Abstract
One hundred nineteen patients with relapsed or refractory Hodgkin's disease (HD) received high-dose therapy followed by autologous hematopoietic progenitor cell transplantation. Three preparatory regimens, selected on the basis of prior therapy and pulmonary status, were employed. Twenty-six patients without a history of prior chest or pelvic irradiation were treated with fractionated total body irradiation, etoposide (VP) 60 mg/kg and cyclophosphamide (Cy) 100 mg/kg. Seventy-four patients received BCNU 15 mg/kg with identical doses of VP and Cy. A group of 19 patients with a limited diffusing capacity or history of pneumonitis received a novel high-dose regimen consisting of CCNU 15 mg/kg, VP 60 mg/kg and Cy 100 mg/kg. Twenty-nine patients (24%) had failed induction therapy and 35 (29%) had progressive HD within 1 year of initial chemotherapy. At 4 years actuarial survival was 52%, event-free survival was 48% and freedom from progression (FFP) was 62%. No significant differences were seen in survival data with the three preparatory regimens. Six patients died within 100 days of transplantation and 5 died at a later date of transplant-related complications. Secondary malignancies have developed in 6 patients, including myelodysplasia/leukemia in four patients and solid tumors in two patients. Regression analysis identified systemic symptoms at relapse, disseminated pulmonary or bone marrow disease at relapse and more than minimal disease at the time of transplantation as significant prognostic factors for overall and event-free survival and FFP. Patients with none of these factors enjoyed an 85% FFP at 4 years compared with 41% for patients with one or more unfavorable prognostic factors (P = .0001). Our results confirm the efficacy of high-dose therapy and autografting in recurrent or refractory HD. Although longer follow-up is necessary to address ultimate cure rates and toxicity, our data indicate that a desire to reduce late effects should drive future research efforts in favorable patients whereas new initiatives are needed for those with less favorable prognoses.
CURRENT combination chemotherapy and radiotherapy treatment programs result in cure of 60% to 85% of patients with Hodgkin's disease (HD).1-4 In the event of failure to completely respond or relapse, the efficacy of second-line therapy is directly related to the duration of initial response. Progression during induction therapy or within 12 months of the completion of treatment has a particularly poor prognosis, with 5-year disease-free survival rates of 0% and 20%, respectively.5,6 Relapses after more durable remissions, those of 12 months or greater, are more amenable to salvage chemotherapy; about half of such patients may enjoy prolonged remissions.5 However, even with prolonged initial remissions, overall survival was only 29% among patients retreated at the National Cancer Institute with MOPP (nitrogen mustard, oncovin, procarbazine, prednisone) chemotherapy, due to cumulative toxicity.6
Multiple series show sustained remissions after high-dose therapy and autografting in patients with refractory or recurrent HD.7-20 Mature data indicate the need for long-term follow-up to assess the ultimate rate of cure as well as the toxicity profile of this treatment.21 The preparatory regimens used in most studies were the CBV (cyclophosphamide, BCNU, VP16) or the BEAM (BCNU, etoposide, cytarabine, melphalan) regimens.11,15 Based on our experience with fractionated total body irradiation (fTBI) and high-dose etoposide (VP) as an effective regimen for acute leukemia, a new combination of fTBI, VP, and cyclophosphamide (Cy) was developed for HD and the non-Hodgkin's lymphomas.14,22 However, as TBI is often contraindicated in HD patients with a history of mantle irradiation, we developed a second high-dose VP regimen, BCNU/VP/Cy, following a dose-escalation study of BCNU in combination with the same doses and schedule of VP and Cy employed in fTBI/VP/Cy. Because either BCNU or irradiation may result in acute or delayed pulmonary toxicity, patients were excluded from treatment with either of these preparatory regimens if they had a significantly impaired diffusing capacity (<60%) or a history of chemical or radiation pneumonitis. A third regimen was designed and tested in a dose-escalation trial of CCNU/VP/Cy in this patient population.23 Selection of CCNU was based on its single agent activity, known to be superior to BCNU, and a lower incidence of respiratory complications reported in the literature.24 We report here the results of three high-dose regimens and autografting in patients with recurrent or refractory HD. The results of an analysis of prognostic factors, performed to assist in the design of future clinical trials, is also presented.
MATERIALS AND METHODS
Patient selection.All patients with a diagnosis of HD referred to the Division of Bone Marrow Transplantation at Stanford University Medical Center (Stanford, CA) were presented at the weekly new patient conference at which time the clinical features were reviewed, extent of current disease was established, protocol eligibility was discussed, and a treatment plan was formulated. From December 1987 through July 1995, 119 patients with refractory or recurrent HD received high-dose therapy and autologous hematopoietic stem cell transplantation at Stanford. Patients were selected on the basis of age ≤ 55, no medical contraindication to the proposed therapy, insurance approval, written informed consent, and pathologically confirmed HD that had either progressed during or after at least one standard chemotherapy combination. Progression was proved by biopsy or fine needle aspiration or demonstrated unequivocally on radiographic studies. After June 1991, absence of myelodysplasia (MDS) on marrow cytogenetics was added as an eligibility criterion based on our previous report that occult MDS resulted in clinical disease following high-dose therapy and autografting.25
Thirty-eight patients who were presented at this conference were not transplanted at Stanford for a variety of reasons. These included: transplant at another institution (5 patients), progressive disease with poor performance status for which transplantation was contraindicated (15 patients), no follow up after initial consultation because of insurance denial/delay or refusal (12 patients), good prognosis with conventional treatment, “too favorable” for high-dose therapy (4 patients), marrow cytogenetics indicative of incipient myelodysplasia (1 patient) and advanced age (1 patient).
Conventional cytoreduction.Before high-dose therapy, most patients received one to three cycles of conventional combination chemotherapy with the goal of achieving a minimal disease state, which was defined as greater than 75% reduction in a bulky (≥10 cm) mass or no individual lymph node >2 cm in maximal horizontal diameter and <10% involvement of the bone marrow. In the event that disease did not regress or actually progressed during one to two cycles of conventional cytoreduction, alternative combination chemotherapy was instituted. The choice of conventional cytoreduction therapy was based on prior treatment and the remission duration. In general, patients who relapsed within 12 months after exposure to a single four-drug regimen such as ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) were treated with a combination such as MOPP, which was considered to be noncross resistant. If disease progression occurred within 12 months of a seven- or eight-drug regimen, the typical combination was DHAP (cisplatin, cytarabine, decadron). Early in the study, patients with disease limited to a site which could be encompassed by a single port received radiotherapy alone or in combination with cytoreductive chemotherapy. Sites irradiated included a mantle field in four patients, base of skull in one patient, para-aortic and/or pelvic field in 10 patients, thoracic or lumbar spine in three patients, axilla in one patient and liver in one patient. Doses ranged from 12.5 to 36 Gy with usual fractionation 1.5 to 2 Gy. Following the observation of pulmonary and hepatic toxicity in the irradiated sites posttransplantation in two patients, routine cytoreductive radiotherapy was discontinued to the thorax or liver after March 1989. Mobilization of peripheral blood progenitor cells (PBPC) with high-dose Cy or VP16 as described below also served to cytoreduce HD in a small subset of patients. In 25 cases, two or more cytoreductive regimens were employed due to inadequate regression or progression on the initial cytoreductive regimen. Six patients had minimal disease on referral and proceeded directly to transplant. The number of cytoreductive regimens is detailed in Table 1.
Initial Stage and Previous Therapy Characteristics
| Characteristic . | CCNU . | BCNU . | FTBI . | Total . |
|---|---|---|---|---|
| . | n = 19 . | n = 74 . | n = 26 . | n = 119 . |
| N Patients | ||||
| Stage at diagnosis* | ||||
| I | 0 | 1 | 0 | 1 |
| II | 8 | 29 | 3 | 40 |
| III | 6 | 31 | 8 | 45 |
| IV | 5 | 13 | 15 | 33 |
| Constitutional symptoms at | ||||
| diagnosis | 9 | 42 | 19 | 70 |
| No. of drugs in initial course | ||||
| of treatment† | ||||
| Four or fewer | 7 | 29 | 9 | 45 |
| More than four | 10 | 31 | 17 | 58 |
| Initial remission duration† | ||||
| Induction failure | 8 | 12 | 9 | 29 |
| 61-365 days | 2 | 23 | 10 | 35 |
| >365 days | 7 | 25 | 7 | 39 |
| Prior RT history* | ||||
| None | 6 | 17 | 23 | 46 |
| Single course of RT alone | 2 | 15 | 0 | 17 |
| Combined modality | ||||
| treatment | 11 | 42 | 3 | 56 |
| Relapse in previously | ||||
| irradiated sites* | ||||
| RT site only | 6 | 20 | 0 | 26 |
| Within and outside RT site | 4 | 21 | 2 | 27 |
| Total no. of prior CT drugs | ||||
| Four or fewer | 6 | 25 | 7 | 38 |
| More than four | 13 | 49 | 19 | 81 |
| No. prior CT courses† | ||||
| One | 12 | 38 | 21 | 71 |
| Two+ | 5 | 22 | 5 | 32 |
| RT alone first course‡ | 2 | 14 | 0 | 16 |
| No. cytoreductive regimens | ||||
| None | 4 | 2 | 0 | 6 |
| One | 9 | 51 | 18 | 78 |
| Two or more | 6 | 12 | 7 | 25 |
| RT | 2 | 12 | 6 | 20 |
| Characteristic . | CCNU . | BCNU . | FTBI . | Total . |
|---|---|---|---|---|
| . | n = 19 . | n = 74 . | n = 26 . | n = 119 . |
| N Patients | ||||
| Stage at diagnosis* | ||||
| I | 0 | 1 | 0 | 1 |
| II | 8 | 29 | 3 | 40 |
| III | 6 | 31 | 8 | 45 |
| IV | 5 | 13 | 15 | 33 |
| Constitutional symptoms at | ||||
| diagnosis | 9 | 42 | 19 | 70 |
| No. of drugs in initial course | ||||
| of treatment† | ||||
| Four or fewer | 7 | 29 | 9 | 45 |
| More than four | 10 | 31 | 17 | 58 |
| Initial remission duration† | ||||
| Induction failure | 8 | 12 | 9 | 29 |
| 61-365 days | 2 | 23 | 10 | 35 |
| >365 days | 7 | 25 | 7 | 39 |
| Prior RT history* | ||||
| None | 6 | 17 | 23 | 46 |
| Single course of RT alone | 2 | 15 | 0 | 17 |
| Combined modality | ||||
| treatment | 11 | 42 | 3 | 56 |
| Relapse in previously | ||||
| irradiated sites* | ||||
| RT site only | 6 | 20 | 0 | 26 |
| Within and outside RT site | 4 | 21 | 2 | 27 |
| Total no. of prior CT drugs | ||||
| Four or fewer | 6 | 25 | 7 | 38 |
| More than four | 13 | 49 | 19 | 81 |
| No. prior CT courses† | ||||
| One | 12 | 38 | 21 | 71 |
| Two+ | 5 | 22 | 5 | 32 |
| RT alone first course‡ | 2 | 14 | 0 | 16 |
| No. cytoreductive regimens | ||||
| None | 4 | 2 | 0 | 6 |
| One | 9 | 51 | 18 | 78 |
| Two or more | 6 | 12 | 7 | 25 |
| RT | 2 | 12 | 6 | 20 |
Abbreviations: CT, chemotherapy; RT, radiotherapy.
Significant (P ≤ .05) differences in distribution.
Excludes 16 patients who received RT alone as primary treatment.
Includes 16 patients who received RT alone as primary treatment.
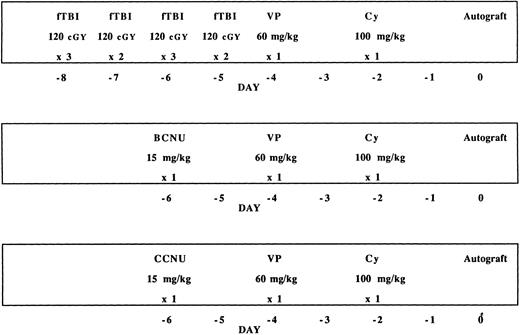
High-dose therapy.Patients were prospectively assigned to one of three high-dose protocols, based on prior treatment, as illustrated in Fig 1. Seventy-four patients who had been previously exposed to mediastinal or pelvic irradiation received carmustine (BCNU) (15 mg/kg, not to exceed 550 mg/m2), VP (60 mg/kg), and Cy (100 mg/kg). These doses had been previously established in a phase I study conducted at Stanford University. A regimen consisting of fTBI (1,200 cGy in 120 cGy fractions), VP (60 mg/kg), and Cy (100 mg/kg), which has been previously described, was given to 26 patients for whom it was not contraindicated (supra vide).14 If actual body weight exceeded ideal body weight, doses of BCNU and Cy were based on the formula: ideal body weight + 25% (actual − ideal body weight). Three patients with recurrent, previously unirradiated mediastinal masses received the BCNU/VP/Cy regimen in anticipation of posttransplantation irradiation. During the course of this study, a subgroup of patients was identified who had impaired pulmonary function, defined as a diffusing capacity <60%, or a history of radiation or chemical pneumonitis. As this patient subgroup did not meet the eligibility criteria for either fTBI/VP/Cy or BCNU/VP/Cy, they were entered on a dose escalation trial in which CCNU was substituted for BCNU and the VP and Cy doses and schedule remained constant. As reported by Chao et al,23 the maximal tolerated dose of CCNU combined with VP and Cy defined by this preliminary study was 15 mg/kg.23
Three high-dose preparatory regimens used in combination with autografting. TBI was delivered in 10 fractions over 4 days. The dose and schedule of VP16 and Cy are identical in all three regimens. Eight of the CCNU/VP/Cy patients received lower doses of CCNU on a dose escalation trial as described in the text.
Three high-dose preparatory regimens used in combination with autografting. TBI was delivered in 10 fractions over 4 days. The dose and schedule of VP16 and Cy are identical in all three regimens. Eight of the CCNU/VP/Cy patients received lower doses of CCNU on a dose escalation trial as described in the text.
The subjects included in the current report include 12 patients who participated in the dose escalation trial and have been previously described.23 These patients received the following doses of CCNU with their respective indications: 6 mg/m2 (one patient: DLCO 31%,), 9 mg/m2 (3 patients: 1 DLCO 46%, 1 bleomycin pneumonitis, 1 active interstitial pneumonitis), 12 mg/m2 (3 patients: 2 with DLCO of 42% and 52% and 1 immediately status-postmantle irradiation), 15 mg/m2 (5 patients: 2 with active pneumonitis, 3 with DLCO <60%). Based on the encouraging results in these compromised patients, experience with this regimen was extended to 7 additional patients, one of whom had recently completed mantle irradiation and another for whom posttransplantation mediastinal irradiation was planned.
Source of hematopoietic progenitor cells.During the course of this study, the methods for procurement of hematopoietic progenitor cells were refined. The initial transplantation of autologous bone marrow (BM) in a patient who had received extensive prior treatment including 12 cycles of MOPP alternating with ABVD, 1 year of single agent CCNU, and irradiation to multiple bony sites was notable for slow engraftment. This patient subsequently received an allograft from a matched sibling donor. Thereafter, all patients were reconstituted with PBPC (dose range 10 to 126 × 108 mononuclear cells/kg) alone, in the case of prior pelvic irradiation, bone or BM disease (n = 44), or together with autologous marrow (n = 74). BM was collected as previously described.26 The procedure for obtaining autologous PBPC has also been previously described.27 After January 1995 BM was no longer procured. PBPC were mobilized with granulocyte colony-stimulating factor (G-CSF ) on commercial availability. In addition, four patients received VP16 at a dose of 2 G/m2 and two patients received cyclophosphamide at a dose of 4 G/m2 for the combined purposes of mobilization of PBPC and cytoreduction of HD.
Supportive care.Hospitalized patients stayed in private rooms with high-efficiency particulate air filtration systems. Nonabsorbable antibiotics or ciprofloxacin were administered to reduce gut contamination. Oral trimethoprim/sulfamethoxazole was initiated coincident with the preparatory regimen for 4 days and reinstituted on day 30, continuing for 30 days. Inhaled pentamidine was substituted in the event of sulfa allergy. From day +1 until discharge from hospital, patients received low dose amphotericin at 0.15 mg/kg daily. In the last year of the study period, patients treated with chemotherapy alone were discharged on day +1 and did not receive amphotericin. Vancomycin was routinely initiated when patients became neutropenic and broad spectrum antibiotics were started on the first febrile episode.
From November 1988 through March 1991, patients were eligible to participate in a double blind randomized study with granulocyte macrophage-CSF (GM-CSF ) beginning on day +1.28 After April 1991, 5 μg/kg G-CSF was initiated on day +1 and continued until the absolute neutrophil count exceeded 500/μL for at least 3 consecutive days. Blood products were irradiated and cytomegalovirus (CMV)− blood products were used for CMV− patients. Platelet counts were maintained at 10,000/μL and packed cell volumes were kept at 30% by transfusion of blood products. Low-dose heparin was given by continuous infusion at 100 μ/kg/d after December 1992 for prevention of hepatic veno-occlusive disease.
Posttransplantation irradiation.Irradiation was given to six carefully selected patients posttransplantation. The use of irradiation in this patient population has been extensively analyzed by Poen et al.29 Four patients received 18 to 40 Gy to the mediastinum as part of the planned treatment, whereas irradiation was administered to two patients with residual disease, one in the humerus and another in the lumbar spine.
Follow-up.Patients were closely evaluated at the medical center for the first 60 days posttransplantation, or longer as indicated. They were subsequently followed by the referring physicians with a recommendation for physical examination, chest x-ray, and blood studies every 1 to 2 months for the first year after transplantation; every 3 months for the second year; every 4 months for the third year; every 6 months for the fourth year; and annually thereafter. Computed tomography (CT) scans were recommended at least six-monthly during the first 2 years posttransplantation, and more often as indicated. Thereafter, CT scans were recommended yearly through 5 years after the autotransplant. BM biopsies were performed at 30, 60, and 100 days to assess engraftment and annually to determine that HD was not present.
Statistics.Initial stage and therapy characteristics as well as characteristics at transplant were summarized for each patient using descriptive statistics and comparisons were made across preparatory regimens by the Pearson chi-square test. Significant differences (P < .05) are reported in Tables 1 and 2. Probabilities of event-free survival, overall survival and freedom from progression over time were estimated with the product-limit method of Kaplan and Meier. Disease progression and death from any cause defined events in the calculation of event-free survival. Progression of HD was the only event defined in freedom from progression; toxic deaths and second malignancies were censored. All calculations were made from the date of transplantation. Differences between preparatory regimens were compared by log-rank statistics.
Characteristics at Transplant
| Characteristic . | CCNU . | BCNU . | FTBI . | Total . |
|---|---|---|---|---|
| . | n = 19 . | n = 74 . | n = 26 . | n = 119 . |
| N Patients | ||||
| Median age at transplant in yrs | 32 | 30.5 | 30.5 | 30.5 |
| Sex | ||||
| Male | 14 | 43 | 9 | 66 |
| Female | 12 | 31 | 10 | 53 |
| Stage at presentation for transplantation | ||||
| I | 2 | 5 | 1 | 8 |
| II | 5 | 33 | 3 | 41 |
| III | 2 | 14 | 7 | 23 |
| IV | 10 | 22 | 15 | 47 |
| Symptoms at presentation for transplantation* | ||||
| No | 10 | 49 | 8 | 65 |
| Yes | 9 | 25 | 18 | 51 |
| Status immediately before high-dose therapy† | ||||
| Minimal disease | 5 | 57 | 22 | 84 |
| Partial remission | 8 | 14 | 4 | 26 |
| Progressive disease | 2 | 1 | 3 | |
| No. extranodal sites at presentation for transplantation | ||||
| None | 9 | 41 | 10 | 60 |
| One | 7 | 21 | 9 | 37 |
| Two or more | 3 | 12 | 7 | 22 |
| Stage IV sites at presentation for transplantation | ||||
| BM | 5 | 7 | 2 | 14 |
| Lung | 3 | 11 | 8 | 22 |
| Bone | 0 | 10 | 5 | 15 |
| Liver | 3 | 1 | 1 | 5 |
| Other | 1 | 8 | 4 | 13 |
| Characteristic . | CCNU . | BCNU . | FTBI . | Total . |
|---|---|---|---|---|
| . | n = 19 . | n = 74 . | n = 26 . | n = 119 . |
| N Patients | ||||
| Median age at transplant in yrs | 32 | 30.5 | 30.5 | 30.5 |
| Sex | ||||
| Male | 14 | 43 | 9 | 66 |
| Female | 12 | 31 | 10 | 53 |
| Stage at presentation for transplantation | ||||
| I | 2 | 5 | 1 | 8 |
| II | 5 | 33 | 3 | 41 |
| III | 2 | 14 | 7 | 23 |
| IV | 10 | 22 | 15 | 47 |
| Symptoms at presentation for transplantation* | ||||
| No | 10 | 49 | 8 | 65 |
| Yes | 9 | 25 | 18 | 51 |
| Status immediately before high-dose therapy† | ||||
| Minimal disease | 5 | 57 | 22 | 84 |
| Partial remission | 8 | 14 | 4 | 26 |
| Progressive disease | 2 | 1 | 3 | |
| No. extranodal sites at presentation for transplantation | ||||
| None | 9 | 41 | 10 | 60 |
| One | 7 | 21 | 9 | 37 |
| Two or more | 3 | 12 | 7 | 22 |
| Stage IV sites at presentation for transplantation | ||||
| BM | 5 | 7 | 2 | 14 |
| Lung | 3 | 11 | 8 | 22 |
| Bone | 0 | 10 | 5 | 15 |
| Liver | 3 | 1 | 1 | 5 |
| Other | 1 | 8 | 4 | 13 |
Significant (P ≤ .05) differences in distribution.
See text for definitions. Data not available for 6 patients.
Patient characteristics were subjected to univariate analysis for each of the outcome variables (survival, event-free survival, freedom from progression). The risk ratio was calculated for each significant variable along with confidence intervals. All variables found to have a P value of <0.10 in univariate evaluation were considered to be candidate variables for the stepwise Cox regression analysis.
Patient population.The characteristics of the 119 participants in this study are outlined in Tables 1 and 2 according to the preparatory regimen. Stage and symptoms at diagnosis and details of previous therapy are described in Table 1 whereas Table 2 illustrates the characteristics of these patients on presentation for consideration of transplantation. The majority of patients received BCNU/VP/Cy on the basis of prior radiotherapy and adequate pulmonary function. Patients treated with fTBI/VP/Cy were more likely to have had stage IV disease and received more than four drugs in their initial course of treatment. Because the fTBI/VP/Cy group was generally radiotherapy-naive, they were less likely to have relapsed in previously irradiated sites and more likely to have radiotherapy incorporated in the cytoreductive regimen.
As indicated in Table 1, 29 patients were considered to be failures of induction. Fourteen patients progressed during primary treatment. Nine additional patients were considered less than complete responders, failures of induction, because they had progressive disease within 4 weeks of best clinical response. Because patients with HD routinely have residual radiographic abnormalities, they are often observed off therapy and the completeness of response is judged retrospectively. Six additional patients were considered to have failed induction therapy as they had progressive HD at 34, 40, 42, 47, 53, and 58 days after their last treatment. As per Reece et al30 we demanded unequivocal progression of HD. The median time to failure in this group was 5 days as compared with 2 months in the group analyzed by Reece et al. The majority (62%) of patients in our series experienced either induction failure or disease progression within 1 year of the completion of primary therapy. Only 39 patients had enjoyed an initial remission following chemotherapy of 1 year or more. Because of the selection criteria for the preparatory regimen, some notable imbalances are apparent in initial stage at diagnosis and primary therapy (Table 1).
The first treatment course for 16 patients was radiotherapy alone; they are not included in the tabulation of the number of drugs in the initial treatment course or the number of prior chemotherapy courses. Thirteen of these patients had failed primary irradiation and salvage chemotherapy and three had failed irradiation and two courses of second-line treatment. Cytoreductive chemotherapy was not considered in the enumeration of prior chemotherapy courses. The majority of patients had been treated with more than four chemotherapy drugs; the remainder had usually received ABVD alone or in combination with radiotherapy.
The median age for all patients presenting for transplantation was 30.5 years (range 15 to 45). The fTBI/VP/Cy subgroup was more likely to have advanced stage disease and constitutional symptoms on presentation for consideration for transplantation than patients treated with BCNU/VP/Cy. As well as compromised pulmonary function, the CCNU/VP/Cy group had particularly refractory disease with a short initial remission duration and failure to achieve a minimal disease state as indicated in Tables 1 and 2. Status immediately before transplant refers to the response to cytoreductive chemotherapy or radiotherapy. Again, typical cytoreduction was one to three cycles of combination chemotherapy selected on the basis of prior treatments whereas a significant minority required more than one cytoreductive regimen. A relatively greater proportion of fTBI/VP/Cy patients were in a minimal disease state before receipt of the preparatory regimen. About half of the patient population had one or more extranodal disease sites on referral for transplantation. According to the Ann Arbor convention, patients with contiguous pleural, pericardial, chest wall, and pulmonary disease were considered to have limited disease with extranodal extension rather than stage IV disease. Nodular disease present in more than one lobe, which could not be encompassed in a single radiotherapy port, was necessary for a diagnosis of stage IV pulmonary parenchymal disease. On this basis, 45 patients had 69 disseminated (stage IV) extranodal disease sites. As indicated in Table 2 lung, bone, and BM were the most prevalent sites of disseminated disease in patients presenting for transplantation.
RESULTS
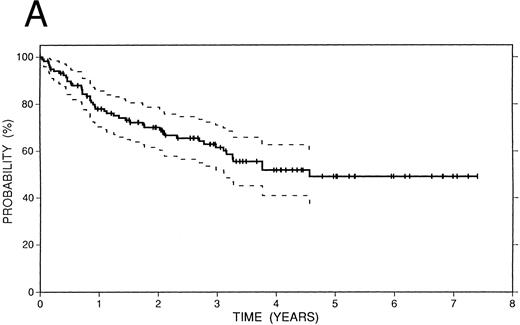
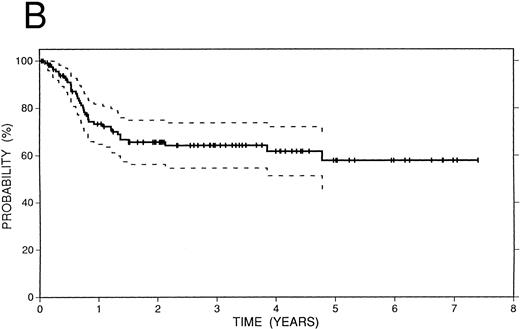
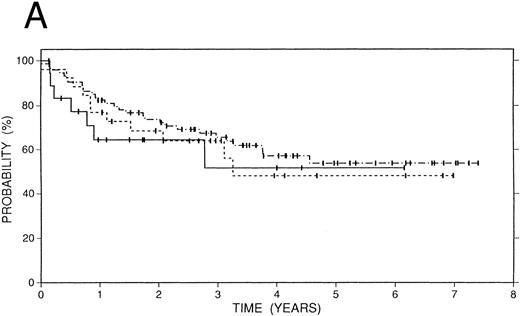
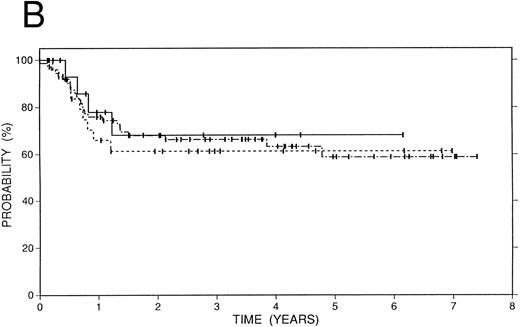
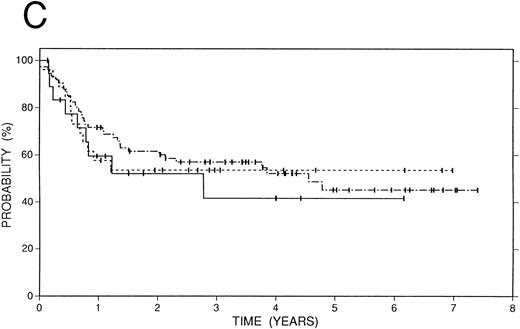
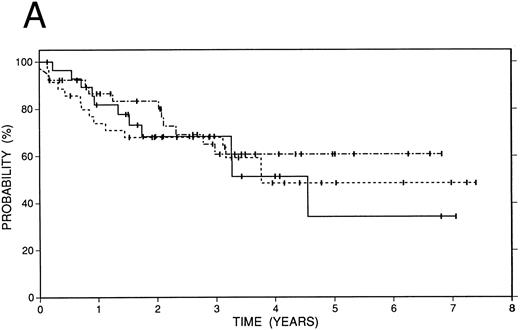
Survival data.The median followup of the entire group of patients is 40 months; it is similar for the BCNU/VP/Cy and fTBI/VP/Cy preparatory regimens but more abbreviated in the CCNU/VP/Cy group. Figure 2 illustrates the Kaplan-Meier survival curves for the entire population. The actuarial 4-year event-free survival was 48% (95% confidence intervals [CI] 38% to 59%). Overall survival at 4 years was 52% (CI 41% to 63%); and freedom from progression at the same time point was 62% (CI 51% to 72%). In Fig 3, these data are expressed according to preparatory regimen, demonstrating no statistically significant differences, despite the differences in clinical features described above.
Survival data in 119 patients with recurrent or refractory HD treated with high-dose therapy and autografting. (A) Overall survival: at 4 years 52% (CI 41% to 63%) of patients are projected to be alive. (B) Freedom from progression: At 4 years 62% (CI 51% to 72%) of patients are projected to be free of progressive HD. (C) Event-free survival: At 4 years 48% (CI 38% to 59%) of patients are projected to be alive and disease-free.
Survival data in 119 patients with recurrent or refractory HD treated with high-dose therapy and autografting. (A) Overall survival: at 4 years 52% (CI 41% to 63%) of patients are projected to be alive. (B) Freedom from progression: At 4 years 62% (CI 51% to 72%) of patients are projected to be free of progressive HD. (C) Event-free survival: At 4 years 48% (CI 38% to 59%) of patients are projected to be alive and disease-free.
Survival data in 119 patients with recurrent or refractory HD according to preparatory regimen received before autografting. CCNU/VP16/Cy (n = 19), solid line; BCNU/VP16/Cy (n = 74), dot and dash line; fTBI/VP16/Cy (n = 26), dotted line. (A) Overall survival. (B) Freedom from progression. (C) Event-free survival.
Survival data in 119 patients with recurrent or refractory HD according to preparatory regimen received before autografting. CCNU/VP16/Cy (n = 19), solid line; BCNU/VP16/Cy (n = 74), dot and dash line; fTBI/VP16/Cy (n = 26), dotted line. (A) Overall survival. (B) Freedom from progression. (C) Event-free survival.
Thirty-eight patients have developed recurrent HD from 1 month to 56 months (median = 8 months) after transplantation. In general, sites of relapse reflected sites of disease before cytoreduction. Only three patients relapsed more than 2 years after transplantation. There have been 46 deaths among the 119 patients of which 29 were due to progressive HD and 17 were due to other causes.
Hematopoietic recovery.The median time to absolute neutrophil count (ANC) >500/μL was 10 days (range 2 to 34). Platelet counts of 25,000/μL or greater were achieved a median time of 21 days after transplantation (range 9 to 195). As detailed below, four patients who did not suffer early transplant-related mortality or recurrence of HD failed to achieve complete engraftment (two for granulocytes and four for platelets). Time to engraftment was prolonged after extensive prior therapy, particularly total nodal irradiation. The time to ANC >500/μL also reflected the use of G-CSF.
Toxicity.Early treatment related mortality, defined as death without recurrent HD within 100 days of transplantation, occurred in six patients (5%). Causes of early death included veno-occlusive disease (one case after fTBI and another case following BCNU with 15 Gy hepatic irradiation included in the cytoreductive treatment), interstitial pneumonitis (one case after BCNU and cytoreduction with a large mediastinal field and another after CCNU in a patient with a history of pneumonitis), and respiratory failure in two patients, both of whom had received CCNU; one had Legionella and the other had active pneumonitis at the time that the high-dose regimen was administered. Five additional deaths occurring at a later time were attributed to the transplantation procedure, including four that were related to cytopenia. In each case, alkylating agent chemotherapy and extensive irradiation had been given earlier in the disease course and all preceded the commercial availability of hematopoietic colony stimulating factors. The remaining transplant-related death occurred at 5.5 months from a respiratory cause and likely represented BCNU toxicity. The incidence of transplant-related mortality declined during the course of autografting (no cases after January 1992), presumably related to better supportive care, including the use of colony stimulating factors and the early recognition and corticosteroid treatment for pneumonitis related to BCNU.
Six patients have developed and died from second malignancies (Table 3). Four patients were diagnosed with acute leukemia or MDS and one case each of breast cancer and lung cancer has occurred. Three of the secondary leukemia/MDS diagnoses (SPN 55, 123, 251) were characterized by loss of chromosome 7, a characteristic karyotypic abnormality seen in HD patients after exposure to alkylating agents alone or in combination with irradiation. The remaining patient had an acute monoblastic (M5) leukemia similar to those associated with topoisomerase II inhibitors. The rapid onset of breast cancer following the high-dose regimen in the field of prior irradiation in one patient was particularly remarkable.
Second Malignancy
| SPN . | Age at AG*/Sex . | Prior Therapy . | Preparatory Regimen . | Interval: Dx+ to Second Malignancy (mos) . | Interval: AG to Second Malignancy (mos) . | Diagnosis . |
|---|---|---|---|---|---|---|
| 55 | 45 M | MOPP × 6 | BCNU/VP/Cy | 121 | 23 | MDS |
| MOPP/ABVD × 6 | ||||||
| MOPP/BAP × 23-154 | ||||||
| Mantle radiation | ||||||
| 123 | 37 M | MOPP/ABVD × 12 | BCNU/VP/Cy | 89 | 50 | MDS |
| TLI3-167 | ||||||
| 251 | 35 M | PAVe × 83-155 | BCNU/VP/Cy | 52 | 12 | MDS |
| TLI | ||||||
| MOPP/ABV × 3 | ||||||
| 1,025 | 34 F | MOPP/ABV × 8 | CCNU/VP/Cy | 47 | 9 | Acute monoblastic leukemia |
| MOPP × 3 | ||||||
| Bronchus radiationρ | ||||||
| High dose VP3-152 | ||||||
| 483 | 48 M | PAVe × 6 | CCNU/VP/Cy | 65 | 27 | Non-small cell lung cancer |
| Mantle radiation | ||||||
| MOPP × 3 | ||||||
| 815 | 28 F | STLI3-160 | BCNU/VP/Cy | 136 | 1 | Breast cancer |
| MOPP × 6 | ||||||
| ABVD × 2 | ||||||
| DHAP × 2 |
| SPN . | Age at AG*/Sex . | Prior Therapy . | Preparatory Regimen . | Interval: Dx+ to Second Malignancy (mos) . | Interval: AG to Second Malignancy (mos) . | Diagnosis . |
|---|---|---|---|---|---|---|
| 55 | 45 M | MOPP × 6 | BCNU/VP/Cy | 121 | 23 | MDS |
| MOPP/ABVD × 6 | ||||||
| MOPP/BAP × 23-154 | ||||||
| Mantle radiation | ||||||
| 123 | 37 M | MOPP/ABVD × 12 | BCNU/VP/Cy | 89 | 50 | MDS |
| TLI3-167 | ||||||
| 251 | 35 M | PAVe × 83-155 | BCNU/VP/Cy | 52 | 12 | MDS |
| TLI | ||||||
| MOPP/ABV × 3 | ||||||
| 1,025 | 34 F | MOPP/ABV × 8 | CCNU/VP/Cy | 47 | 9 | Acute monoblastic leukemia |
| MOPP × 3 | ||||||
| Bronchus radiationρ | ||||||
| High dose VP3-152 | ||||||
| 483 | 48 M | PAVe × 6 | CCNU/VP/Cy | 65 | 27 | Non-small cell lung cancer |
| Mantle radiation | ||||||
| MOPP × 3 | ||||||
| 815 | 28 F | STLI3-160 | BCNU/VP/Cy | 136 | 1 | Breast cancer |
| MOPP × 6 | ||||||
| ABVD × 2 | ||||||
| DHAP × 2 |
AG, autograft.
Dx, diagnosis.
VP, 2 G/m2.
ρ Bronchus radiation, 800 cGy.
PAVe, procarbazine, alkeran, vinblastine.
BAP, bleomycin, adriamycin, prednisone.
TLI, total lymphoid irradiation.
STLI, subtotal lymphoid irradiation.
Regression analyses.Univariate analyses of the characteristics outlined in Tables 1 and 2 were undertaken to select covariates for multivariate analyses. Characteristics with a P value of .10 or less, as illustrated in Table 4, were identified for the three endpoints of interest: freedom from progression, event-free survival, and overall survival. Stage and number of extranodal sites were evaluated as continuous variables as reported in Table 4. They were also found to achieve significance when appraised as dichotomous variables as limited versus advanced stage and no versus one or more extranodal sites. Status immediately prior to transplant was considered to be complete remission/minimal disease versus more than minimal disease. On Cox regression analysis, three factors, each of which was descriptive of patient characteristics on presentation for autotransplantation, were consistently identified as predictive for these outcome parameters (Table 5). They included stage IV involvement of the pulmonary parenchyma or BM, constitutional symptoms, and the presence of more than minimal residual HD before the preparatory regimen. Overall, 44% of patients had B symptoms on presentation for transplantation; 30% had lung or BM involvement; and 18% had more than minimal disease immediately before transplant.
Univariate Analysis
| Endpoint/Variable . | P . | Risk Ratio . |
|---|---|---|
| Freedom from progression | ||
| Lung or marrow involvement at presentation for transplantation | .001 | 4.086 |
| No. of extranodal sites at presentation for transplantation | .007 | 1.465 |
| Stage at presentation for transplantation | .008 | 1.625 |
| Status immediately before transplant | .010 | 2.055 |
| Symptoms at presentation for transplantation | .023 | 2.132 |
| Cytoreductive RT | .096 | 0.366 |
| Overall survival | ||
| Lung or marrow involvement at presentation for transplantation | .003 | 2.410 |
| Symptoms at presentation for transplantation | .010 | 2.171 |
| No. of extranodal sites at presentation for transplantation | .035 | 1.321 |
| Status immediately before transplant | .054 | 1.692 |
| No. of prior CT courses | .086 | 1.478 |
| Stage at presentation for transplantation | .083 | 1.303 |
| Event-free survival | ||
| Lung or marrow involvement at presentation for transplantation | .002 | 2.412 |
| Symptoms at presentation for transplantation | .003 | 2.257 |
| Status immediately before transplant | .037 | 1.697 |
| No. of extranodal sites at presentation for transplantation | .042 | 1.299 |
| Stage at presentation for transplantation | .065 | 1.297 |
| No. of cytoreductive regimens | .087 | 1.400 |
| Endpoint/Variable . | P . | Risk Ratio . |
|---|---|---|
| Freedom from progression | ||
| Lung or marrow involvement at presentation for transplantation | .001 | 4.086 |
| No. of extranodal sites at presentation for transplantation | .007 | 1.465 |
| Stage at presentation for transplantation | .008 | 1.625 |
| Status immediately before transplant | .010 | 2.055 |
| Symptoms at presentation for transplantation | .023 | 2.132 |
| Cytoreductive RT | .096 | 0.366 |
| Overall survival | ||
| Lung or marrow involvement at presentation for transplantation | .003 | 2.410 |
| Symptoms at presentation for transplantation | .010 | 2.171 |
| No. of extranodal sites at presentation for transplantation | .035 | 1.321 |
| Status immediately before transplant | .054 | 1.692 |
| No. of prior CT courses | .086 | 1.478 |
| Stage at presentation for transplantation | .083 | 1.303 |
| Event-free survival | ||
| Lung or marrow involvement at presentation for transplantation | .002 | 2.412 |
| Symptoms at presentation for transplantation | .003 | 2.257 |
| Status immediately before transplant | .037 | 1.697 |
| No. of extranodal sites at presentation for transplantation | .042 | 1.299 |
| Stage at presentation for transplantation | .065 | 1.297 |
| No. of cytoreductive regimens | .087 | 1.400 |
Multivariate Analysis
| Endpoint/Variable . | P . | Risk Ratio . |
|---|---|---|
| Freedom from progression | ||
| Lung or marrow involvement at presentation for transplantation | .001 | 3.065 |
| Symptoms at presentation for transplantation | .033 | 2.050 |
| Status immediately before transplant | .047 | 1.797 |
| Overall survival | ||
| Status immediately before transplant | .012 | 2.348 |
| Lung or marrow involvement at presentation for transplantation | .024 | 2.074 |
| Symptoms at presentation for transplantation | .041 | 1.875 |
| Event-free survival | ||
| Symptoms at presentation for transplantation | .014 | 2.014 |
| Status immediately before transplant | .016 | 2.191 |
| Lung or marrow involvement at presentation for transplantation | .036 | 1.926 |
| Endpoint/Variable . | P . | Risk Ratio . |
|---|---|---|
| Freedom from progression | ||
| Lung or marrow involvement at presentation for transplantation | .001 | 3.065 |
| Symptoms at presentation for transplantation | .033 | 2.050 |
| Status immediately before transplant | .047 | 1.797 |
| Overall survival | ||
| Status immediately before transplant | .012 | 2.348 |
| Lung or marrow involvement at presentation for transplantation | .024 | 2.074 |
| Symptoms at presentation for transplantation | .041 | 1.875 |
| Event-free survival | ||
| Symptoms at presentation for transplantation | .014 | 2.014 |
| Status immediately before transplant | .016 | 2.191 |
| Lung or marrow involvement at presentation for transplantation | .036 | 1.926 |
The risk associated with the number of these factors present, none to 3, was then further considered. The P values for the number of risk factors were highly significant for each endpoint as follows: freedom from progression — .0001, overall survival — .0003, event-free survival — .0001. The risk ratios associated with these endpoints were: freedom from progression 2.24, overall survival 2.18, and event-free survival 1.97. The correlation of the number of factors present with each of the outcome variables suggested that these could reliably form the basis of a prognostic factors index. This possibility was further assessed by exploring each of the possible combinations for these factors (23 = 8). As outlined in Table 6 for each of the outcome variables, the different combinations consistently show the relationship of the number of prognostic factors with outcome.
Survival Data By Factor Distribution
| N Factors . | Symptoms . | Lung/Marrow . | Status . | OS% . | FFP% . | EFS% . |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 77 | 82 | 71 |
| 1 | X | 0 | 0 | 64 | 71 | 54 |
| 0 | X | 0 | 62 | 52 | 51 | |
| 0 | 0 | X | 56 | 68 | 47 | |
| 2 | X | X | 0 | 45 | 33 | 29 |
| X | 0 | X | 38 | 52 | 26 | |
| 0 | X | X | 35 | 28 | 23 | |
| 3 | X | X | X | 17 | 12 | 7 |
| N Factors . | Symptoms . | Lung/Marrow . | Status . | OS% . | FFP% . | EFS% . |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 77 | 82 | 71 |
| 1 | X | 0 | 0 | 64 | 71 | 54 |
| 0 | X | 0 | 62 | 52 | 51 | |
| 0 | 0 | X | 56 | 68 | 47 | |
| 2 | X | X | 0 | 45 | 33 | 29 |
| X | 0 | X | 38 | 52 | 26 | |
| 0 | X | X | 35 | 28 | 23 | |
| 3 | X | X | X | 17 | 12 | 7 |
Figure 4 illustrates the Kaplan-Meier plot of freedom from progression according to the number of risk factors present. This endpoint was selected because it was anticipated that it would become increasingly important as patients are considered for autotransplantation earlier in their disease course and early transplant-related mortality continues to decline. The 3-year FFP was 85%, 57%, 41%, and <20% for 0, 1, 2, and 3 factors, respectively (Fig 4A). In Fig 4B, the actuarial freedom from progression for patients with no unfavorable factors (n = 46) is contrasted with those with one or more (n = 68). The difference between the two-survival plots, with 4-year FFP of 85% and 41%, is highly statistically significant (P = .0001).
Freedom from progression in 119 patients with recurrent or refractory HD treated with high-dose therapy and autografting according to number of prognostic factors. See text for description of prognostic factors. (A) None (n = 46), solid line; one (n = 39), dotted line, two (n = 23), dot and dash line; three (n = 6), grey line. (B) None (n = 46), solid line or 1 to 3 unfavorable prognostic factors (n = 68), dotted line.
Freedom from progression in 119 patients with recurrent or refractory HD treated with high-dose therapy and autografting according to number of prognostic factors. See text for description of prognostic factors. (A) None (n = 46), solid line; one (n = 39), dotted line, two (n = 23), dot and dash line; three (n = 6), grey line. (B) None (n = 46), solid line or 1 to 3 unfavorable prognostic factors (n = 68), dotted line.
DISCUSSION
Since its introduction more than 15 years ago, high-dose therapy and autografting has become the treatment of choice for many patients with HD who have failed or relapsed after induction chemotherapy. The increased popularity of the high-dose approach can be attributed to a marked reduction in early transplant-related mortality, highly encouraging disease-free survival from multiple centers and the widespread availability of this approach. Major issues to be addressed in comparative clinical trials include the ideal timing of high-dose therapy, the optimal preparatory regimen, definition of widely adopted prognostic factors, the role of additional conventional therapy and the reduction of adverse late effects.
In contrast to the early transplant literature where relapse rates in excess of 60% and transplant-related mortality rates of 20% or greater were reported, more recent results, such as those from Stanford, show lower relapse rates, 30% to 50%, and less than 10% early transplant related mortality.7-21 Maturation of data from several major centers strongly suggests that the high-dose therapy and autografting results are superior to those achieved with conventional therapy, and this is particularly true of patients who progress during or within 12 months of induction chemotherapy.13,30,31 Comparison of high-dose and conventional therapy approaches outside of a randomized trial will always be subject to criticism because of issues of patient selection. We have conducted a retrospective analysis of patients with refractory disease or in first relapse, matched for demographic and disease parameters, who were treated at our institution with conventional chemotherapy or high-dose therapy and autografting. The results of this analysis, in which significant improvements in overall and event-free survival and freedom from progression were seen with high-dose therapy and autografting in HD patients who progressed within 12 months of induction chemotherapy, are presented in a separate manuscript.32
Based on a number of assumptions, in many cases lacking actual data, Desch et al33 concluded from decision analysis that optimal timing of autografting would be in second relapse unless seven or eight drugs were used for primary induction therapy. Support for the use of high-dose therapy in first relapse comes from the retrospective analyses of the mature data from Nebraska and the M.D. Anderson as well as the very excellent results reported from Vancouver.21,31 However, Chopra et al,11 found that patients in second or third relapse actually enjoyed disease-free survival superior to those in first relapse. This apparent contradiction relates to the biologic aggressiveness of the underlying HD, as many of the first relapse patients in the London series were selected for particularly refractory HD. The variable biology of HD points out the necessity for randomized trials and long-term followup.
Regarding the optimal high-dose regimen, Bierman et al21 report minimal or no transplant-related mortality with conventional dose CBV in contrast to that seen with the augmented doses used in Vancouver and at the City of Hope (COH) and Stanford.11,19 Although excellent outcomes have been reported with the higher dose BCNU regimens used in these institutions, it is not clear that augmented BCNU or TBI-containing regimens are superior to the lower dose CBV regimen or BEAM chemotherapy. Although a number of authors reported pulmonary toxicity with high-dose BCNU (>600 mg/m2), no difference in interstitial pneumonitis was seen in a nonrandomized study of allograft recipients in Seattle whether a dose of 300 mg/m2 or 600 mg/m2 was given.19 34-36 Since it is intuitive that higher doses of BCNU may translate into greater lung or marrow toxicity, a toxicity question might reasonably be addressed in the subgroup of recurrent HD patients with favorable prognostic features.
Survival data after high-dose therapy and autografting in 103 patients according to duration of initial chemotherapy remission: induction failure (n = 29), solid line; remission 1 year or less (n = 35), dotted line; remission greater than 1 year (n = 39), dot and dash line. (A) Survival. (B) Freedom from progression.
Survival data after high-dose therapy and autografting in 103 patients according to duration of initial chemotherapy remission: induction failure (n = 29), solid line; remission 1 year or less (n = 35), dotted line; remission greater than 1 year (n = 39), dot and dash line. (A) Survival. (B) Freedom from progression.
Prognostic factors associated with high-dose therapy and autografting include bulk of disease at transplant,9,11,12,31 number of prior treatment regimens,9,11,17,21,31 duration of initial remission,31 systemic symptoms at relapse,9,17,31 performance status,21,37 extranodal disease at relapse17,31 and relapse within a prior radiotherapy field.12 Different definitions and the variable distribution of prognostic factors make it difficult to compare results across studies. For instance, in the series from the COH the most important variable was the number of prior regimens.17 Although fewer patients in the COH series had failed just one prior regimen compared with the Stanford series, Nademanee et al17 transplanted fewer patients with extranodal disease or constitutional symptoms, which were significant features in the current series. Likewise, it is difficult to compare the generally outstanding preliminary results reported by Reece et al31 in patients in first relapse of HD, where marrow disease was an exclusion parameter, with the current study in which BM involvement was a powerful prognostic factor. While we did not find initial remission duration, calculated only for patients who received combination chemotherapy as their initial treatment, to be an independently significant variable, a trend toward fewer relapses was seen in the subgroup of patients who enjoyed an initial remission longer than 1 year (Fig 5).
Disseminated pulmonary involvement was also an unfavorable factor in the Stanford experience, in agreement with some previous reports.38,39 The University College of London group (London, UK) reported no differences in patients with or without pulmonary HD in their transplant series but they did not discriminate between contiguous versus disseminated disease and included thoracic disease of multiple types, both before and after relapse.40 Despite these differences, a consistent theme which emerges is that the most significant patient characteristics are those at presentation for transplantation, including the prior treatment history, the extent of disease and systemic symptoms, and the response to conventional therapy given immediately before the preparatory regimen.
Although we incorporated conventional cytoreduction therapy into our treatment plan, the impact of this strategy on disease-free survival is unclear. Certainly such a practice allows some measure of drug sensitivity. Whereas the majority of patients in the Stanford series were in a minimal disease state immediately before the administration of high-dose therapy, a significant number (30%) were relatively resistant to their initial cytoreductive chemotherapy. Bulky disease may be a biologic surrogate representing the need for more effective as well as simply greater cumulative cytoreduction. This may explain the observation of better outcomes in untested relapse, presumably a patient population with significantly less tumor volume, by investigators from London and Omaha.11,41 Based on the relatively small proportion of patients who were eligible for fTBI in our series, the unacceptable toxicity profile of pretransplant irradiation (at least to the thorax and liver), the reduced relapse rate noted in sites that were irradiated peritransplant in our patients as analyzed by Poen et al,29 and the known dose-response characteristics of HD, we currently prefer to incorporate radiotherapy after transplantation at doses of 30 to 36 Gy directed to a smaller volume.
Given the high rates of cure anticipated for patients newly diagnosed with Hodgkin's disease of all stages, current research efforts are directed toward reducing early and late morbidity and the incidence of intercurrent death. Increasing success with high-dose therapy is bringing these same considerations to bear in the recurrent disease setting. We have piloted the CCNU/VP/Cy regimen to address BCNU pulmonary toxicity in patients at very high risk for early or late morbidity. In addition to a possibly more favorable toxicity profile, CCNU is established to be the superior nitrosurea in untreated HD.24 Although the experience with CCNU/VP/Cy was not without respiratory toxicity, it proved to be feasible in the majority of patients in this high-risk population.23 Lower doses of BCNU or the concomitant use of a chemoprotectant might also be anticipated to lessen the incidence of clinically significant, early or late pulmonary toxicity.
MDS/secondary leukemia, a complication of HD treatment known to be associated with cumulative doses of alkylating agents and possibly further promoted by wide-field radiotherapy, is emerging as a major cause of morbidity and mortality in patients successfully treated with high-dose therapy and autografting.25 42-45 Because alkylating agents comprise a mainstay of high-dose therapy, one strategy might be to reserve this category of drugs for second-line treatment. In contrast to reports in the non-Hodgkin's lymphomas, where the combination of TBI and alkylating agents has been implicated, all cases of MDS/secondary leukemia in our series occurred in patients who received a chemotherapy-only preparatory regimen. Secondary leukemia may also result from topoisomerase II inhibitors such as VP, suspected in one of our patients who received a total VP dose of 4.2 G/m2, in which case the clinical picture usually involves a monoblastic morphology, short interval to diagnosis and an 11q23 cytogenetic abnormality. Whereas toxicity issues are assuming greater significance in the subgroup of recurrent or refractory HD patients with a relatively favorable prognosis after high-dose therapy, a significant subset of patients with a 50% or greater risk of failure with current high-dose approaches persists. New therapeutic initiatives are required for these patients.
It must be emphasized that long-term follow-up is requisite to address these important issues of efficacy and toxicity. Data from Stanford University with conventional salvage therapy define a clear plateau on the disease-free survival curve after about 7 years.32 Similarly, the combined data from Nebraska and the M.D. Anderson show a continuous pattern of failure for about 7 to 8 years after transplantation. Although the failures after 2 years appeared to be less frequent with higher doses of BCNU in the Stanford University and Vancouver series, both of these studies require longer follow-up for validation. A desire to reduce late effects at 10 or more years is currently driving clinical research efforts in untreated HD. History will no doubt repeat itself in the setting of recurrent disease, where we must first reliably accomplish high rates of cure, after which we can turn our attention to the quality of life following high-dose therapy. It is now time to bring the discipline of randomized trials, designed to address major questions, to the setting of high-dose therapy and autografting for refractory or recurrent HD.
Supported in part by the Grant No. NIH CA 49605 (K.G.B.), Marrow Grafting for Lymphoma, Project II: Bone Marrow-Grafting for Leukemia and Lymphoma).
Address reprint requests to Sandra J. Horning, MD, 1000 Welch Rd, Suite 202, Palo Alto, CA 94304.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal