Abstract
Virtually no progress has been made during more than 2 decades of clinical trials for multiple myeloma (MM) involving standard therapy (ST). Recent studies suggest that dose intensification requiring hematopoietic stem cell support results in higher complete response (CR) rates and extended disease control. “Total Therapy” (TT) consisting of non–cross-resistant induction regimens, followed by a double autotransplant (AT) procedure, was administered to 123 untreated patients with symptomatic MM. Upon hematologic recovery, interferon (IFN) maintenance (3 million units [MU]/m2 subcutaneously thrice weekly) was given until disease recurrence/progression. Results were compared with the outcome of untreated patients receiving ST according to Southwest Oncology Group (SWOG) trials. One hundred sixteen pair mates were selected from both TT and among 1,123 patients to match for the major prognostic features. TT induced CR in 40% of all 123 patients (intent-to-treat). By 12 months, 7% had died, including 4% from treatment-related complications. With a median follow-up of 31 months, median durations of event-free survival (EFS) and overall survival (OS) are 49 and 62+ months, respectively. Abnormalities of chromosomes 11q and 13 were associated with inferior outcome, whereas CR within 6 months after induction was a favorable prognostic feature for both EFS and OS. In comparison to ST, TT induced higher PR rates (85% v 52%, P < .0001) (CR rates not available on SWOG trials) and extended EFS (49 v 22 months, P = .0001) and OS (62+ v 48 months, P = .01). Compared to ST, dose intensification with double AT markedly augments tumor cytoreduction, effecting not only higher CR rates but also significantly extending EFS and OS in previously untreated patients with MM.
AFTER ALMOST 30 YEARS of clinical trial research, standard therapy (ST) for multiple myeloma (MM) remains ineffective, with few complete responses (CR; ≤ 5%) and a median survival of only 30 to 36 months.1 Increasing treatment intensity with high-dose melphalan 140 mg/m2 (MEL 140) as a means of overcoming drug resistance yielded CRs even in patients with high-risk and advanced MM.2 More recently, peripheral blood stem cells (PBSC; procured after chemotherapy and/or growth factor priming) afforded rapid hematopoietic recovery and lowered mortality, so that double autotransplants (AT) became feasible with CR rates approaching 50%.3 A phase II evaluation of “Total Therapy” (TT) was initiated in August 1990 combining all therapeutic avenues available at that time to achieve maximal tumor cytoreduction in newly diagnosed patients with MM.
The purpose of this report is to identify prognostic parameters associated with the outcome of TT patients and to compare TT results with those of ST administered to comparable patients according to protocols of the Southwest Oncology Group (SWOG).
PATIENTS AND METHODS
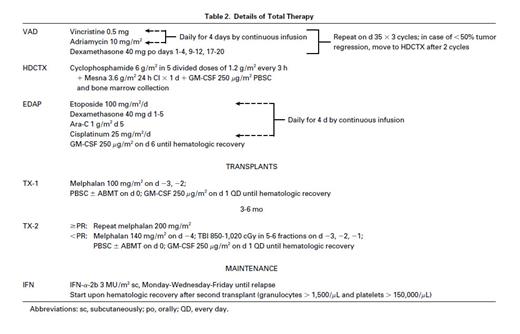
Total therapy.Patient characteristics were typical for newly diagnosed subjects with MM referred to this center (Table 1). Table 2 summarizes the details of TT, using VAD (vincristine, doxorubicin, dexamethasone)4 because of its marked and speedy tumor cell kill without inflicting hematopoietic stem cell compromise; followed by high-dose cyclophosphamide (HDCTX) and granulocyte-macrophage colony-stimulating factor (GM-CSF ) for PBSC collection5; EDAP (etoposide, dexamethasone, cytarabine, cisplatin)6 was included to target a more immature tumor cell compartment believed to be present in most patients with MM.7 In the absence of tumor progression (>25% increase in tumor mass), patients proceeded through the entire induction phase and were offered a first AT in support of MEL 200. In case of sustained partial remission (PR, see below) or CR, a second AT with MEL 200 was performed (79 patients); the remaining patients received MEL 140 plus total body irradiation (TBI; 1,125 cGy) (10 patients) or high-dose combination chemotherapy (5 patients). The intent was to complete both AT within 3 to 6 months and, indeed, only 8 patients had their second AT delayed beyond 6 months for medical, social, or insurance reasons. However, patients were kept on study if the interval between 2 AT did not exceed 1 year. PBSC were collected as previously described.3 A minimum of 6 × 108 mononuclear cells per kilogram had to be collected for the safe conduct of 2 AT. Interferon (IFN) maintenance (Intron-A; Schering-Plough Corp, Kenilworth, NJ) (3 million units [MU]/m2 subcutaneously thrice weekly) was started when granulocytes exceeded 1,500/μL and platelets 150,000/μL, and continued until disease relapse.
Characteristics of Total Therapy Patients (N = 123)
| Parameter . | Percent . |
|---|---|
| Age > 50 yr | 50 |
| Male | 64 |
| Ig Isotype G | 66 |
| A | 15 |
| Other | 19 |
| Albumin < 3.5 g/dL | 31 |
| Hb < 10 g% | 31 |
| Creatinine ≥ 2 mg/dL | 11 |
| β2M ≥ 3 mg/L | 54 |
| CRP > 0.4 mg/dL | 48 |
| Parameter . | Percent . |
|---|---|
| Age > 50 yr | 50 |
| Male | 64 |
| Ig Isotype G | 66 |
| A | 15 |
| Other | 19 |
| Albumin < 3.5 g/dL | 31 |
| Hb < 10 g% | 31 |
| Creatinine ≥ 2 mg/dL | 11 |
| β2M ≥ 3 mg/L | 54 |
| CRP > 0.4 mg/dL | 48 |
Eligibility criteria for TT included symptomatic MM, an upper age limit of 70 years, and adequate cardiopulmonary function (systolic cardiac ejection fraction ≥50%, carbon monoxide diffusion capacity ≥50%). At protocol entry, patients could have renal failure (creatinine >2 mg/dL; 9%), hypercalcemia (calcium >10.5 mg/dL; 28%), or poor performance status (Zubrod >2; 8%) related to MM. Renal function had to improve after VAD so that serum creatinine levels could not exceed 2 mg/dL before HDCTX as well as before the first and second AT.
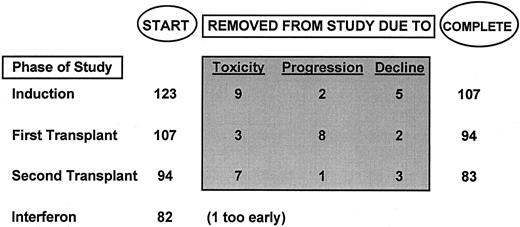
One hundred thirty-four untreated patients were registered at least 15 months before this analysis (December 15, 1995); 3 were denied insurance coverage and 8 opted for an allograft for their second transplant. Of the remaining 123 patients, who are the subjects of this report, 87% have completed one and 76% two autotransplants.
All patients signed an informed consent in keeping with guidelines of the National Cancer Institute, which had reviewed the TT program, and of the Institutional Review Board of the University of Arkansas for Medical Sciences and the Arkansas Cancer Research Center. In addition to standard laboratory parameters, cytogenetic analysis was performed on bone marrow samples by standard techniques.8 Patient follow-up was done according to protocol guidelines, usually on a monthly basis, to appreciate treatment-related toxicity and antitumor effect.
CR required the disappearance of monoclonal gammopathy in serum and urine on immunofixation analysis and attainment of normal marrow aspirate and biopsy with less than 1% light chain-restricted plasma cells on flow cytometry, on at least two successive occasions at least 2 months apart. PR implied ≥75% tumor mass reduction including a normal marrow aspirate and biopsy and, in the case of Bence Jones proteinuria, reduction to less than 100 mg per day.
SWOG trials.For comparison of outcome with TT, 116 ST patients were selected among 1,123 untreated patients with symptomatic MM registered on SWOG trials 82299 and 8624,10 to match for age (within a decade) (P = .8), β2-microglobulin (β2M) levels (within 2 mg/L) (P = .9), and serum creatinine concentrations (within 1 mg/dL) (P = 1.0). Those three parameters had been recognized as the dominant prognostic variables with these SWOG ST. The median time interval between registrations of SWOG pair mates and TT patients is 40 months; and the median follow-up of living patients is 63 months with ST and 31 months with TT. SWOG 8229 (614 patients) used chemotherapy with VMCP-VBAP, comparing an alternating versus syncopated regimen, yielding no difference between the two treatment arms.9 SWOG 8624 (509 patients) compared VMCP/VBAP versus identical therapy with more intensive prednisone (VMCPP/VBAPP) versus standard VAD.10
Statistical analysis.Survival curves and event-free survival (EFS) curves were estimated by the product-limit method and compared using the log-rank test. Cox regression was used to compare overall survival (OS) and EFS of subgroups receiving TT. A stratified logrank test was used for the matched comparison of TT versus SWOG ST. All P values are two-tailed.
RESULTS
Total therapy.Of the 123 patients, 87% completed one AT and 76% two AT (Fig 1). Ninety percent of first and second AT were completed by 7.5 and 13 months, respectively, with a median time interval between AT of 4.5 months (range, 2.5 to 11 months). Upon hematologic recovery, 82 started IFN maintenance at a median time of 2 months; 1 patient had not yet begun IFN at 3.5 months.
Using an intent-to-treat approach for all 123 patients, the frequency of ≥PR (CR) was 41% (5%) after VAD and increased progressively to 61% (9%) after HDCTX, 69% (16%) after EDAP, and 78% (25%) after one transplant, reaching 85% (40%) by the time the second AT should have been completed. Among the 94 patients actually completing 2 AT, 92% achieved at least PR and 48% CR. If PR had already been attained after VAD, the ultimate incidence of CR was 72%; CR rates decreased to 20% and 11%, respectively, if PR was not attained until after the first (10 patients) or second AT (8 patients) (overall P = .002).
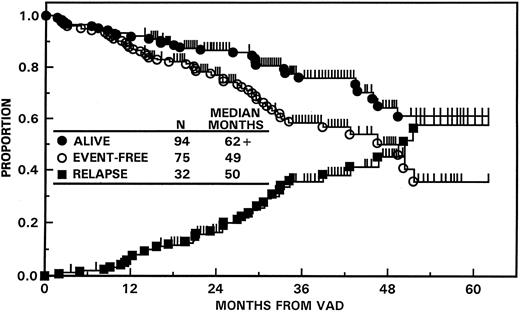
Figure 2 portrays EFS and OS for all 123 patients along with times to relapse for patients attaining at least a PR. Twelve months after VAD, 4% were alive with progressive disease and 7% had died, including 4% from treatment-related complications. Median durations of EFS and OS were 49 and 62+ months, respectively, and the projected 5-year EFS and OS rates (±1 SD) were 36% (±16%) and 61% (±14%). On multivariate analysis of 10 pretreatment parameters, the absence of abnormalities of chromosomes 11q and 13 (13q−, −13) (“unfavorable karyotypes,” see ref 8) (104 patients) was the dominant favorable feature for both EFS (50 v 21 months; P = .0001) and OS (62+ v 34 months; P = .0001). In a landmark analysis of 119 patients surviving at least 6 months, CR was a significant favorable parameter for both EFS (P < .005) and OS (P < .01) in addition to cytogenetics.
EFS and OS with “total therapy” from initiation of VAD (N = 123). The median EFS has been reached at 49 months, whereas OS exceeds 62 months. The median time to relapse from PR or CR was 50 months.
EFS and OS with “total therapy” from initiation of VAD (N = 123). The median EFS has been reached at 49 months, whereas OS exceeds 62 months. The median time to relapse from PR or CR was 50 months.
Toxicities.Severe granulocytopenia (<500/μL) and thrombocytopenia (<50,000/μL) were absent with VAD, and their median duration did not exceed 1 week with HDCTX, EDAP, and first or second AT. Patients were hospitalized for a median of 14 days with the first and only 6 days with the second AT; in fact, 38% and 46%, respectively, were transplanted entirely in an outpatient setting. Treatment-related mortality was 3% with VAD, 0% with HDCTX, 1% each with EDAP and with the first AT, and 2% with the second AT. The cumulative treatment-related mortality during the first 12 months was 4%.
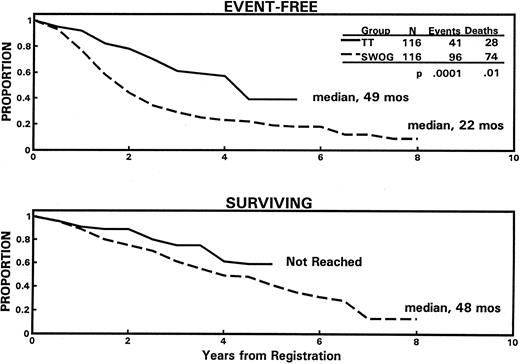
Total therapy versus SWOG standard therapy.Using an intent-to-treat approach, TT was superior to ST, resulting in a higher ≥PR rate of 86% versus 52% (P = .0001) (CR rates not available for SWOG trials) and longer median durations of EFS (49 v 22 months; P = .0001) and OS (62+ v 48 months; P = .01), with projected 5-year rates of EFS of 36% versus 19% and OS of 61% versus 39% (Fig 3).
Superior EFS (top panel) and OS (bottom panel) among 116 newly diagnosed patients receiving “total therapy” (TT) compared with 116 closely matched “pair mates” receiving standard therapy according to SWOG protocols. The median times of follow-up of living patients on “total therapy” and SWOG studies are 31 and 63 months, respectively.
Superior EFS (top panel) and OS (bottom panel) among 116 newly diagnosed patients receiving “total therapy” (TT) compared with 116 closely matched “pair mates” receiving standard therapy according to SWOG protocols. The median times of follow-up of living patients on “total therapy” and SWOG studies are 31 and 63 months, respectively.
DISCUSSION
For the first time, TT evaluated non–cross-resistant remission induction regimens followed by tandem ATs in newly diagnosed patients. Despite its complexity, the cytotoxic portion of TT (including 2 AT) was completed within 15 months in 96% of patients. Treatment-related mortality was low (4% at 12 months, 7% overall) and short-term efficacy was high (40% CR). Early response predicted higher ultimate CR rates with TT, implying greater responsiveness to subsequent induction and AT regimens in VAD-sensitive disease. The absence of abnormalities of chromosome 11q and/or partial or complete deletion of chromosome 13 was the dominant favorable prognostic factor, as reported earlier.8
Although a randomization between standard and high-dose therapies is the accepted strategy to unequivocally define the relative merits of different treatments, most patients came to our center specifically for AT because of promising results of earlier pilot trials. Therefore, our TT results were compared with the published data of newly diagnosed MM patients treated on two consecutive SWOG protocols between 1982 and 1990.9,10 Appreciating the difficulty of drawing firm conclusions from historical controls, an effort was undertaken to account for differences in relevant prognostic factors by matching patients closely for the three most important pretreatment parameters affecting EFS and OS after ST (age, B2M, creatinine). Among 116 pairs, significantly higher PR rates (P = .0001) and prolonged EFS (P = .0001) and OS durations (P = .01) were observed in TT patients. Not unexpectedly, as a result of matching, the 116 selected ST patients had superior EFS (22 v 19 months) and OS (48 v 36 months) compared with the entire group of 1,123 patients.9 10
The French Myeloma Intergroup (IFM) has recently updated results of a randomized trial of ST with VMCP/VBAP versus one AT, demonstrating a higher CR rate (22 v 5%, P < .001) and superior 5-year EFS (28 v 10%, P < .01) and OS rates (52 v 10%, P < .03) on the AT arm.11 The results of the IFM trial and TT are entirely consistent in terms of safety, clear benefit from greater dose intensity, and the importance of CR as the first crucial step toward durable disease control. Virtually identical CR rates (25% with TT, 22% with IFM study) after one AT (intent-to-treat analysis) despite different preparatory regimens suggest that the longer EFS duration of 49 months with TT as opposed to only 27 months on the IFM transplant arm most likely results from additional tumor cytoreduction achieved with a second AT course, which raised the CR rate of TT to 40% (intent-to-treat; 48% among double AT recipients). The current IFM trial, randomizing between one versus two AT, will settle the clinically important issue whether further dose intensification indeed improves the outcome of patients with MM.
Our earlier findings12 provided the basis for the ongoing randomized trial (INT141) among the 3 major cooperative groups in the United States and Canada, comparing a single PBSC autotransplant in support of melphalan 140 mg/m2 + TBI 1,200 cGy with standard VBMCP chemotherapy. Because of promising pilot data with “Total Therapy,”12 and the results of the IFM trial, the intergroup study, INT141, requires early PBSC collection also in patients randomized to standard therapy before significant damage to hematopoietic stem cells has occurred; furthermore, transplants are recommended upon relapse or disease progression on VBMCP therapy. Thus, in addition to comparing response rates and EFS, this trial will also examine whether OS is prolonged when myeloablative therapy is instituted early as opposed to being used for salvage, an important issue since preliminary data by Fermand et al13 suggest comparable survival among patients randomized to autotransplants and those receiving high-dose therapy after relapse or progression on standard chemotherapy.
We elected to offer all patients who had completed the double-transplant procedure IFN maintenance therapy, based on encouraging results observed after conventional chemotherapy12 and high-dose melphalan with autotransplantation,13 especially in patients who had attained a major tumor reduction. Subsequent studies using IFN maintenance after conventional chemotherapy have failed to show any impact on OS,10,14 although EFS may be prolonged.14 A recent update by the Royal Marsden group continues to demonstrate a significant survival advantage for MM autotransplant patients, who had attained a CR, but not for PRs and nonresponders. However, the data are somewhat difficult to interpret because of the large variability in time between the transplant and the start of the IFN maintenance.15 Moreover, there is some concern that IFN maintenance therapy may lead to an increased incidence of second neoplasms as recently reported for hairy cell leukemia,16 and that IFN may not be effective in MM patients, overexpressing bcl-2,17 a very common finding in this disease.
The poor prognosis, even with TT, in 15% of patients presenting with abnormalities of chromosomes 11 and/or 13 (EFS, 21 months; OS, 34 months) demands new and potentially more hazardous treatment approaches such as allotransplants,18 which have recently been shown to exert disease control by way of a graft-versus-myeloma effect.19 20
ACKNOWLEDGMENT
The manuscript is dedicated to Emil J. Freireich for his pioneering work in developing effective treatment for acute leukemia, which has inspired the design of “Total Therapy.”
The authors greatly acknowledge the support provided by the Bone Marrow Transplant Team of the University of Arkansas for Medical Sciences, Arkansas Cancer Research Center. We are also indebted to the many referring physicians who entrusted us with their patients' care. Connie Walker provided invaluable assistance in the preparation of this manuscript.
Supported in part by Grants No. CA55819, CA59340, CA38926 and CA32102 from the National Cancer Institute, Bethesda, MD.
Address reprint requests to Bart Barlogie, MD, Division of Hematology/Oncology, University of Arkansas for Medical Sciences, Little Rock, AR 72205.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal