Abstract
We have retrospectively assessed the neurological manifestations in 34 patients with hemophagocytic lymphohistiocytosis (HLH) in a single center. Clinical, radiological, and cerebrospinal fluid (CSF ) cytology data were analyzed according to treatment modalities. Twenty-five patients (73%) had evidence of central nervous system (CNS) disease at time of diagnosis, stressing the frequency of CNS involvement early in the time course of HLH. Four additional patients who did not have initial CNS disease, who did not die early from HLH complications, and who were not transplanted, also developed a specific CNS disease. Therefore, all surviving and nontransplanted patients had CNS involvement. Initially, CNS manifestations consisted of isolated lymphocytic meningitis in 20 patients and meningitis with clinical and radiological neurological symptoms in nine patients. For these nine patients, neurological symptoms consisted of seizures, coma, brain stem symptoms, or ataxia. The outcome of patients treated by systemic and intrathecal chemotherapy and/or immunosuppression exclusively (n = 16) was poor, as all died following occurrence of multiple relapses or CNS disease progression in most cases. Bone marrow transplantation (BMT) from either an HLA identical sibling (n = 6) or haplo identical parent (n = 3) was performed in nine patients, once first remission of CNS and systemic disease was achieved. Seven are long-term survivors including three who received an HLA partially identical marrow. All seven are off treatment with normal neurological function and cognitive development. In four other patients, BMT performed following CNS relapses was unsuccessful. Given the frequency and the poor outcome of CNS disease in HLH, BMT appears, therefore, to be the only available treatment procedure that is capable of preventing HLH CNS disease progression and that can result in cure when performed early enough after remission induction.
HEMOPHAGOCYTIC lymphohistiocytosis (HLH) is a lethal disease characterized by the occurrence of a hemophagocytic syndrome including high fever, hepatosplenomegaly, pancytopenia, low fibrinogen level, hemodilution, elevation of liver enzymes, and hypertriglyceridemia.1,2 Most reported cases indicate an autosomal recessive inheritance, but the cause of the disease remains unknown. The main histopathologic feature is a nonmalignant diffuse infiltration by lymphocytes and macrophages, most typically with hemophagocytosis, into visceral organs, lymph nodes, bone marrow, and central nervous system (CNS). This appears to reflect a massive activation of T lymphocytes3,4 and cells of the macrophage-histiocyte lineage.5
Neurological manifestations of HLH have been recognized,6-11 but their frequencies, characteristics, and influence on the outcome remain relatively ill-defined. In the present study, we reviewed the characteristics of the neurological manifestations that occurred in 34 consecutive patients with HLH followed in a single center. The outcome according to treatment regimen and CNS disease status has been assessed.
PATIENTS AND METHODS
Patients.Thirty-four patients (19 girls, 15 boys) with HLH were consecutively hospitalized from 1981 to 1993 at Hôpital Necker-Enfants Malades (Paris, France). In this single center retrospective study, the diagnosis of HLH was based in 25 patients on the occurrence of a hemophagocytic syndrome with a family history of consanguinity or of siblings affected by the same syndrome. In nine cases, the patient was the first affected child of the family and there was no consanguinity. In these cases, the diagnosis was based on the occurrence of: (1) a hemophagocytic syndrome without detection of concomitant infection known to induce this condition, and (2) the recurrence of hemophagocytic syndrome once a complete remission was observed either spontaneously or following appropriate treatment. Treatment modalities for 26 of these patients have been previously reported.12-14
Involvement of the CNS was documented by clinical evaluation, examination of cerebrospinal fluid (CSF ), and neuroradiological studies. The clinical neurological evaluation excluded the observation of isolated “hypotonia” or “hypertonia” during high fever or painful episodes of systemic hemophagocytic syndrome. Patients with CNS involvement were classified as suffering from either “meningitis” (no neurological symptoms except meningismus and abnormal CSF ) or from “neurological symptoms” (any neurological deficit or seizures associated with CSF abnormalities). In this last group of patients, brain stem involvement was defined by the presence of a coma with opisthotonos and irregularities in cardiac or respiratory rates.
Nineteen patients were treated by chemotherapy alone and 15 patients (mostly in more recent years), after initial chemotherapy, also underwent a bone marrow transplantation (BMT). In all cases, an intensive supportive care protocol was used during hemophagocytic syndromes. During the maintenance phases, the children received intravenous gammaglobulin every 3 weeks and trimethoprim-sulfamethoxazole orally.
Patients were treated according to previously published regimens.12-15 Between 1981 and 1990, chemotherapy consisted of steroids, VP16-213 (etoposide) and intrathecal methotrexate injections (MTX IT) (6 to 12 mg according to age) (n = 19).12,13 Since 1991, VP16-213 was replaced by Cyclosporine A (CSA) and rabbit antithymocyte globulin (ATG) (Institut Merieux, Lyon, France) (n = 15).14 During the maintenance therapy of both regimens, MTX IT was given usually every month. The number of MTX IT injections depended on the intensity of CNS disease and the analysis of CSF performed before each IT injection.
The conditioning regimen of BMT consisted of VP16-213, busulfan, and cyclophosphamide.13 Graft-versus-host disease (GVHD) prophylaxis consisted of the associaton of methotrexate (10 mg/m2 on days 1, 3, 6, and 10) and CSA.16 Patients who received a haplo identical BMT were also treated by monoclonal anti-leukocyte function adhesion 1 (anti-LFA1) and anti-CD2 antibodies (n = 4)17 or by anti-LFA1 antibody alone (n = 3) to prevent graft rejection.18 Prevention of GVHD was based on T-cell depletion by E-rosetting (four patients) or by Campath 1-M plus human complement (three patients).19
Until 1990, the decision to perform BMT was based on the occurrence of relapse and on the availability of an HLA-identical sibling. From 1990 to 1993, BMT was performed in case of relapse even when no HLA-identical sibling was available. After 1993, BMT was performed as soon as a complete remission was achieved.
Methods.CSF studies consisted of determination of the protein level and microscopic analysis of cells detected on the cytospin preparation. After staining by Giemsa, immunohistochemistry was performed using anti-HLA DR (major histocompatibility complex [MHC] class II) antibody. Neuroradiological studies were performed in 19 patients and consisted of neuroradiologic magnetic resonance (MR) imaging with administration of gadopentate dimeglumine and/or computed tomographic (CT) scans.
RESULTS
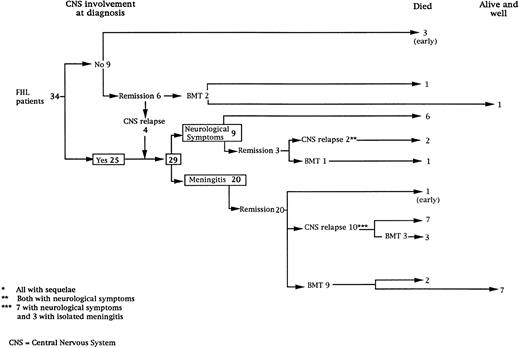
Thirty-four consecutive patients were diagnosed with HLH. Twenty-five patients had CNS involvement at the time of the diagnosis (Fig 1). Among the nine patients without CNS involvement at diagnosis, three died within weeks of the diagnosis at 3, 4, and 8 months, respectively, of age and two received a BMT before the occurrence of any neurological symptoms (one, transplanted at the age of 4 months, is alive and well 5 years post-BMT and the second, transplanted at the age of 12 months, died of graft failure). The remaining four patients developed CNS disease (Fig 1). Together, 29 patients had CNS involvement as defined above and were studied further.
Description of the initial CNS manifestations.The initial CNS manifestations consisted of meningitis in 20 patients and neurological symptoms in nine (Fig 1). Mean age at time of meningitis was 6.9 months (median, 3; range, 1 to 31), while mean age at the time of neurological symptoms was 16 months (median, 4; range, 1 to 58) (P = .05). The CNS involvement was associated with a systemic hemophagocytic syndrome in all cases.
Meningitis characteristics were detection of 20 to 80 lymphocytes/μL of CSF with an elevated spinal fluid protein varying between 0.5 and 1 g/L. Cytospin preparation of the CSF showed hemophagocytosis in 11 cases, the presence of HLA DR-positive activated lymphocytes in eight cases (both in five cases), while neither hemophagocytosis nor HLA DR(+) lymphocytes were detected in six cases. Cultures of CSF for bacterial, viral, and fungal infectious agents were negative in all cases. In 9 of the 20 patients with meningitis, a neuroradiological study was performed; brain imaging was abnormal in 4 patients, demonstrating pericerebral diffuse subdural dilatation and white matter abnormalities in 2 cases each.
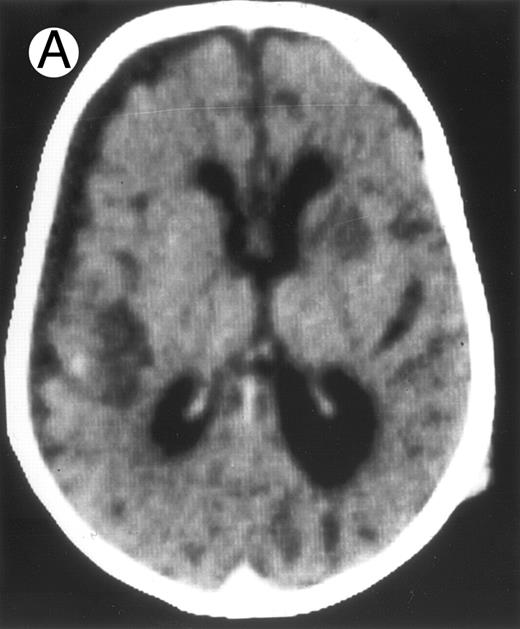
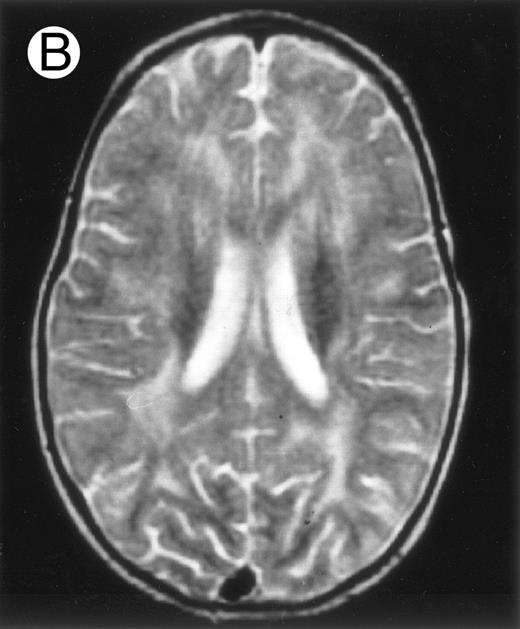
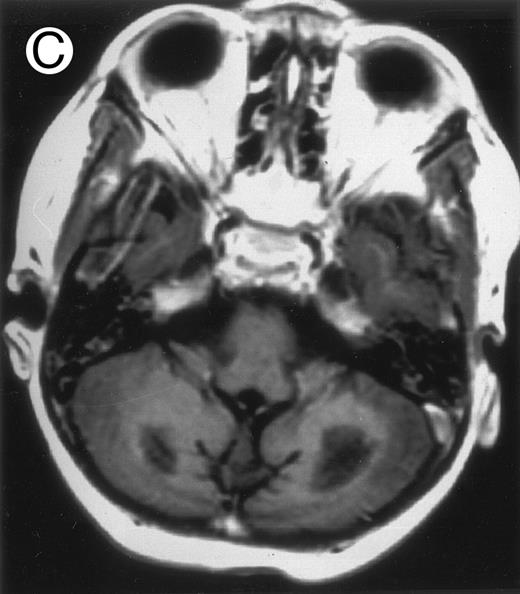
Among the nine children with initial neurological symptoms, seizures were the most frequent initial symptom in the youngest patients, whereas ataxia was found in the two oldest patients (46 and 58 months old, respectively) (Table 1). All nine patients had the same CSF abnormalities as patients with meningitis only. Eight of these nine patients had a neuroradiological study performed at the time of their first neurological symptoms (Table 1). The two most frequent lesions were focal necrosis with parenchymal volume loss and atrophy (Fig 2A and C) and white matter abnormalities (Fig 2B). Several small focal lesions with hypersignal at MR imaging that enhanced after administration of gadopentate dimeglumine (or contrast on CT scan) were also observed in two cases.
Characteristics of CNS Disease in 16 Patients at Time of Their First Overt Clinical Neurological Manifestations
| Age (mo) . | Primary (I) or Secondary Onset (II) . | Clinical Symptoms . | Radiological Symptoms . |
|---|---|---|---|
| 1 | I | Coma | MRI: White matter abnormalities |
| Seizures | CT Scan: Calcifications | ||
| Brain stem symptoms | Abnormal contrast enhancement | ||
| 1.5 | I | Brain stem symptoms | ND |
| 2 | I | Coma | CT Scan: Abnormal contrast enhancement |
| Subdural space dilatation | |||
| 3 | I | Coma | CT Scan: Severe necrotic areas |
| Seizures | White matter abnormalities | ||
| Brain stem symptoms | Subdural space dilatation | ||
| 4 | I | Seizures | CT Scan: Severe atrophy |
| Brain stem symptoms | |||
| 17 | I | Coma | CT Scan: Severe atrophy |
| Seizures | |||
| 19 | I | Coma | CT Scan: Necrotic areas |
| Seizures | |||
| Hemiplegia | |||
| 46 | I | Ataxia | CT Scan: White matter abnormalities |
| Somnolence | |||
| Meningism | |||
| 58 | I | Ataxia | CT Scan: White matter abnormalities |
| 6 | II | Coma | |
| Tetraparesis | |||
| Brain stem symptoms | ND | ||
| Developmental delay | |||
| 7 | II | Developmental delay | CT Scan: Calcifications |
| 8 | II | Seizures | CT Scan: Severe atrophy |
| 13 | II | Developmental delay | MRI: White matter abnormalities |
| CT Scan: Calcifications | |||
| Abnormal contrast enhancement | |||
| 13 | II | Developmental delay | MRI: White matter abnormalities |
| Coma | CT Scan: Normal | ||
| Seizures | |||
| Tetraparesis | |||
| 14 | II | Coma | MRI: White matter abnormalities |
| 57 | II | Ataxia | MRI: Necrotic areas |
| White matter abnormalities |
| Age (mo) . | Primary (I) or Secondary Onset (II) . | Clinical Symptoms . | Radiological Symptoms . |
|---|---|---|---|
| 1 | I | Coma | MRI: White matter abnormalities |
| Seizures | CT Scan: Calcifications | ||
| Brain stem symptoms | Abnormal contrast enhancement | ||
| 1.5 | I | Brain stem symptoms | ND |
| 2 | I | Coma | CT Scan: Abnormal contrast enhancement |
| Subdural space dilatation | |||
| 3 | I | Coma | CT Scan: Severe necrotic areas |
| Seizures | White matter abnormalities | ||
| Brain stem symptoms | Subdural space dilatation | ||
| 4 | I | Seizures | CT Scan: Severe atrophy |
| Brain stem symptoms | |||
| 17 | I | Coma | CT Scan: Severe atrophy |
| Seizures | |||
| 19 | I | Coma | CT Scan: Necrotic areas |
| Seizures | |||
| Hemiplegia | |||
| 46 | I | Ataxia | CT Scan: White matter abnormalities |
| Somnolence | |||
| Meningism | |||
| 58 | I | Ataxia | CT Scan: White matter abnormalities |
| 6 | II | Coma | |
| Tetraparesis | |||
| Brain stem symptoms | ND | ||
| Developmental delay | |||
| 7 | II | Developmental delay | CT Scan: Calcifications |
| 8 | II | Seizures | CT Scan: Severe atrophy |
| 13 | II | Developmental delay | MRI: White matter abnormalities |
| CT Scan: Calcifications | |||
| Abnormal contrast enhancement | |||
| 13 | II | Developmental delay | MRI: White matter abnormalities |
| Coma | CT Scan: Normal | ||
| Seizures | |||
| Tetraparesis | |||
| 14 | II | Coma | MRI: White matter abnormalities |
| 57 | II | Ataxia | MRI: Necrotic areas |
| White matter abnormalities |
Abbreviations: MRI, magnetic resonance imaging; CT Scan, computed tomographic scans; ND, not determined.
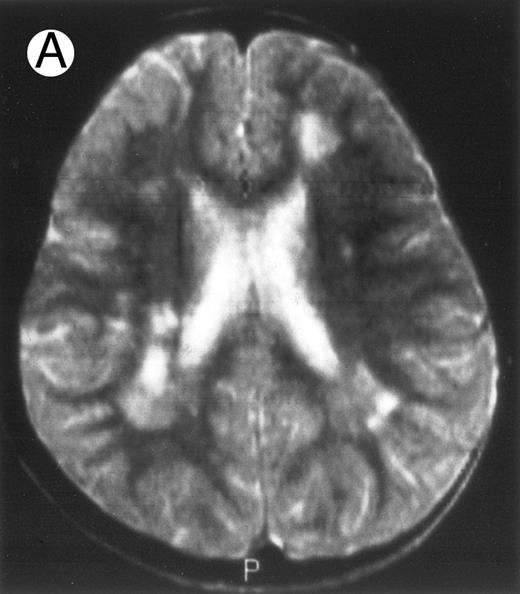
Three different aspects of brain imaging in HLH patients. (A) CT scan of a 3-month-old baby showing a large subdural effusion, several necrotic areas and hypodensities of the white matter. (B) Brain magnetic resonance of a 14-month-old boy showing large confluent areas of hypersignal in T2-weighted images. (C) Large symmetrical necrotic areas of cerebellar white matter in a 41/2-year-old girl (MRI).
Three different aspects of brain imaging in HLH patients. (A) CT scan of a 3-month-old baby showing a large subdural effusion, several necrotic areas and hypodensities of the white matter. (B) Brain magnetic resonance of a 14-month-old boy showing large confluent areas of hypersignal in T2-weighted images. (C) Large symmetrical necrotic areas of cerebellar white matter in a 41/2-year-old girl (MRI).
Subsequent neurological manifestations according to treatment regimen.Intravenous (IV) and IT chemotherapy led to complete systemic and neurological remission in the 20 patients with initial meningitis (Fig 1). Among the nine patients who presented neurological symptoms as the initial CNS manifestation, IV and IT chemotherapy led to a complete remission in only three patients who, nonetheless, developed neurological sequelae.
Once complete remission was achieved (n = 23), one patient died of sepsis 2 months following initial chemotherapy (Fig 1). In 12 patients, maintenance treatment only was given, and CNS relapses occurred within a median delay of 6 months (range, 1 to 26) associated with systemic disease in eight patients. Nine of the 12 had new clinical neurological manifestations. At time of relapse, 2 patients were still under IV and IT treatment, 4 were under IV treatment with the last MTX IT injection performed respectively, 2, 5, 10, and 30 months earlier, and 3 were off therapy for 1, 2, and 3 months, respectively.
Together, 19 patients had primary severe CNS disease progression or CNS relapse (Fig 1). Three of 19 had a CNS relapse characterized by meningitis only. Neurological symptoms occurred in 16 of these 19 patients (9 from the onset and 7 after the initial meningitis) (Fig 1). No clinical or neuroradiological difference was detected between the patients who developed neurological symptoms as the initial CNS manifestation or after initial meningitis (Table 1). The occurrence of these neurological manifestations marked a turning point in the evolution of the disease, as chemotherapy led to an improvement of the neurological symptoms in only three patients despite the use of intensive treatment by MTX IT. In these three patients, neurological sequelae persisted after the first symptoms and a relapse occurred within 3 to 10 months. Brain imaging was repeated during chemotherapy for four patients with progression of the CNS disease: in three patients, a severe brain atrophy developed (Fig 3A and B) and in the last patient who initially had contrast enhancement in cerebellar white matter, a cerebellum parenchymal loss was observed (Fig 2C). All of these 19 patients treated by chemotherapy only (n = 15) died during a last episode of coma and brain stem symptoms associated with a systemic hemophagocytic syndrome, 6 ± 6 months after the first neurological symptom (irrespective of the age at this first event). Four of these 19 patients were transplanted (one patient received an HLA-identical BMT, three an HLA-partially identical BMT). Median delay between diagnosis and BMT was 14 months (range, 2 to 20 months). All of these four patients died of BMT-related toxicity and/or disease progression.
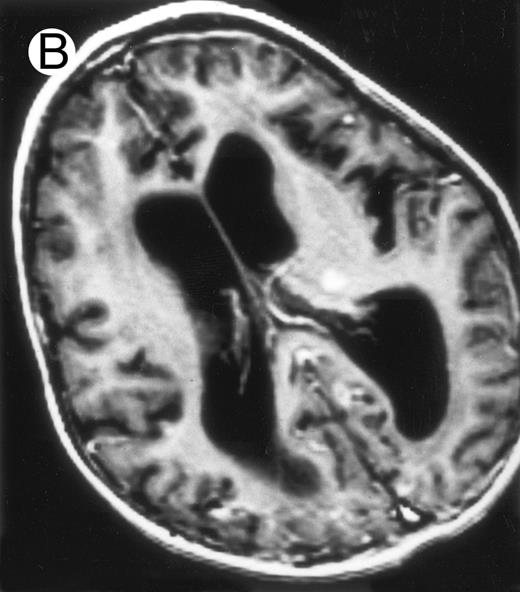
Evolution of brain lesions during HLH. (A) MRI of a 41/2-year-old girl showing focal white matter hypersignal on T2-weighted images. (B) MRI of the same child 1 year later with an important atrophy of both the white matter and the cortices.
Evolution of brain lesions during HLH. (A) MRI of a 41/2-year-old girl showing focal white matter hypersignal on T2-weighted images. (B) MRI of the same child 1 year later with an important atrophy of both the white matter and the cortices.
Nine patients were transplanted soon after the remission of initial CNS and systemic manifestations. All of these patients had meningitis only as the CNS manifestation. The median delay between diagnosis and BMT was 4 months (range, 2 to 14 months). Two patients died of BMT-related toxicity (both recipients of an HLA-identical BMT). Seven patients are alive and well with normal neurological examination, normal CSF tap, and normal cognitive development after a follow-up of 18 to 132 months (median, 55 months) (four recipients of an identical BMT and three of a partially-identical BMT). After BMT, chimerism studies demonstrated full or partial engraftment in all of these seven patients. MRI was performed before and after the transplantation in three cases. Two patients had a normal MRI or an isolated subarachnoidal and subdural space dilatation before transplantation and a normal MRI, respectively 4 years and 18 months after transplantation. One patient had white matter abnormalities before transplantation, which remained identical 2 years after transplantation (Fig 4).
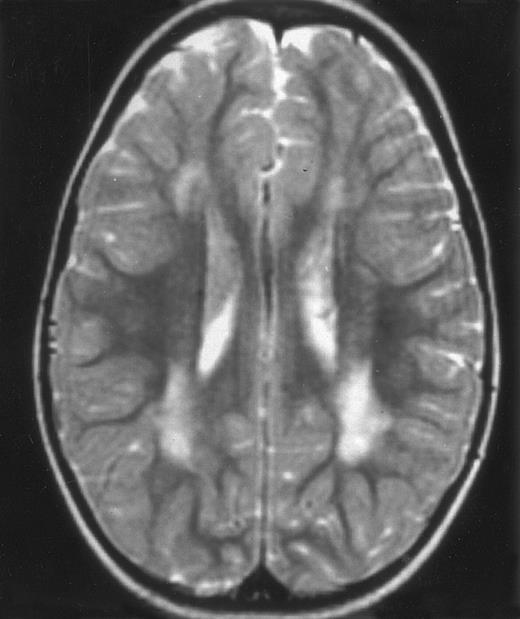
MRI of a symptom-free 31/2-year-old child who received BMT at 18 months showing the persistent white matter abnormalities.
MRI of a symptom-free 31/2-year-old child who received BMT at 18 months showing the persistent white matter abnormalities.
DISCUSSION
We have retrospectively reviewed the frequency, characteristics, and outcome of neurological manifestations of HLH. Twenty-five of 34 HLH patients (73%) had CNS disease at time of diagnosis. In addition, 4 of 9 patients without CNS involvement at diagnosis later developed a CNS relapse, while the 5 others either died early or received a BMT. Therefore, 100% of evaluable patients developed CNS lesions in the absence of transplantation. The observed frequency of CNS disease at diagnosis appears slightly higher than that reported in other studies. Janka1 reported CSF abnormalities in 17 of a series of 33 patients and neurological symptoms in less than 10% of the patients. In a multicenter retrospective study, Arico et al20 reported CSF abnormalities at diagnosis in 55 of 94 patients (58%). Hirst et al21 found clinical neurological manifestations at diagnosis in seven of 23 patients and CSF abnormalities in 13 of 17 evaluable patients, and Henter et al22 reported CSF abnormalities in four of seven patients with neurological symptoms in three cases. No data are available in the literature that describe the frequency of CNS disease occurrence during the course of HLH disease.
Despite the chemotherapy-induced normalization of CSF after initial meningitis, neurological symptoms developed within months in all evaluable patients treated by chemotherapy alone, regardless of the chemotherapy regimen and frequency and number of IT methotrexate injections. Onset of an overt neurological disease represented a major milestone in the outcome since, subsequently, repeated relapses and CNS disease progression led to more severe neurological lesions and ultimately death within months.
Abnormalities on brain imaging appeared to roughly parallel the severity of clinical manifestations. All patients with neurological symptoms, but also half of the patients with initial meningitis, had abnormal brain imaging usually consisting of a combination of diffuse white matter abnormalities and necrotic areas with parenchymal volume loss, as previously described.8,23 In patients with isolated meningitis, however, only white matter abnormalities were detected, whereas necrotic lesions and cerebral atrophy were found in patients with neurological symptoms. These lesions progressed despite chemotherapy. Previous neuropathological studies have demonstrated infiltration by monocytes and activated lymphocytes of leptomeninges and brain parenchyma along penetrating vessels.24,25 Infiltration is associated with focal and confluent areas of myelin pallor, as well as neuronal loss, tissue necrosis, and cavitation,24,25 findings that were also demonstrated in the neuropathologic study of five patients in our series. Leukocytes infiltrating the CNS probably secrete cytokines and other neurotoxic factors, such as tumor necrosis factor-α (TNF-α), which may be responsible for the myelinic alteration observed in neurologically asymptomatic patients. Infiltrating leukocytes could also activate in parallel the numerous resident brain macrophages (the microglial cells) and astrocytes, which in turn, can secrete neurotoxic glutamate and free radicals.26
The efficiency of allogenic BMT in preventing relapses of peripheral HLH manifestations has already been reported, including by our group.13,27,28 In this study, we describe the remarkable effect of BMT on the prevention of subsequent neurological manifestations and the good cognitive and neurological evolution of the seven transplanted children who are long-term survivors. In addition, brain imaging performed several months or years after BMT showed a normal aspect or a stabilization of white matter abnormalities. These seven patients had initial meningitis, but never developed neurological symptoms before BMT. Therefore, this study does not allow conclusions on the potential beneficial effects of BMT in patients with neurological symptoms. Nevertheless, it is likely that when atrophy and/or necrotic lesions are present, BMT could probably, at best, lead to stabilization of lesions carrying a risk of neurological and cognitive sequelae. We, therefore, believe that the optimal strategy for the treatment of HLH patients is to perform a BMT as early as possible once complete (systemic and neurological) remission has been achieved. Today, BMT from alternative donors, ie, matched unrelated29 and haplo-identical related donors (Jabado et al submitted), can also be considered. However, this approach raises the question of the accuracy of HLH diagnosis in patients without a family history or consanguinity. In this setting, it may be more appropriate to wait until relapse to confirm the HLH diagnosis, although the risk of severe CNS lesion occurring in the meantime is significant.
Address reprint requests to Elie Haddad, MD, Unité d'Immunologie et d' Hématologie Pédiatriques, Département de Pédiatrie, Hôpital Necker-Enfants Malades, 149 rue de Sèvres, 75749 Paris Cedex 15, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal