Abstract
Between August 1990 and August 1995, 231 patients (median age 51, 53% Durie-Salmon stage III, median serum β-2-microglobulin 3.1 g/L, median C-reactive protein 4 g/L) with symptomatic multiple myeloma were enrolled in a program that used a series of induction regimens and two cycles of high-dose therapy (“Total Therapy”). Remission induction utilized non–cross-resistant regimens (vincristine-doxorubicin-dexamethasone [VAD], high-dose cyclophosphamide and granulocyte-macrophage colony-stimulating factor with peripheral blood stem cell collection, and etoposide-dexamethasone-cytarabine-cisplatin). The first high-dose treatment comprised melphalan 200 mg/m2 and was repeated if complete (CR) or partial (PR) remission was maintained after the first transplant; in case of less than PR, total body irradiation or cyclophosphamide was added. Interferon--2b maintenance was used after the second autotransplant. Fourteen patients with HLA-compatible donors underwent an allograft as their second high-dose therapy cycle. Eighty-eight percent completed induction therapy whereas first and second transplants were performed in 84% and 71% (the majority within 8 and 15 months, respectively). Eight patients (3%) died of toxicity during induction, and 2 (1%) and 6 (4%) during the two transplants. True CR and at least a PR (PR plus CR) were obtained in 5% (34%) after VAD, 15% (65%) at the end of induction, and 26% (75%) after the first and 41% (83%) after the second transplants (intent-to-treat). Median overall (OS) and event-free (EFS) survival durations were 68 and 43 months, respectively. Actuarial 5-year OS and EFS rates were 58% and 42%, respectively. The median time to disease progression or relapse was 52 months. Among the 94 patients achieving CR, the median CR duration was 50 months. On multivariate analysis, superior EFS and OS were observed in the absence of unfavorable karyotypes (11q breakpoint abnormalities, -13 or 13-q) and with low β-2-microglobulin at diagnosis. CR duration was significantly longer with early onset of CR and favorable karyotypes. Time-dependent covariate analysis suggested that timely application of a second transplant extended both EFS and OS significantly, independent of cytogenetics and β-2-microglobulin. Total Therapy represents a comprehensive treatment approach for newly diagnosed myeloma patients, using multi-regimen induction and tandem transplantation followed by interferon maintenance. As a result, the proportion of patients attaining CR increased progressively with continuing therapy. This observation is particularly important because CR is a sine qua non for long-term disease control and, eventually, cure.
CURE HAS REMAINED an elusive goal of myeloma therapy. With standard melphalan-prednisone (MP) or combination chemotherapy using additional cytotoxic drugs, stringently defined complete remission (CR) rates have not exceeded 5% and median survival has not been extended beyond 3 years.1,2 When high-dose therapy with melphalan (MEL) was introduced in the mid 1980s, CRs were observed more frequently, and attainment of CR became a primary trial objective as a potential prelude to long-term disease control.3-7 Extensive phase II studies, initially with autologous bone marrow transplants (ABMT) and, more recently, with mobilized peripheral blood stem cell (PBSC) support (decreasing the duration of marrow aplasia and treatment-related mortality [TRM]), indicated that drug resistance could be overcome by dose escalation. Thus, in newly diagnosed disease, up to 50% of patients achieved stringently defined CR.8-13 With PBSC, the duration of bone marrow aplasia was shortened to a few days so that TRM could be reduced to well under 5%.14 A randomized trial by the Intergroupe Francais du Myélome (IFM) showed better outcome with high-dose therapy than with standard chemotherapy among 200 patients with newly diagnosed disease.15
In 1989, the “Total Therapy” program was designed using all treatment tools available at the time to maximize the chance of CR induction in newly diagnosed myeloma patients.16 After induction chemotherapy with non–cross-resistant regimens of vincristine, doxorubicin, dexamethasone (VAD); high-dose cyclophosphamide (HDCTX); and etoposide, dexamethasone, cytarabine, cisplatin (EDAP) patients underwent two cycles of MEL-based high-dose therapy with PBSC support and subsequent interferon (IFN) maintenance. With a median follow-up of 4.2 years among surviving patients, we now report the final results of this trial. A comparative analysis of a subset of 123 untreated patients receiving Total Therapy with patients receiving standard therapy according to Southwest Oncology Group protocols has been reported previously.17
MATERIALS AND METHODS
Total Therapy.
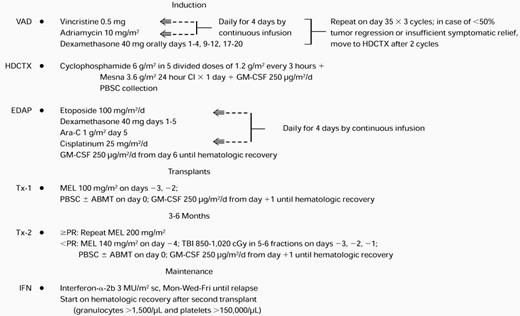
The treatment plan is outlined in Table 1. The VAD regimen was applied in standard fashion for two to three cycles because of its marked and speedy tumor cell kill without inflicting hematopoietic stem cell compromise.18 After intravenous hydration for 12 hours, HDCTX was administered at doses of 1.5 g/m2 every 4 hours ×4 for a total of 6 g/m2 along with mesna 6 g/m2 followed by subcutaneous administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) 250 μg/m2 beginning on day 2 (modified from Gianni et al19). On hematopoietic recovery with platelets reaching at least 50,000/μL, PBSC collection was performed as described previously.20 Daily aphereses of approximately 15 L of blood were continued until at least 5 × 106 CD34 cells/kg were collected (a quantity judged necessary for the safe conduct of two autotransplants). As a safety precaution, the first 69 patients also had autologous bone marrow collected under general anesthesia provided that bone marrow plasmacytosis did not exceed 30%. On completion of PBSC collection, the EDAP chemotherapy was administered on an outpatient basis,21 consisting of etoposide 100 mg/m2daily ×4 by continuous infusion, dexamethasone 40 mg orally daily ×4, cytarabine 1 g/m2 intravenously over 2 hours on day 5, and cisplatin 25 mg/m2 daily ×4 by continuous infusion, followed by GM-CSF 250 μg/m2 beginning on day 6 and administered subcutaneously on a daily basis until granulocytes exceeded 2,000/μL for 3 days. The rationale for inclusion of EDAP was to target immature tumor cells believed to be present in most patients with myeloma.22
Total Therapy Regimen
Abbreviations: sc, subcutaneous; Mon, Monday; Wed, Wednesday; Fri, Friday.
In the absence of tumor progression (>25% increase in tumor mass), patients proceeded through the entire induction phase and were then offered a first high-dose therapy cycle with MEL 200 mg/m2(in two doses of 100 mg/m2 on days −3 and −2) followed by administration of autologous stem cells on day 0. Standard supportive care included antibacterial, antifungal, and antiviral prophylaxis and blood cell component support as needed. In case of sustained partial remission (PR, see below) or CR, a second autotransplant with MEL 200 mg/m2 was performed. The remaining patients received either MEL 140 mg/m2 plus total body irradiation (TBI) 850-1125 cGy or other regimens, usually MEL 200 mg/m2 plus HDCTX 120 mg/kg in case prior radiotherapy prevented the application of TBI. Eleven patients under the age of 50 years with HLA-compatible siblings were allografted after a first autotransplant. The conditioning regimen consisted of MEL 140 mg/m2 plus TBI 1125 cGy. Three additional patients received a T-cell–depleted matched unrelated donor (MUD) bone marrow transplant after conditioning with thiotepa 10 mg/kg, HDCTX 120 mg/kg, and TBI 1375 cGy in 11 fractions of 125 cGy each. The intent was to complete the second transplant within 3 to 6 months of the first. After completion of two autotransplants, IFN maintenance was commenced at 3 million U/m2 subcutaneously thrice weekly when granulocytes exceeded 1,500/μL and platelets exceeded 100,000/μL and continued until disease relapse. The protocol had a provision for potential insurance denial, in which case, at the end of induction with EDAP, MEL 70 mg/m2 with subsequent subcutaneous GM-CSF 250 μg/m2 was administered, followed by cyclical administration of VAD and intermediate-dose cyclophosphamide (1 g/m2) along with IFN for 2 years, followed by IFN maintenance indefinitely as with autotransplant recipients. There were only seven patients in whom this nontransplant strategy was applied.
All patients signed an informed consent in keeping with guidelines of the National Cancer Institute, which had reviewed the Total Therapy program, and of the Institutional Review Board of the University of Arkansas for Medical Sciences and the Arkansas Cancer Research Center. Patient entry began in August 1990 and continued through August 1995. More than 95% of consecutive eligible patients (see below) were enrolled during this time interval; only a few patients declined study participation.
Eligibility criteria.
Eligible patients had to have symptomatic multiple myeloma using standard diagnostic criteria.23 They had to be previously untreated or had to have received only one cycle of standard chemotherapy. Prior local radiation was permitted. A modified Durie-Salmon staging system was applied whereby bone lesions were enumerated not according to the actual number of lesions but according to the number of sites of involvement, using skull, cervical, thoracic, and lumbar spine as well as pelvis and long bones as distinct sites. An upper age limit of 70 years was imposed, although protocol exception was obtained for one patient who was enrolled at the age of 71 years because his physiological status was deemed adequate to withstand the potential toxicity from the Total Therapy program. Adequate cardiopulmonary function had to be present at the time of entry on study including a systolic cardiac ejection fraction ≥50%. Patients could have renal function impairment (creatinine >2 mg/dL, 9%) at protocol entry or poor performance status related to multiple myeloma (Zubrod >2, 8% at diagnosis). However, renal function had to improve after VAD so that serum creatinine could not exceed 2 mg/dL at the time of initiation of HDCTX as well as before first and second transplant. In addition to standard laboratory parameters, cytogenetic24 and morphological bone marrow examinations were performed.25 Patient follow-up was performed according to protocol guidelines, usually on a monthly basis, to record treatment-related toxicity and antitumor effect.
Response, event-free survival (EFS), and overall survival (OS).
CR required the disappearance of monoclonal gammopathy in serum and urine on immunofixation analysis and attainment of normal bone marrow aspirate and biopsy with <1% light chain-restricted plasma cells on flow cytometry, on at least two successive occasions at least 2 months apart. PR implied ≥75% tumor mass reduction including a normal marrow aspirate and biopsy and, in case of Bence Jones proteinuria, reduction to <100 mg/day. For computation of PR and CR rates, all patients were eligible; those dying early before antitumor effect could be established were considered treatment failures. TRM included any death within 60 days for autotransplants and within 100 days for allotransplants. EFS and OS were dated from the time of initiation of the first cycle of VAD, whereas CR duration was computed from the onset of CR. Events included disease progression/relapse or death from any cause. Relapse was defined as recurrence of monoclonal protein on immunofixation or bone marrow plasmacytosis in case of CR and a 25% increase from minimal tumor mass in case of PR. Disease progression for nonresponsive patients implied at least a 25% increase in tumor mass. Unless otherwise specified, all analyses were performed on an intent-to-treat basis.
Statistical analysis.
Data were analyzed as of September 1997. EFS and OS distributions were estimated by the product-limit method.26 EFS and OS among categorical prognostic variables measured before start of therapy were compared using the log-rank test.27 EFS and OS comparisons among categorical variables measured after start of therapy were made using landmark analysis.28 Cox regression was used to examine continuous and categorical univariate and multivariate effects of prognostic features on EFS and OS.29 Variables measured after start of therapy were incorporated in the Cox models as time-dependent covariates. Cumulative incidence distributions were calculated for the competing risks of disease-related progression/death (time to progression) and TRM.30 The chi-square test or Fisher’s exact test (where appropriate) were used to compare cross tabulations of categorical variables.
RESULTS
Patient and disease characteristics.
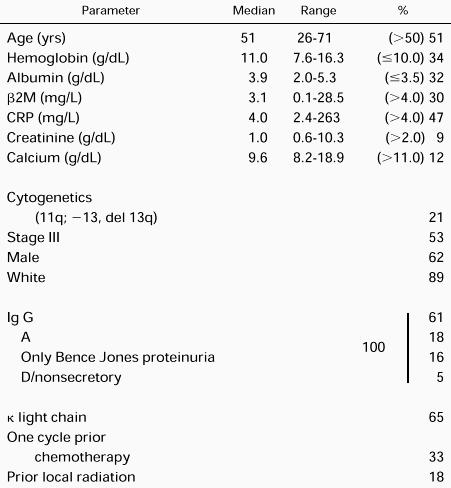
Table 2 depicts the pertinent demographic features, which are representative of our referral population of newly diagnosed patients. As indicated, tumor staging was modified so that bone lesions were enumerated according to the number of regional bone sites rather than the number of individual lesions (see Eligibility criteria). Considering hemoglobin (<8.5 g/dL), calcium (>12 mg/dL), albumin (<3.0 g/dL), β-2-microglobulin (β2M; >6 mg/L) and C-reactive protein (CRP; >6 mg/L), 30% had at least one, 14% had two, 4% had three, and 2% had four high-stage–associated features. Sixty-seven percent were untreated, 33% had one cycle of prior chemotherapy, and 18% had prior local radiation.
Patient flow through total therapy (Table3 and Fig 1).
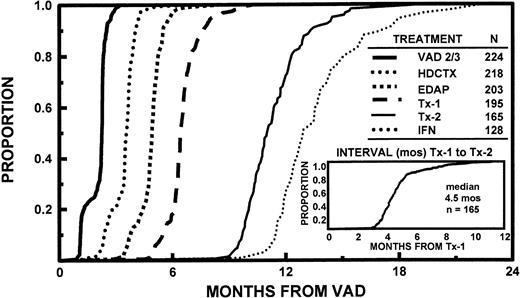
All 231 patients completed one cycle of VAD, 224 received two cycles, and 174 received three cycles. By protocol design, HDCTX could be administered after completion of two cycles of VAD in case >50% tumor mass reduction was not obtained or insufficient subjective improvement, especially in pain, had not been effected. Remission induction deaths included 1 patient with the first and 4 additional patients with the second and third cycle of VAD (total of 3 suicides related to dexamethasone-induced psychosis), and 1 after HDCTX and 2 after EDAP for a total of 8 (3% of 231 patients). Additional off-study reasons pertained to excessive toxicity in 8, disease progression in 6, or decline in further trial participation in 7 patients; only 7 patients were denied insurance coverage and were entered on the nontransplant arm of the study (see Materials and Methods and Table 3). One hundred ninety-five patients (84%) completed one transplant and 165 patients (71%) completed two transplants, 84% within 6 months and all within 1 year of the first transplant. The time interval between first and second transplant ranged from 2.4 to 11.2 months (median, 4.5 months) (Fig 1). There was no significant relationship between the interval of the two transplants and the time interval to first transplant. The second transplant was autologous in 151 and allogeneic in 14 patients, including 3 who received a MUD transplant. Reasons for failure to proceed to second transplant included toxicity with first transplant in 11 patients (2 deaths), disease progression in 9, and patient preference in 10 patients. One hundred twenty-one patients attaining and sustaining PR underwent a second autotransplant with MEL 200 mg/m2. The conditioning regimen for the remaining patients consisted of MEL 140 mg/m2 and TBI 1125 cGy (n = 20) or other regimens (n = 10; 8 received MEL 200 mg/m2 +HDCTX 120 mg/kg). After two autotransplants, 128 patients (85% of tandem autotransplant recipients) started IFN maintenance, whereas 37 came off-study for reasons listed in Table 3. The time course to initiation of the different phases of study shows that >95% of patients had completed remission induction by 6 months, first transplant by 8 months, and second transplant by 15 months (Fig 1).
Patient Flow on Total Therapy
| Started Treatment . | Off-Study Reasons . | |||||
|---|---|---|---|---|---|---|
| Phase of Therapy . | N . | % . | Toxicity (deaths) . | Platelets <50,000/μL . | Progression . | Declined . |
| Induction | 231 | 100 | 16 (8) | 0 | 6 | 7/73-150 |
| Tx-1 | 195 | 84 | 11 (2) | 0 | 9 | 10 |
| Tx-2 | 165 | 71 | 12 (6) | 10 | 2 | 13 |
| IFN | 128 | 56 | 34 (0) | 0 | 25 | 16 |
| Started Treatment . | Off-Study Reasons . | |||||
|---|---|---|---|---|---|---|
| Phase of Therapy . | N . | % . | Toxicity (deaths) . | Platelets <50,000/μL . | Progression . | Declined . |
| Induction | 231 | 100 | 16 (8) | 0 | 6 | 7/73-150 |
| Tx-1 | 195 | 84 | 11 (2) | 0 | 9 | 10 |
| Tx-2 | 165 | 71 | 12 (6) | 10 | 2 | 13 |
| IFN | 128 | 56 | 34 (0) | 0 | 25 | 16 |
Seven patients without insurance coverage proceeded to nontransplant arm of study (see text).
Time course of initiating successive treatment regimens used in Total Therapy, consisting of VAD × 2 to 3 (see text), HDCTX + GM-CSF with subsequent PBSC collection, EDAP + GM-CSF; followed by two cycles of high-dose therapy with hematopoietic stem cell support (Tx-1 and Tx-2); followed by IFN maintenance after completion of two autotransplants (151 patients). Note that >95% of patients completed remission induction by 6 months, first transplant by 8 months, and second transplant by 15 months (for further details, see text). The time interval between first and second transplant regimen ranged from 2.4 to 11.2 months with a median of 4.5 months; 80% completed both transplants within 1 year of study enrollment.
Time course of initiating successive treatment regimens used in Total Therapy, consisting of VAD × 2 to 3 (see text), HDCTX + GM-CSF with subsequent PBSC collection, EDAP + GM-CSF; followed by two cycles of high-dose therapy with hematopoietic stem cell support (Tx-1 and Tx-2); followed by IFN maintenance after completion of two autotransplants (151 patients). Note that >95% of patients completed remission induction by 6 months, first transplant by 8 months, and second transplant by 15 months (for further details, see text). The time interval between first and second transplant regimen ranged from 2.4 to 11.2 months with a median of 4.5 months; 80% completed both transplants within 1 year of study enrollment.
Antitumor effect and survival.
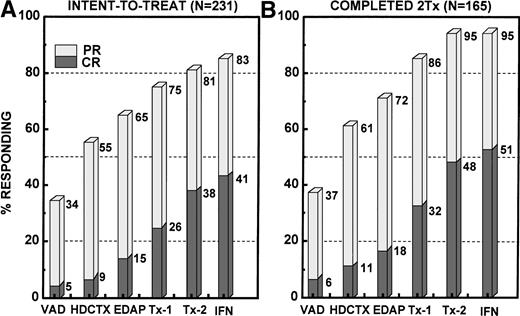
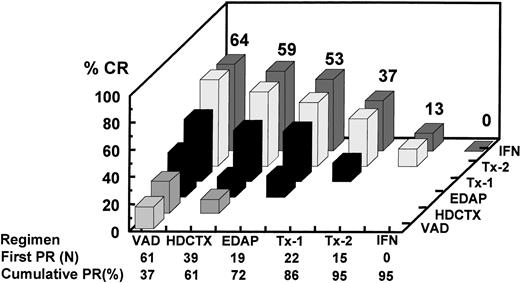
The incremental increase in both PR and CR rates in relationship to the different phases of study is shown in Fig 2for all 231 patients on an intent-to-treat basis, whether the designated treatment portion was actually applied or not, and separately for the 165 patients who actually completed two transplants (including allotransplants). Among all patients, the CR rate increased progressively from 5% after VAD to 15% at the end of remission induction to 26% after one transplant and 38% after two transplants, and to 41% with IFN. The corresponding PR + CR rates were 34%, 65%, 75%, 81%, and 83%, respectively. Among the subgroup of 165 patients completing two transplants, the corresponding CR(≥PR) rates were 6%(37%), 18%(72%), 32%(86%), 48%(95%), and 51%(95%), respectively. The response status was upgraded in 61% (7% to CR) of 28 patients not achieving PR (no response [NR]) after the first transplant and in 30% of 84 PR patients, so that altogether 38% of 112 patients with ≤PR experienced further tumor cytoreduction. Four patients with PR and 1 with CR had disease progression before second transplant. Among 165 tandem transplant recipients, the final CR rate was higher if PR status was attained early; thus, the 61 patients achieving PR after VAD had the highest CR rate of 64% after two transplants and IFN compared with only 13% when PR status was not obtained until after the second transplant (Fig 3; P = .004).
Response to Total Therapy in relationship to the individual treatment components, depicting ≥PR by the stippled columns and CR by the shaded bars. (A) Intent-to-treat analysis including all 231 patients enrolled. PR and CR rates increased steadily with the progression through the different phases of Total Therapy. PR and CR rates are depicted in a cumulative fashion, regardless of whether the individual treatment components were actually administered. (B) PR and CR increments among the 165 patients who actually completed two transplants (128 of 151 tandem autotransplant recipients also started IFN).
Response to Total Therapy in relationship to the individual treatment components, depicting ≥PR by the stippled columns and CR by the shaded bars. (A) Intent-to-treat analysis including all 231 patients enrolled. PR and CR rates increased steadily with the progression through the different phases of Total Therapy. PR and CR rates are depicted in a cumulative fashion, regardless of whether the individual treatment components were actually administered. (B) PR and CR increments among the 165 patients who actually completed two transplants (128 of 151 tandem autotransplant recipients also started IFN).
Analysis of CR rate in relationship to time of onset of first PR among the 165 patients completing tandem transplants including 14 who received an allograft. The horizontal axis depicts the serial components of Total Therapy along with the number of patients achieving first PR status as a result of the indicated therapeutic intervention along with the cumulative PR rate. For example, 61 patients already responding to VAD (37%) had the highest CR rate after two transplants (64%) compared with a CR rate of 37% among the 22 patients attaining PR only after the first cycle of MEL 200 mg/m2. Thus, CR rate was highest in VAD-sensitive myeloma.
Analysis of CR rate in relationship to time of onset of first PR among the 165 patients completing tandem transplants including 14 who received an allograft. The horizontal axis depicts the serial components of Total Therapy along with the number of patients achieving first PR status as a result of the indicated therapeutic intervention along with the cumulative PR rate. For example, 61 patients already responding to VAD (37%) had the highest CR rate after two transplants (64%) compared with a CR rate of 37% among the 22 patients attaining PR only after the first cycle of MEL 200 mg/m2. Thus, CR rate was highest in VAD-sensitive myeloma.
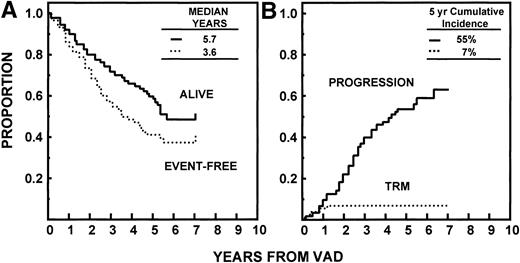
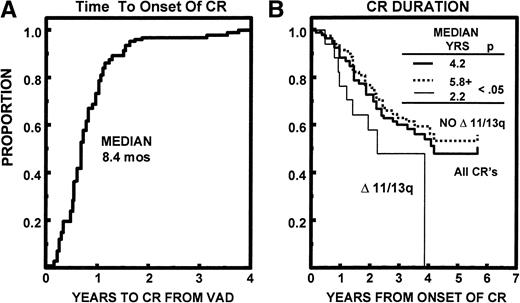
Median durations of OS and EFS have been reached at 68 and 43 months, respectively. The median time to relapse/progression was 52 months. All 7% of TRM events occurred within 24 months (Fig 4). Of the 94 patients achieving CR, the median time to CR was 8.4 months (range, 0.5 to 45 months). The median CR duration was 50 months and was longer in the absence of abnormalities of chromosomes 11 and 13 (≥69 v 26 months, P < .05; Fig 5).
(A) EFS and OS with Total Therapy from initiation of VAD. Median durations have been reached at 3.6 and 5.7 years, respectively. (B) Time to disease progression or relapse (see text) since initiation of therapy, with toxic or incidental deaths not related to disease progression/relapse being censored. TRM was 7% by 2 years.
(A) EFS and OS with Total Therapy from initiation of VAD. Median durations have been reached at 3.6 and 5.7 years, respectively. (B) Time to disease progression or relapse (see text) since initiation of therapy, with toxic or incidental deaths not related to disease progression/relapse being censored. TRM was 7% by 2 years.
(A) Time to onset of CR status among 94 patients eventually achieving CR; 90% attained CR status within 18 months. (B) CR duration from first onset of CR was 50 months. The 17 patients (18%) with abnormalities of chromosomes 11 and/or 13 (Δ 11/13) had a significantly shorter CR duration than the remaining patients (P < .05).
(A) Time to onset of CR status among 94 patients eventually achieving CR; 90% attained CR status within 18 months. (B) CR duration from first onset of CR was 50 months. The 17 patients (18%) with abnormalities of chromosomes 11 and/or 13 (Δ 11/13) had a significantly shorter CR duration than the remaining patients (P < .05).
Table 4 depicts the clinical outcome dated from second transplant in relationship to response status and treatment regimen. Among autograft recipients, those already in CR and receiving MEL 200 mg/m2 had the longest EFS and OS durations. Similar durations of disease control were observed for the 70 patients in PR (but not CR) receiving autotransplant-supported MEL 200 mg/m2 and the 28 patients with less than PR (NR) receiving combination therapy. The 14 allograft recipients (all in PR) had inferior EFS/OS durations compared with the other three groups, but displayed a higher frequency of adverse cytogenetics (50% v19% with autograft recipients; P = .006) (see below).
Clinical Outcome After Second Transplant (N = 165)
P values >.05 are not reported.
Allogeneic transplants, all others are autologous.
Abbreviations: ED, early death ≤60 days; MEL 200, melphalan 200 mg/m2; MEL-TBI, melphalan 140 mg/m2 + total body irradiation (850-1,125 cGy); HDCTX, high-dose cyclophosphamide 120 mg/kg; Thio, thiotepa 10 mg/kg.
Prognostic factors.
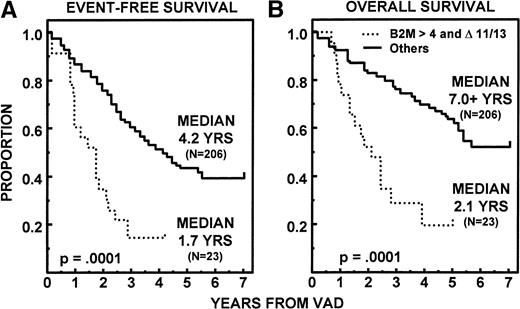
On univariate analysis of 15 pretreatment variables, 10 had significant associations with OS, 7 with EFS, and 6 with both EFS and OS (Table 5 for categorical variables; this was also true for continuous variables where applicable, data not shown). Two parameters retained independent significance on multivariate analysis among the 218 patients in whom all parameters were available, using continuous variables where appropriate. Thus, superior EFS and OS were noted in the absence of unfavorable cytogenetics (11q breakpoints and/or partial or complete deletions of chromosome 13 [13q−, −13]) (EFS, P = .0001; OS, P = .0001) and with low levels of β2M (EFS, P = .001; OS,P = .0007). On further scrutiny, the presence of unfavorable cytogenetics (“▵ 11/13”) identified a subgroup of 23 patients among the 68 with β2M > 4 mg/L whose median EFS and OS were only 1.7 and 2.1 years, respectively (Fig6). This group of 10% of all patients fared distinctly worse than the other three cytogenetic/β2M subgroups. CR duration was significantly longer with early onset of CR (P = .01) and favorable cytogenetics (P = .03). To appreciate the potential impact of response and regimen intensity delivered with tandem transplants, a time-dependent covariate analysis was conducted among the 229 patients with both β2M and cytogenetic data available. Multivariate regression analysis considered the additional importance of time to PR, CR, and one or two transplants alone and in combination (Table 6). In addition to cytogenetics and β2M, application of a second transplant (yes or no) and the timeliness of this approach extended both EFS and OS markedly. Whereas significant on univariate analysis for both EFS (P = .02) and OS (P = .02), attaining CR in a timely fashion was less significant once dose intensity parameters had been included.
Prognostic Factors at Diagnosis
| Parameter . | N . | EFS (mos) . | P . | OS (mos) . | P . |
|---|---|---|---|---|---|
| Karyotype | |||||
| No Δ 11/13 | 182 | 52 | .0002 | 84+ | .0001 |
| Δ 11/13 | 49 | 25 | 34 | ||
| Bartl grade | |||||
| ≤1 | 142 | 53 | .002 | 81+ | .0004 |
| >1 | 60 | 28 | 43 | ||
| β2M (mg/L) | |||||
| ≤4.0 | 161 | 51 | .0002 | 84+ | .003 |
| >4.0 | 68 | 26 | 50 | ||
| CRP (mg/L) | |||||
| ≤4.0 | 120 | 57 | .0003 | 84+ | .008 |
| >4.0 | 93 | 30 | 62 | ||
| Hgb (g/dL) | |||||
| >10.0 | 151 | 53 | .0008 | 84+ | .009 |
| ≤10.0 | 80 | 29 | 60 | ||
| Creat (g/dL) | |||||
| ≤2.0 | 211 | 46 | .05 | 84+ | .01 |
| >2.0 | 20 | 28 | 44 | ||
| Albumin (g/dL) | |||||
| >3.5 | 158 | 43 | .9 | 84+ | .02 |
| ≤3.5 | 73 | 52 | 55 | ||
| Isotype | |||||
| Non-Ig | 190 | 50 | .04 | 84+ | .03 |
| IgA | 41 | 33 | 47 | ||
| Age (yrs) | |||||
| ≤60 | 181 | 50 | .09 | 84+ | .03 |
| >60 | 50 | 32 | 50 | ||
| Calcium (g/dL) | |||||
| ≤11.0 | 204 | 49 | .06 | 68 | .05 |
| >11.0 | 27 | 23 | 44 | ||
| LDH (U/L) | |||||
| ≤190 | 181 | 49 | .2 | 82+ | .09 |
| >190 | 49 | 33 | 57 | ||
| BM PC (%) | |||||
| ≤50% | 121 | 50 | .1 | 80+ | .1 |
| >50% | 91 | 34 | 62 | ||
| Stage | |||||
| <III | 108 | 43 | .3 | 84+ | .2 |
| III | 123 | 46 | 65 | ||
| Sex | |||||
| Male | 143 | 41 | .5 | 68 | .4 |
| Female | 88 | 53 | 82+ | ||
| Race | |||||
| White | 206 | 43 | .7 | 65 | .6 |
| Non-white | 25 | 49 | 78+ | ||
| Total | 231 | 43 | 68 |
| Parameter . | N . | EFS (mos) . | P . | OS (mos) . | P . |
|---|---|---|---|---|---|
| Karyotype | |||||
| No Δ 11/13 | 182 | 52 | .0002 | 84+ | .0001 |
| Δ 11/13 | 49 | 25 | 34 | ||
| Bartl grade | |||||
| ≤1 | 142 | 53 | .002 | 81+ | .0004 |
| >1 | 60 | 28 | 43 | ||
| β2M (mg/L) | |||||
| ≤4.0 | 161 | 51 | .0002 | 84+ | .003 |
| >4.0 | 68 | 26 | 50 | ||
| CRP (mg/L) | |||||
| ≤4.0 | 120 | 57 | .0003 | 84+ | .008 |
| >4.0 | 93 | 30 | 62 | ||
| Hgb (g/dL) | |||||
| >10.0 | 151 | 53 | .0008 | 84+ | .009 |
| ≤10.0 | 80 | 29 | 60 | ||
| Creat (g/dL) | |||||
| ≤2.0 | 211 | 46 | .05 | 84+ | .01 |
| >2.0 | 20 | 28 | 44 | ||
| Albumin (g/dL) | |||||
| >3.5 | 158 | 43 | .9 | 84+ | .02 |
| ≤3.5 | 73 | 52 | 55 | ||
| Isotype | |||||
| Non-Ig | 190 | 50 | .04 | 84+ | .03 |
| IgA | 41 | 33 | 47 | ||
| Age (yrs) | |||||
| ≤60 | 181 | 50 | .09 | 84+ | .03 |
| >60 | 50 | 32 | 50 | ||
| Calcium (g/dL) | |||||
| ≤11.0 | 204 | 49 | .06 | 68 | .05 |
| >11.0 | 27 | 23 | 44 | ||
| LDH (U/L) | |||||
| ≤190 | 181 | 49 | .2 | 82+ | .09 |
| >190 | 49 | 33 | 57 | ||
| BM PC (%) | |||||
| ≤50% | 121 | 50 | .1 | 80+ | .1 |
| >50% | 91 | 34 | 62 | ||
| Stage | |||||
| <III | 108 | 43 | .3 | 84+ | .2 |
| III | 123 | 46 | 65 | ||
| Sex | |||||
| Male | 143 | 41 | .5 | 68 | .4 |
| Female | 88 | 53 | 82+ | ||
| Race | |||||
| White | 206 | 43 | .7 | 65 | .6 |
| Non-white | 25 | 49 | 78+ | ||
| Total | 231 | 43 | 68 |
Significantly shorter EFS (A) and OS (B) in 23 patients (10%) with β2M > 4 and unfavorable karyotypes (▵ 11/13) compared with the 206 remaining patients (161 with β2M ≤ 4 mg/L and 45 with β2M > 4 mg/L but absence of ▵ 11/13).
Significantly shorter EFS (A) and OS (B) in 23 patients (10%) with β2M > 4 and unfavorable karyotypes (▵ 11/13) compared with the 206 remaining patients (161 with β2M ≤ 4 mg/L and 45 with β2M > 4 mg/L but absence of ▵ 11/13).
Multivariate Analysis of Pretreatment and Postintervention Variables (N = 229)
| EFS . | RR . | P . | OS . | RR . | P . |
|---|---|---|---|---|---|
| No Δ 11/13q | .481 | .0004 | No Δ 11/13q | .386 | .0001 |
| β2M ≤4.0 mg/L | .534 | .0006 | Any second Tx | .200 | .0002 |
| Any second Tx | .307 | .001 | Time to second Tx | 1.008 | .0005 |
| Time to second Tx | 1.005 | .03 | Time to first CR | 1.001 | .02 |
| Any first CR | NA6-150 | .17 | β2M ≤4.0 mg/L | .609 | .03 |
| Time to first CR | NA6-150 | .42 | Any first Tx | NA6-150 | .06 |
| EFS . | RR . | P . | OS . | RR . | P . |
|---|---|---|---|---|---|
| No Δ 11/13q | .481 | .0004 | No Δ 11/13q | .386 | .0001 |
| β2M ≤4.0 mg/L | .534 | .0006 | Any second Tx | .200 | .0002 |
| Any second Tx | .307 | .001 | Time to second Tx | 1.008 | .0005 |
| Time to second Tx | 1.005 | .03 | Time to first CR | 1.001 | .02 |
| Any first CR | NA6-150 | .17 | β2M ≤4.0 mg/L | .609 | .03 |
| Time to first CR | NA6-150 | .42 | Any first Tx | NA6-150 | .06 |
RR estimates are not calculated for variables excluded from the final model.
Abbreviation: RR, relative risk.
When serial landmark analyses were performed at 11, 12, 13, 14, 15, and 16 months, EFS and OS both were longer among the patients who had received a second transplant within 13 months (when nearly 85% of second transplants had been completed) compared with the others receiving their second cycle of high-dose therapy later or not at all. The proportion of high-risk patients (unfavorable cytogenetics and high β2M) was not different regardless of whether a second transplant had been performed at any of the landmarks examined. Interestingly, the difference in outcome between the group completing a second transplant and the remaining patients gradually emerged by 12 months and was lost by 15 months. This pattern supports the results of the Cox regression model that identified timelines of a second transplant as a significant variable for clinical outcome.
Toxicities.
The VAD regimen used, by design, virtually nonmyelosuppressive doses of doxorubicin so that the profound immunosuppressive effects of high-dose dexamethasone would not be associated with significant neutropenia and hence enhanced risk of serious infections. Median durations of critical neutropenia (<500/μL) and thrombocytopenia (<50,000/μL) typically did not exceed 1 week for the remainder of induction regimens and both transplants. Grade III/IV extramedullary toxicities are summarized in Table 7. No serious toxicity was noted in more than two thirds with VAD, in approximately one third with HDCTX, in three quarters with EDAP, and in one third with MEL 200 mg/m2 administered with either first or second transplant, even when HDCTX was added to MEL 200 mg/m2. However, as anticipated, with added TBI more toxicity was encountered so that only 10% had no serious extramedullary toxicity. TRM was 2% with VAD; 1% with HDCTX, EDAP, and first transplant; 2% with second autotransplant using MEL 200 mg/m2 alone or with added HDCTX and rose to 5% with added TBI. Among the 14 allotransplant recipients, 21% died within 100 days and 50% within 12 months, mainly from transplant-related complications. With the various induction regimens, bacteremia/pneumonia occurred in 10% to 30% of patients; thromboembolic events were observed in about 10% each with VAD and HDCTX. Capillary leak syndrome, mainly related to GM-CSF administration, was noted among 10% of patients during the HDCTX plus GM-CSF portion of the trial. With autologous transplants, the incidence of grade ≥ III mucositis was 30% to 40%, serious diarrhea occurred in 10% to 15% of patients, and pneumonia/sepsis was more common with TBI during the second transplant (40%) as opposed to 25% to 30% with MEL 200 mg/m2 ± HDCTX. Most of the induction regimens were administered in the outpatient setting except for mandatory admission to accommodate hydration (for 2 to 3 days) with the HDCTX phase of therapy. With first and second autotransplants, the median durations of hospitalization were 13 and 14 days, respectively, and over one third of the patients were hospitalized for no more than 1 week.
Grade III/IV Nonhematologic Toxicity
| Toxicity7-150 . | VAD (N = 224) . | HDCTX (N = 218) . | EDAP (N = 203) . | Tx-1 (N = 195) . | Tx-2 . | |
|---|---|---|---|---|---|---|
| No TBI (N = 131) . | (TBI)7-151 (N = 20) . | |||||
| None | 68 | 36 | 75 | 33 | 35 | 10 |
| Nausea/ vomiting | 2 | 10 | 3 | 26 | 24 | 35 |
| Mucositis | 2 | 1 | 1 | 39 | 29 | 35 |
| Diarrhea | 1 | 5 | 1 | 13 | 11 | 10 |
| Bacteremia/ pneumonia | 17 | 28 | 11 | 25 | 31 | 40 |
| Thrombo- embolic events | 10 | 11 | 2 | 2 | 0 | 0 |
| Cap leak syndrome | 0 | 10 | 0 | 0 | 1 | 0 |
| Early death | 2 | 1 | 1 | 1 | 2 | 5 |
| Toxicity7-150 . | VAD (N = 224) . | HDCTX (N = 218) . | EDAP (N = 203) . | Tx-1 (N = 195) . | Tx-2 . | |
|---|---|---|---|---|---|---|
| No TBI (N = 131) . | (TBI)7-151 (N = 20) . | |||||
| None | 68 | 36 | 75 | 33 | 35 | 10 |
| Nausea/ vomiting | 2 | 10 | 3 | 26 | 24 | 35 |
| Mucositis | 2 | 1 | 1 | 39 | 29 | 35 |
| Diarrhea | 1 | 5 | 1 | 13 | 11 | 10 |
| Bacteremia/ pneumonia | 17 | 28 | 11 | 25 | 31 | 40 |
| Thrombo- embolic events | 10 | 11 | 2 | 2 | 0 | 0 |
| Cap leak syndrome | 0 | 10 | 0 | 0 | 1 | 0 |
| Early death | 2 | 1 | 1 | 1 | 2 | 5 |
Percent of N by phase of therapy.
Excludes 11 allotransplants and 3 MUD transplants.
IFN maintenance.
IFN maintenance was commenced in 85% of 151 patients completing two autotransplants, with a median time to starting IFN of 2 months ( range, 1 to 14 months). Reasons for not commencing IFN included incomplete platelet recovery (<100,000/μL, 9%), patient refusal (3%), or physician judgment that the anticipated toxicity from IFN would interfere with still ongoing recovery of performance status from a second transplant (2%); 2 patients (1%) died within 76 days after second transplant. The duration of IFN maintenance ranged from 0.5 to ≥65 months (median, 22 months). IFN was discontinued because of disease progression or relapse in 25 patients and because of toxicity in 34 patients (mainly persistent malaise). Delayed (>6 months) PR to CR conversion was observed in 7 patients, including 3 on IFN maintenance (8, 18, and 41 months).
DISCUSSION
The Total Therapy program represents the first trial for newly diagnosed patients with multiple myeloma that used multi-regimen induction followed by two cycles of high-dose cytotoxic therapy (“tandem transplant”), regardless of responsiveness to treatment, followed by IFN maintenance. As a result of this approach that incorporated all treatment strategies available at the time of initiation of this trial, we were able to show a progressive increase in stringently defined CR rates during the induction sequence and, especially, as a result of the two high-dose therapy cycles, raising the final CR to the 40% range using an intent-to-treat analysis. It is difficult to evaluate the contribution of TBI or added cytotoxic drugs to MEL to disease control for those patients with less than PR at the time of second autotransplant. However, among autograft recipients, the incidence of 7% CR with combination therapy among 28 patients with less than PR status before second transplant compared with 37% with MEL 200 mg/m2 for 70 patients in PR (but not yet in CR) suggests that the addition of TBI or other drugs to MEL may not be superior to MEL 200 mg/m2 alone (P = .003). This is substantiated by comparable durations of EFS (52 v 37 months,P = .2) and OS (68 v ≥81 months, P= .9) after MEL 200 versus combination therapy (Table 4). Superior EFS and OS after MEL 200 mg/m2 compared with other high-dose regimens have also been reported by Powles et al.11 The 7 patients who were denied insurance coverage and received intermediate dose MEL 70 mg/m2 and cyclical chemoimmunotherapy (VAD, cyclophosphamide, IFN) faired well (EFS 43 months, OS 62 months), although only 1 patient had unfavorable cytogenetics, β2M elevation, or hemoglobin levels ≤10 g/dL. The allogeneic transplant population is too small to comment on a graft-versus-myeloma effect.
The observation of higher CR rates after successful completion of two transplants the sooner PR status was attained suggests that sensitivity to high-dose MEL alone or in combination with TBI or other drugs is greater when sensitivity to standard VAD is preserved. Conversely, resistance to glucocorticoids, multidrug resistance (MDR)-associated agents like doxorubicin and etoposide, and other alkylators such as cyclophosphamide and cisplatin may confer a relative degree of resistance to myeloablative therapy as well. The observation of a modest CR rate of 15% at the end of five induction cycles with three different regimens raises the question whether a similar outcome could have been obtained if remission induction had been limited to a few cycles of dexamethasone pulsing to control patients’ symptoms and disease-related complications. Randomized trials of minimal remission induction versus maximum tumor cytoreduction before transplant are needed to answer this important issue.
Analysis of prognostic factors identified the presence of certain cytogenetic abnormalities as critically important.31 As reported previously, the exact molecular mechanisms resulting from these chromosomal abnormalities and the inferior prognosis conferred have yet to be identified. β2M before treatment was confirmed as a dominant prognostic variable.32 To appreciate the independent prognostic contributions of disease sensitivity to treatment (onset of PR and CR) and of dose intensity (one and two transplants), a time-dependent covariate analysis was performed in the context of a multivariate regression approach. In the presence of these four additional variables, timely completion of two transplants emerged as a highly significant factor for both EFS and OS in addition to cytogenetics and β2M (Table 6). Serial landmark analyses showed that application of two transplants within 13 months from initiation of therapy improved both EFS and OS. The optimal timing seemed to be within 13 months from study entry or within 6 months from first transplant. Delaying the second high-dose therapy cycle beyond this time limit probably permits significant tumor growth so that net tumor cytoreduction beyond the residual tumor burden post–first transplant could not be effected. The greater importance of “dose-dense” therapy rather than attaining CR status (which was significant on univariate analysis) may reflect the heterogeneity in residual tumor burden among CR patients. Collectively, these data support the importance of high-dose therapy for overcoming resistance mechanisms with which myeloma cells are abundantly endowed, even before any therapeutic intervention, explaining the low incidence of true CR in the 5% range with standard therapy and the lack of cure with traditional treatment.1 A comparison of Total Therapy with other high-dose regimens is difficult because of heterogeneity of induction and high-dose regimens as well as response requirements before transplant. Cunningham reported on 53 chemotherapy-responsive patients receiving MEL 200 mg/m2 and ABMT, who experienced 2% TRM, “CR” of 75%, and median EFS/OS durations of 2.0/6.7 years.8 An update by Powles et al11 of 195 consecutive newly diagnosed patients under age 70 receiving modified VAD induction and mainly MEL 200 mg/m2–based autotransplants (72% of initial patients) reported a CR rate of 53% and EFS/OS durations of 2.0/4.5 years. Using conditioning with combination chemotherapy comprising MEL, carmustine (BCNU), HDCTX, etoposide, and TBI 1,200 cGy to 63 mainly untreated patients with a median age of 44 years, Fermand et al12 observed TRM of 11%, CR of 20%, and EFS/OS durations of 3.6/6.4 years. Harousseau et al,13 treating 133 newly diagnosed patients with MEL 140 mg/m2 + TBI 850 cGy noted 4% TRM, 37% CR, and EFS/OS of 2.0/3.8 years. A recent update of the IFM-90 trial33 comparing standard chemotherapy with a single autotransplant (MEL 140 mg/m2 + TBI 800 cGy) in 200 untreated patients under age 65 showed median EFS/OS durations of 2.3/4.8 years after transplant compared with 4.2/≥6.8 years among the 140 patients under age 65 without any prior therapy enrolled in Total Therapy. In light of the above data, Total Therapy clearly effects an outstanding clinical outcome at acceptable morbidity and a low treatment (not just transplant)–related mortality of 7% at 2 years. Despite its complexity, all phases of Total Therapy could be applied in a timely fashion, so that 95% of patients will have completed induction by 6 months, first transplant (performed in 84% of all patients) by 8 months, and second transplant (performed in 71% of all patients) by 15 months. Obvious questions concern the need for multiple induction regimens before transplant and for two cycles of high-dose therapy. The latter is currently being addressed by the IFM-94 trial randomizing patients to one versus two transplants using MEL 140 mg/m2 + TBI 800 cGy as the myeloablative regimen in both arms, preceded by MEL 140 mg/m2 in the tandem transplant group.34 A preliminary analysis of the first 200 patients with a median follow-up of 2 years from diagnosis failed to show a difference in CR rate or EFS and OS. The lack of difference in CR rate is difficult to explain, although divergent results of the IFM-90 trial comparing standard with high-dose therapy emerged only beyond 2 years.
When documented, most pretransplant induction regimens yield CR rates of no more than 5% after 4 cycles of either VAD or VMCP (vincristine, melphalan, cyclophosphamide, prednisone)/VBAP (vincristine, carmustine, doxorubicin, prednisone) that do not seem to increase when such therapy is continued as typically practiced in standard therapy trials.33 However, with Total Therapy, the incidence of at least PR (≥75% tumor cytoreduction and normal bone marrow morphology) had increased from 34% to 65% whereas the CR rate had risen from 5% to 15%. Whether the final PR + CR rate of 83% and the CR rate of 41% at the completion of Total Therapy (intent-to-treat) would also have been accomplished with minimal induction such as dexamethasone pulsing or VAD is difficult to determine except in the context of a randomized trial. However, in as much as CR status is a sine qua non for long-term disease control, ideally, each cycle of therapy should be maximally cytoreductive and contribute to raising the CR rate as accomplished in Total Therapy. Unfortunately, compared with acute myeologenous leukemia where CR is obtained in the majority of patients within one cycle of cytarabine-anthracycline combination, the median time to CR even with complex induction and tandem transplant exceeded 8 months. From the available literature, it is clear that high CR rates (50% range) require myeloablative therapy either with a single course using more hazardous chemoradiotherapy12 or repeated applications of high-dose therapy as practiced with Total Therapy. The latter approach seems to be better tolerated and applicable to patients up to age 70 and, more importantly, permits adjustments in dosing and timing depending on tolerance of and responsiveness to the first cycle. Based on the lack of deleterious effects of advanced age35and renal insufficiency,36 we recommend that autotransplants should be considered for all patients with symptomatic myeloma, which requires strict avoidance of potentially stem cell–toxic standard therapy before PBSC collection.
Based on analysis of tandem transplant trials in 551 patients with prior therapy of variable durations that identified three dominant risk factors for EFS, namely cytogenetics, β2M, and duration of therapy before first transplant,37 our current approach uses a risk-based algorithm matching disease risk with the anticipated risk of therapeutic intervention. Tandem autotransplants with MEL 200 mg/m2 represent the standard backbone of therapy. Posttransplant, idiotype, or dendritic cell vaccination is evaluated in good-risk patients (all three favorable variables present)38-41 and combination chemotherapy with dexamethasone, cyclophosphamide, etoposide, and cisplatin42in those with high risk. In this fashion, we hope to decrease the risk of relapse and increase the fraction of patients enjoying sustained CR, now obtained in one half of patients entering CR (≥5 years). Longer follow-up will be required to determine the fraction of patients that can be considered cured with Total Therapy, because approximately 20% of patients achieving CR have not relapsed at 10 years even after a single mainly TBI-based autotransplant.43
Future clinical trial questions in myeloma should build on the Total Therapy experience. Until more specific myeloma therapy has been developed in phase I and II trials for advanced and refractory disease, front line regimens should consider a Total Therapy–like approach with cure as an objective of treatment which hence needs to aim at increasing CR rates beyond the current level of 40% to 50%. Thus, fine-tuning strategies are not yet warranted. Rather, standard dose consolidation chemotherapy versus additional cycles of PBSC-supported high-dose therapy after an MEL-based tandem transplant deserves exploration.
ACKNOWLEDGMENT
We acknowledge the support provided by the Bone Marrow Transplant Team of the University of Arkansas for Medical Sciences and the Arkansas Cancer Research Center. We particularly thank the many referring physicians who entrusted us with their patients’ care, the Myeloma Data Management Team for their tireless effort, and Caran Hammonds for excellent assistance in manuscript preparation.
Supported in part by Grant No. CA55819 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
Further analysis of data indicated that the adverse implications of chromosome 11 and 13 abnormalities (49 patients) are entirely due to partial or complete deletions of chromosome 13,44 present in 41 patients (18%).
Author notes
Address reprint requests to B. Barlogie, MD, PhD, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, 4301 W Markham Slot 776, Little Rock, AR 72205.