4 fractions over a 4-day period (days −6, −5, −4, −3) without lung shielding plus HDM140 administered on day −2 by infusion over 30 minutes. In arm B, HDM200 was administered on day −2 by infusion over 30 minutes. PBSC transplantation was carried out on day 0. Hematopoietic growth factor support with granulocyte-macrophage CSF (GM-CSF) was recommended from day 7 after transplantation until granulocyte recovery. HDT was performed in protected units.
After completion of HDT, treatment with recombinant interferon-α (3 × 106 U subcutaneously thrice weekly) was given in both arms to all patients when granulocytes exceeded 1500/μL and platelets exceeded 100 × 109/L (100 000/μL) during the first year after transplantation. Interferon was discontinued at the physician's discretion, in case of disease progression, or when it induced severe and persistent adverse effects.
Response criteria
A complete remission (CR) was defined as the absence of a monoclonal gammopathy in serum and urine on immunofixation analysis and 5% or fewer plasma cells with normal morphologic features on bone marrow aspiration. A VGPR was defined as a decrease of 90% in the serum paraprotein level; partial response (PR) as a decrease of 50% in the serum paraprotein level and a decrease of 90% in Bence Jones protein; stable disease as no change or less than 50% decrease in the paraprotein level; progressive disease as an increase of 25% in the serum paraprotein level observed after 3 cycles of VAD; and a relapse as the reappearance of the paraprotein, the recurrence of bone marrow infiltration in a patient with a CR, or both, and a 25% increase in the paraprotein above the “plateau” level in 2 samples obtained 4 weeks apart in a patient with a response.
Statistical analysis
The main end point was CR rate after ASCT. The random assignment of at least 140 patients to each treatment group was needed to ensure a 5% level of significance and a power of 80% if the true probabilities of CR after HDT were 25% in arm A1 and 40% in arm B.4 Secondary end points were OS and EFS. Data were analyzed as of December 2000. The proportions of patients with a given characteristic were compared by the χ2 test or by the Fisher exact test. Differences in the means of continuous measurements were tested by the Student t test and checked by the Mann-Whitney U test. All tests were 2-tailed. The duration of EFS was calculated from the date of transplantation until the time of progression of disease, relapse, death, or the date the patient was last known to be in remission. The duration of OS was calculated from the date of transplantation until the time of death. Curves for EFS and OS were plotted according to the method of Kaplan and Meier and were compared by the log-rank test.
Results
From July 1995 to January 1999, 399 patients from 42 centers met eligibility criteria and received at least one course of VAD. A total of 101 (25.3%) enrolled patients did not proceed to randomization because of severe complications or disease progression during the cycles of VAD regimen. After randomization, 16 patients (4%, 8 in arm A and 8 in arm B) did not proceed to HDT because of disease progression, protocol violation, or infectious death before ASCT and were considered inevaluable (Table 1). Thus, 282 patients (70.7%) were evaluable: 140 patients in arm A and 142 patients in arm B were treated according to the whole protocol.
Patient flow, IFM 9502 trial
| No. of patients enrolled | 399 (100%) |
| Exclusion during VAD regimen | 101 (25.3%) |
| Progressive disease | 39 |
| Severe infectious complications | 29 |
| Cardiac failure | 9 |
| Patient's decision | 8 |
| Protocol violation | 5 |
| Renal failure | 5 |
| Toxic death | 5 |
| Suicide | 1 |
| No. of patients randomized | 298 (74.7%) |
| No. of patients inevaluable for HDT | 16 (4%) |
| Progression before ASCT | 7 |
| Patient's decision | 4 |
| Infectious death before ASCT | 2 |
| Protocol violation | 2 |
| Suicide | 1 |
| No. of patients evaluable | 282 (70.7%) |
| Arm A: TBI + HDM140 | 140 |
| Arm B: HDM200 | 142 |
| No. of patients enrolled | 399 (100%) |
| Exclusion during VAD regimen | 101 (25.3%) |
| Progressive disease | 39 |
| Severe infectious complications | 29 |
| Cardiac failure | 9 |
| Patient's decision | 8 |
| Protocol violation | 5 |
| Renal failure | 5 |
| Toxic death | 5 |
| Suicide | 1 |
| No. of patients randomized | 298 (74.7%) |
| No. of patients inevaluable for HDT | 16 (4%) |
| Progression before ASCT | 7 |
| Patient's decision | 4 |
| Infectious death before ASCT | 2 |
| Protocol violation | 2 |
| Suicide | 1 |
| No. of patients evaluable | 282 (70.7%) |
| Arm A: TBI + HDM140 | 140 |
| Arm B: HDM200 | 142 |
Characteristics of evaluable patients
Baseline characteristics were identical in the 2 treatment arms (Table 2). The median number of courses of VAD regimen, disease response to VAD, and modalities of stem cell collection were comparable in the 2 arms (Table3). Only 5 of 140 grafts in arm A and 4 of 142 in arm B contained fewer than 2 × 106 CD34 cells per kilogram (P = .98), and the median number of CD34 cells infused was significantly lower in patients treated with HDM200 as compared with those treated with TBI/HDM140 (Table 3).
Main characteristics at diagnosis of the 282 evaluable patients according to treatment group
| . | Arm A TBI + HDM140 (n = 140) . | Arm B HDM 200 (n = 142) . | P . |
|---|---|---|---|
| Age | 60 (36-65) | 61 (42-65) | .16 |
| Male gender | 58 (41) | 65 (46) | .61 |
| Durie-Salmon stage | |||
| I | 10 (7) | 8 (6) | .78 |
| II | 20 (14) | 27 (19) | .37 |
| III | 110 (79) | 107 (75) | .62 |
| M component | |||
| IgG | 80 (57) | 79 (56) | .89 |
| IgA | 36 (26) | 37 (26) | .94 |
| Bence Jones | 24 (17) | 26 (18) | .92 |
| Hemoglobin (g/dL) | 10.9 (5.4-16.8) | 11.1 (5.6-15.2) | .3 |
| Serum calcium (mM) | 2.4 (2-4) | 2.4 (1.9-3.5) | .51 |
| Serum creatinine (μM/L) | 95 (59-395) | 88 (50-306) | .24 |
| Serum β2-microglobulin (mg/L) | 3.1 (1.1-16.2) | 3.2 (1-20) | .46 |
| C-reactive protein (mg/L) | 5 (1.7-200) | 5 (1-75) | .96 |
| . | Arm A TBI + HDM140 (n = 140) . | Arm B HDM 200 (n = 142) . | P . |
|---|---|---|---|
| Age | 60 (36-65) | 61 (42-65) | .16 |
| Male gender | 58 (41) | 65 (46) | .61 |
| Durie-Salmon stage | |||
| I | 10 (7) | 8 (6) | .78 |
| II | 20 (14) | 27 (19) | .37 |
| III | 110 (79) | 107 (75) | .62 |
| M component | |||
| IgG | 80 (57) | 79 (56) | .89 |
| IgA | 36 (26) | 37 (26) | .94 |
| Bence Jones | 24 (17) | 26 (18) | .92 |
| Hemoglobin (g/dL) | 10.9 (5.4-16.8) | 11.1 (5.6-15.2) | .3 |
| Serum calcium (mM) | 2.4 (2-4) | 2.4 (1.9-3.5) | .51 |
| Serum creatinine (μM/L) | 95 (59-395) | 88 (50-306) | .24 |
| Serum β2-microglobulin (mg/L) | 3.1 (1.1-16.2) | 3.2 (1-20) | .46 |
| C-reactive protein (mg/L) | 5 (1.7-200) | 5 (1-75) | .96 |
Median values are given for continuous variables, while the number of patients (%) is given otherwise.
Response to VAD regimen and stem cell collection
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| Median no. of courses of VAD (range) | 4 (3-6) | 4 (3-6) | .11 |
| Response to VAD regimen | |||
| CR (%) | 8 (6) | 7 (5) | .97 |
| VGPR (%) | 18 (13) | 17 (12) | .96 |
| PR (%) | 81 (58) | 86 (61) | .73 |
| Stable disease (%) | 33 (23) | 32 (22) | .95 |
| Stem cell collection | |||
| G-CSF priming (%) | 107 (76) | 107 (75) | .94 |
| Stem cell factor + G-CSF (%) | 5 (4) | 6 (4) | .98 |
| Cyclophosphamide + G-CSF (%) | 28 (20) | 29 (21) | .95 |
| Median no. of CD34 (106/kg) infused (range) | 7.3 (0.7-164) | 5 (1.2-132) | .03 |
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| Median no. of courses of VAD (range) | 4 (3-6) | 4 (3-6) | .11 |
| Response to VAD regimen | |||
| CR (%) | 8 (6) | 7 (5) | .97 |
| VGPR (%) | 18 (13) | 17 (12) | .96 |
| PR (%) | 81 (58) | 86 (61) | .73 |
| Stable disease (%) | 33 (23) | 32 (22) | .95 |
| Stem cell collection | |||
| G-CSF priming (%) | 107 (76) | 107 (75) | .94 |
| Stem cell factor + G-CSF (%) | 5 (4) | 6 (4) | .98 |
| Cyclophosphamide + G-CSF (%) | 28 (20) | 29 (21) | .95 |
| Median no. of CD34 (106/kg) infused (range) | 7.3 (0.7-164) | 5 (1.2-132) | .03 |
Engraftment and treatment-related toxicity analysis
Table 4 shows engraftment and treatment-related toxicity analysis. The use of hematopoietic growth factors after ASCT was equivalent in both groups. Hematologic recovery was significantly faster for the duration of both neutropenia and thrombocytopenia in the HDM200 arm. Transfusion requirements were also significantly lower in the HDM200 arm for both red blood cells and platelets. Overall, the median duration of hospitalization (considered from the day of melphalan infusion to discharge) was significantly shorter in arm B as compared with arm A.
Engraftment, hospitalization time, and transplantation-related toxicity
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| G/GM-CSF (%) | 111 (79) | 107 (75) | .52 |
| Duration of G/GM-CSF, d (median) | 0-25 (8) | 0-23 (7) | .37 |
| Duration of neutropenia, d (median) | 4-34 (10) | 4-34 (8) | < .001 |
| Duration of thrombocytopenia, d (median) | 0-110 (7) | 0-30 (7) | < .001 |
| No. of platelet transfusions (median) | 0-30 (2) | 0-18 (1) | < .001 |
| No. of red blood cell transfusions (median) | 2 (0-22) | 2 (0-9) | < .001 |
| Duration of hospitalization, d (median) | 12-77 (23) | 11-47 (19) | < .001 |
| Duration of intravenous antibiotics, d (median) | 0-60 (11) | 0-30 (8) | < .001 |
| Cardiac toxicity grade 3-4 (%) | 5 (3.6) | 1 (0.7) | .21 |
| Mucositis grade 3-4 (%) | 71 (51) | 42 (30) | < .001 |
| Pulmonary toxicity grade 3-4 (%) | 9 (6.4) | 2 (1.4) | .06 |
| Renal toxicity grade 3-4 (%) | 5 (3.6) | 3 (2.1) | .7 |
| Liver toxicity grade 3-4 (%) | 2 (1.4) | 1 (0.7) | .99 |
| Toxic death (%) | 5 (3.6) | 0 | .07 |
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| G/GM-CSF (%) | 111 (79) | 107 (75) | .52 |
| Duration of G/GM-CSF, d (median) | 0-25 (8) | 0-23 (7) | .37 |
| Duration of neutropenia, d (median) | 4-34 (10) | 4-34 (8) | < .001 |
| Duration of thrombocytopenia, d (median) | 0-110 (7) | 0-30 (7) | < .001 |
| No. of platelet transfusions (median) | 0-30 (2) | 0-18 (1) | < .001 |
| No. of red blood cell transfusions (median) | 2 (0-22) | 2 (0-9) | < .001 |
| Duration of hospitalization, d (median) | 12-77 (23) | 11-47 (19) | < .001 |
| Duration of intravenous antibiotics, d (median) | 0-60 (11) | 0-30 (8) | < .001 |
| Cardiac toxicity grade 3-4 (%) | 5 (3.6) | 1 (0.7) | .21 |
| Mucositis grade 3-4 (%) | 71 (51) | 42 (30) | < .001 |
| Pulmonary toxicity grade 3-4 (%) | 9 (6.4) | 2 (1.4) | .06 |
| Renal toxicity grade 3-4 (%) | 5 (3.6) | 3 (2.1) | .7 |
| Liver toxicity grade 3-4 (%) | 2 (1.4) | 1 (0.7) | .99 |
| Toxic death (%) | 5 (3.6) | 0 | .07 |
The duration of intravenous antibiotics was significantly shorter in patients treated with HDM alone. Regarding cardiac, renal function, and liver enzymes tests, there were no differences in grade 3-4 toxicities (World Health Organization scale) between the 2 arms. Pulmonary toxicity was slightly increased in the TBI arm. The main difference between the 2 arms was the incidence of severe mucositis, which was significantly higher in the TBI arm. One venoocclusive disease was documented in arm A (TBI/HDM140) and 0 in arm B (HDM200). Five toxic deaths (3.6%) were observed in the TBI arm, as compared with 0 in the HDM200 arm (P = .07).
Response to HDT
Table 5 shows the response to HDT. After HDT, the CR rate was comparable in the 2 arms (29% in arm A vs 35% in arm B, P = .41), but the rate of CR plus VGPR was slightly increased in arm B (55% vs 43%, P = .06). The number of patients with a PR or patients who did not respond to HDT was similar in both arms. The number of patients who received interferon treatment and the duration of this maintenance therapy were equivalent in both groups after HDT.
Response to HDT
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| CR (%) | 41 (29) | 49 (35) | .41 |
| VGPR (%) | 19 (14) | 29 (20) | .17 |
| CR + VGPR (%) | 60 (43) | 78 (55) | .06 |
| PR (%) | 65 (46) | 56 (39) | .28 |
| Stable or progressive disease (%) | 10 (7) | 8 (6) | .78 |
| Toxic death (%) | 5 (4) | 0 (0) | .07 |
| . | Arm A HDM140/TBI (n = 140) . | Arm B HDM200 (n = 142) . | P . |
|---|---|---|---|
| CR (%) | 41 (29) | 49 (35) | .41 |
| VGPR (%) | 19 (14) | 29 (20) | .17 |
| CR + VGPR (%) | 60 (43) | 78 (55) | .06 |
| PR (%) | 65 (46) | 56 (39) | .28 |
| Stable or progressive disease (%) | 10 (7) | 8 (6) | .78 |
| Toxic death (%) | 5 (4) | 0 (0) | .07 |
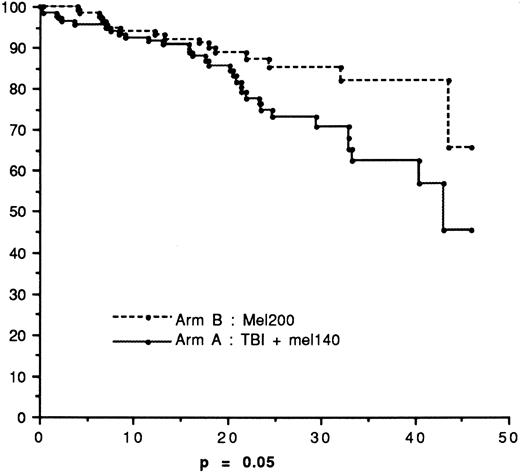
Overall survival
Figure 2 depicts OS. At the reference date, 32 and 18 patients died in arms A and B, respectively. The 45-month survival was 65.8% in arm B versus 45.5% in arm A (P = .05). The median survival was not reached in arm B and was 43 months in arm A. The median follow-up times for living patients were 20 months (range 3-53.5 months) in arm A and 20.5 months (range 3-55 months) in arm B, respectively.
Event-free survival
Figure 3 shows EFS. At the reference date, relapse or death had occurred in 76 patients in arm A compared with 69 patients in arm B. The median duration of EFS was 21 months in arm A versus 20.5 months in arm B (P = .6).
Survival advantage in arm B
The survival rate after HDT was higher in arm B versus arm A with borderline significance (P = .05), although the EFS rates were strictly comparable in the 2 groups of patients. This difference might be attributed in part to better salvage regimen after relapse in arm B compared with arm A. The survival curves of relapsed patients from the date of relapse (Figure4) showed a trend for a better outcome for patients treated with HDM200 compared with TBI + HDM140 (P = .10). The treatment of relapse was not standardized, but half the patients in arm B received a second cycle of intensive therapy with ASCT, compared with only 25% in arm A.
Discussion
In newly diagnosed patients with MM treated with HDT, the goal of the conditioning regimen is to achieve the best response rate, with minimum toxicity. At present, the most widely used conditioning regimens are probably HDM200 and HDM140 + TBI,7 but some other regimens have shown apparently similar results.10-12 To date, no randomized studies comparing different regimens have been reported, and most data suggest that none of the conditioning regimens are superior to another.6 7We have thus designed a prospective randomized trial comparing the 2 most common regimens used for MM.
Our results clearly show a lower toxicity for HDM200 compared with HDM140 + TBI regarding severe mucositis, duration of neutropenia and thrombocytopenia, number of red blood cell and platelet transfusions, number of days on antibiotics, and duration of hospitalization. At least 3 nonrandomized trials have compared HDM200 with HDM140 + TBI and have shown similar results. First, in the total therapy program Barlogie has used HDM200 for the second transplantation in responding patients and HDM140 + 8.5 to 10 Gy TBI for the others.5 He has found that with added TBI more toxicity was encountered so that only 10% of the patients had no serious extramedullary toxicity. TRM was 2% with the second autotransplantation using HDM200 alone and rose to 5% with added TBI. Secondly, Goldschmidt has reported a single-center experience with 261 MM patients comparing 3 regimens: HDM140 + TBI, HDM200 once, and 2 sequential cycles of HDM200.13 The dose of TBI was higher in this trial (14.4 Gy). The toxicity of HDM140 + TBI was higher regarding mucositis, days on total parenteral nutrition, days with fever, days on antibiotics, and number of platelet and red blood cell transfusions. Thirdly, a Spanish group has retrospectively evaluated the efficacy and toxicity of 3 regimens: HDM200, HDM140 + TBI, and HDM140 + 12 mg/kg busulphan in 563 evaluable patients.7 Although specific organ toxicity data are not available in this study, the duration of hospitalization was significantly reduced in the HDM200 cohort as compared with HDM140 + TBI or HDM140 + busulfan (days to discharge from PBSC infusion 18, 21, and 24, respectively, P = .007).
Our study showed slower engraftment after HDM140 + TBI, despite the higher number of CD34 cells infused. This delayed hematologic recovery might be attributed to stromal damage following TBI. It has already been shown that HDT followed by ASCT can induce a persistent impairment of the stem cell and stromal cell compartments.14 In a series of 33 patients treated with HDT and autologous bone marrow transplantation, decreased production of granulocyte macrophage colony-forming units and a defect in stromal layer confluence in long-term marrow cultures were observed as compared with 42 pregraft samples and 17 normal control samples.14Statistical analysis in this series of patients revealed that conditioning regimens with TBI led more frequently to nonconfluent stromal layers. Another study has analyzed the fibroblast colony-forming units, the precursor compartment for the microenvironmental lineages essential to hematopoietic stem cell survival, proliferation, and differentiation.15Pretransplantation conditioning included TBI in nearly all cases. In 15 cases, marrows were analyzed before and at serial times after transplantation, and the authors showed that regardless of age the marrow damage occurred as early as 20 days after transplantation, implying that the damage may be due to the pretransplantation conditioning regimen. In a series of 51 cases treated with myeloablative conditioning and ASCT, Bentley et al observed patients who remained platelet- and/or red cell transfusion–dependent for 100 days or more after transplantation after good neutrophil recovery. A significantly higher proportion of these patients had received TBI as part of their conditioning regimen.16 Together with these data, our results also suggest that TBI may in some cases impair the early and late capacities of the marrow microenvironment to support transplanted stem cells. This could explain the differences regarding hematologic recovery in the 2 arms of our trial.
In the present study the EFS rates were equivalent in both groups, but the survival rate was higher in the HDM200 arm with borderline significance (P = .05). Barlogie and coworkers have indeed suggested that the addition of TBI or other drugs to melphalan might not be superior to HDM200 alone in their total therapy program.5 Comparable OS and EFS were observed in their trial after HDM200 versus combination therapy, but HDM200 was used for patients in PR and TBI for patients with less than PR status before the second transplantation. The retrospective study from the Spanish registry did not show any differences regarding the 5-year OS (43% for the 102 patients treated with HDM140 + TBI vs 37% for the 245 cases treated with HDM200) and the 5-year EFS (21% vs 16%, respectively).7 In the retrospective German study comparing HDM140 + TBI versus HDM200, the remission status after transplantation, the EFS, and OS were equivalent in both groups of patients.13 In both univariate and multivariate analysis, the European Group for Blood and Marrow Transplantation registry's results in 1905 patients have shown that OS as well as progression-free survival were significantly higher when patients received HDM alone compared with HDM + TBI.6 Superior EFS and OS after HDM200 compared with other high-dose regimens without TBI have also been reported by Powles et al.17 Our results might be explained by the treatment of relapse, which was not standardized in the protocol. More patients received a subsequent autotransplantation after relapse in the HDM200 arm compared with relapse after TBI + HDM140. The role of ASCT in patients relapsing after a prior transplantation is not yet clearly defined,18 19 but this approach might increase survival.
Based on these data, we conclude that HDM200 is a less toxic and at least as effective conditioning regimen compared with HDM140 + 8 Gy TBI. This regimen should be considered as the standard of care before ASCT in MM.
Supported by research grants from the Programme Hospitalier de Recherche Clinique, University Hospital, Nantes, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Luc Harousseau, Dept of Hematology, University Hospital Hôtel-Dieu, Place Alexis Ricordeau, 44093 Nantes cedex 01, France; e-mail: jlharousseau@sante.univ-nantes.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal