Abstract
Stem cell factor (SCF) has been shown to synergize with filgrastim to mobilize CD34+ cells into the peripheral blood. To determine if addition of SCF to chemotherapy and filgrastim reduces the number of leukaphereses required to achieve a target yield of 5 × 106 CD34+ cells/kg, 102 patients with multiple myeloma were randomized to receive mobilization chemotherapy with cyclophosphamide (4 g/m2) and either SCF (20 μg/kg/d) combined with filgrastim (5 μg/kg/d) or filgrastim alone (5 μg/kg/d), administered daily until leukaphereses were completed. After collection, patients were treated with myeloablative therapy supported by autologous peripheral blood progenitor cell (PBPC) infusion and filgrastim (5 μg/kg/d). There was a significant difference between the treatment groups in the number of leukaphereses required to collect 5 × 106 CD34+ cells/kg (median of 1 v 2 for SCF + filgrastim and filgrastim alone, respectively, P = .008). Patients receiving the combination of SCF plus filgrastim had a 3-fold greater chance of reaching 5 × 106 CD34+ cells/kg in a single leukapheresis compared with patients mobilized with filgrastim alone. The median CD34+ cell yield was significantly increased for the SCF group in the first leukapheresis (11.3 v 4.0 × 106/kg, P = .003) and all leukaphereses (12.4v 8.2 × 106/kg, P = .007). Total colony-forming unit–granulocyte-macrophage (CFU-GM) and mononuclear cell counts were also significantly higher in the SCF group in the first leukapheresis and in all leukaphereses. As expected for patients mobilized to an optimal CD34+ cell yield, the time to engraftment was similar between the 2 treatment groups. Cells mobilized with the combination of SCF plus filgrastim were thus considered effective and safe for achieving rapid engraftment. Treatment with SCF plus filgrastim was well tolerated, with mild to moderate injection site reactions being the most frequently reported adverse events. There were no serious allergic-like reactions to SCF. The addition of SCF to filgrastim after cyclophosphamide for PBPC mobilization resulted in a significant increase in CD34+cell yield and a concomitant reduction in the number of leukaphereses required to collect an optimal harvest of 5 × 106CD34+ cells/kg.
INTENSIVE TREATMENT with autologous hematopoietic support has become the treatment of choice for multiple myeloma patients up to 65 years of age.1,2 Peripheral blood progenitor cells (PBPC) are currently preferred for transplantation, because a hematopoietic recovery after transplantation is faster than with bone marrow transplants.3-5 In multiple myeloma, mobilization of stem cells in the peripheral blood is usually achieved with repeated daily injections of cytokines (granulocyte colony-stimulating factor [G-CSF] or granulocyte-macrophage colony-stimulating factor [GM-CSF]) after VAD6(vincristine, adriamycin, dexamethasone) or cyclophosphamide.7,8 After PBPC transplantation, the time to hematopoietic recovery is correlated with the number of CD34+ progenitor cells infused.9 Recent studies suggest that infusion of ≥5 × 106 CD34+cells/kg results in rapid and consistent engraftment in a large proportion of patients.10 11 Conversely, high-dose chemotherapy is usually not administered to patients with CD34+ cell yields less than 1 to 2 × 106/kg.
Stem cell factor (SCF) is a glycoprotein growth factor that acts on hematopoietic blood cell progenitors.12 Whereas SCF alone exerts little colony-stimulating activity on normal human bone marrow cells in vitro, the combination of recombinant SCF and other recombinant hematopoietic cytokines results in a synergistic increase in the numbers of colonies.13 The addition of SCF to recombinant G-CSF (filgrastim) synergistically increases PBPC mobilization compared with filgrastim alone.14-17 Several clinical trials have reported the ability of the combination of SCF with filgrastim to mobilize PBPC in patients with lymphoma, multiple myeloma, and breast and ovarian cancers.18-24 Combination of SCF with filgrastim has been observed to improve CD34+cell mobilization in heavily pretreated lymphoma20,25 or myeloma21 patients, who are known to be at risk of poor mobilization.
We report here the results of a large randomized and controlled trial evaluating the addition of SCF to filgrastim for the mobilization of PBPC in the chemotherapy-based mobilization setting. The study was conducted in patients with multiple myeloma, most of whom were newly diagnosed. The primary objective was to determine whether the addition of SCF could reduce the number of leukaphereses required to achieve a target yield of 5 × 106 CD34+ cells/kg.
PATIENTS AND METHODS
Patient Eligibility
The study was reviewed and approved by the relevant institutional ethics committees and all patients gave written informed consent before study entry. Patients were eligible if they were between 18 and 65 years of age and if they had either newly diagnosed symptomatic stage I, II, or III (Durie and Salmon staging) multiple myeloma or chemosensitive myeloma in relapse eligible for autologous transplantation. Eligible patients had to have an ECOG performance status of 0-2; a life expectancy with treatment of at least 6 months; an absolute neutrophil count (ANC) ≥1.5 × 109/L ; a platelet count ≥100 × 109/L; and adequate major organ function as defined by serum creatinine ≤150 mmol/L, bilirubin, asparate aminotransferase (AST), and alanine aminotransferase (ALT) less than twice the upper limit defined at the investigating laboratory.
Patients were not included if they had received prior high-dose chemotherapy with autologous progenitor cell support or had presented with another malignancy within the preceding 5 years, with the exception of surgically cured basal cell carcinoma of the skin or in situ carcinoma of the cervix. Because of the possibility of systemic allergic-like reactions, patients with severe allergic history (seasonal/recurrent asthma, anaphylactic-type events, angioedema/recurrent urticaria, and allergy to insect venoms) were not included. Other exclusion criteria included active infection or fever, human immunodeficiency virus seropositivity, known allergy toEscherichia coli-derived products, or significant nonmalignant disease. The concurrent use of β-adrenergic blocking agents was prohibited due to potential interactions with the SCF premedications.
Study Design
This was a randomized, open-label, multicenter study. It consisted of a collection phase, a treatment phase, and a 90-day follow-up (Fig 1).
Collection phase.
Patients were randomized in a 1:1 ratio to 1 of the 2 stem cell mobilization regimens. The mobilization regimen consisted of 4 g/m2 cyclophosphamide administered by intravenous (IV) infusion to all patients followed 24 hours later by either 20 μg/kg/d SCF (r-metHuSCF; Amgen Inc, Thousands Oaks, CA) subcutaneously (SC) plus 5 μg/kg/d filgrastim (Neupogen; Amgen Inc) SC (SCF group) or 5 μg/kg/d filgrastim alone SC (filgrastim group) administered daily, at separate sites of the body, until all leukaphereses were completed. All patients randomized to treatment with SCF were premedicated with H1 and H2 antihistamines (cetirizine and ranitidine, respectively) and an inhaled bronchodilator (salbutamol). Leukaphereses were initiated when the white blood count (WBC) was ≥4 × 109/L after the cyclophosphamide-induced nadir. Leukaphereses were performed using a Baxter Fenwall CS3000 (Baxter, Deerfield, IL) or a comparable machine. A blood volume of approximately 10 L was processed at each leukapheresis. An aliquot from each leukapheresis harvest was sent to a central laboratory (Haematology Lab, AZ VUB, Brussels, Belgium) for CD34+ cell enumeration using the HPCA2 anti-CD34+ fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (Becton Dickinson, Mountain View, CA). Daily leukapheresis continued until a total of ≥5 × 106 CD34+cells/kg body weight were collected, based on the central laboratory results, or until a total of 4 leukaphereses had been performed. Patients with less than 5 × 106 CD34+ cells/kg proceeded to treatment phase at the discretion of the investigator. No assessment of CD34+ cell subsets or malignant clone cells was performed on the leukaphereses product.
Treatment phase.
After a rest period of a maximum of 8 weeks, patients received myeloablative therapy followed by autologous PBPC infusion and observation of hematopoietic recovery. Administration of chemotherapy was allowed between the last day of leukapheresis and the first day of conditioning therapy, at the discretion of the investigator. The myeloablative treatment regimen consisted of either melphalan alone (200 mg/m2, IV) or melphalan (140 mg/m2, IV) plus total body irradiation (8 to 10 Gy). PBPC were infused on day 0, 24 hours after the last dose of cytotoxic therapy. Filgrastim (5 μg/kg/day, IV or SC) was administered from day 1 until neutrophil recovery.
Follow-up phase.
Patients were assessed on day 90 post-PBPC infusion for maintenance of engraftment, disease status, and survival. Patients continue to be observed for survival on a separate protocol.
Statistical Methods
The primary study endpoint was the number of leukaphereses required to achieve a target yield of 5 × 106 CD34+cells/kg body weight. Secondary study endpoints were CD34+cell, colony-forming unit–granulocyte-macrophage (CFU-GM), and mononuclear cell (MNC) numbers in the first leukapheresis product and over all required leukaphereses in the collection phase; the time to the first of 3 days with platelet count ≥20 × 109/L independent of platelet transfusions, time to platelet transfusion independence, number of days and number of platelet transfusions, number of days and number of red blood cell (RBC) transfusions, and time to ANC ≥0.5 × 109/L in the treatment phase.
The number of leukaphereses required to reach the target was analyzed using Kaplan Meier analysis and the Gehan-Wilcoxon test. The numbers of CD34+ cells, CFU-GM, and MNC and the number of days of platelet and RBC transfusions were compared using the Wilcoxon rank-sum test. Time to ANC recovery and time to platelet recovery were analyzed using Kaplan-Meier analysis and the logrank test.
The effect of SCF and melphalan and their interaction on CD34+ cell yields were assessed using analysis of variance on the log CD34+ cell yields (to satisfy the assumptions of normality and equal variances).
RESULTS
One hundred two patients (55 in the SCF group and 47 in the filgrastim group) were enrolled and randomized from March 1996 to October 1997 at 15 sites in France (8 sites), Spain (4 sites), and Belgium (3 sites). All patients were included in the intent-to-treat analysis.
Of the 102 patients randomized, 101 had at least 1 leukapheresis. Ninety-seven patients (95%) completed the collection phase and entered the treatment phase of the study and 95 patients (93%) completed the study according to protocol. Five patients withdrew during the collection phase: 2 patients died of progressive disease, 1 before undergoing any leukapheresis, and 3 patients were withdrawn due to reported poor CD34+ cell yields (2 in the SCF group and 1 in the filgrastim group; CD34+ cell yields: 2.9, 0.12, and 0.26 × 106/kg, respectively). In the treatment phase, 2 patients withdrew: 1 died during autotransplantation of multiple organ failure and the other was lost to follow-up after autotransplantation.
Treatment groups were balanced for demographics, disease stage, ECOG score, number of prior therapy cycles, and prior radiotherapy (Table 1) and for all key hematology and biochemistry parameters (data not shown). Twenty-three patients (24%; 11 in the SCF group and 12 in the filgrastim group) were administered chemotherapy between the collection and the treatment phase: 18 patients received 1 cycle, 3 patients received 2 cycles, 1 patient received 3 cycles, and 1 patient received 4 cycles. The number of cycles of chemotherapy administered during this period was balanced between treatment groups.
Patient Demographics and Baseline Disease Characteristics
| . | SCF + Filgrastim . | Filgrastim . | Total . |
|---|---|---|---|
| n | 55 | 47 | 102 |
| Sex (%) | |||
| Male | 36 (65) | 24 (51) | 60 (59) |
| Female | 19 (35) | 23 (49) | 42 (41) |
| Age (yrs) | |||
| Median | 60 | 59 | 59 |
| Range | 38-66 | 37-65 | 37-66 |
| Stage (DS) at diagnosis (%) | |||
| I | 1 (2) | 3 (6) | 4 (4) |
| II | 10 (18) | 10 (21) | 20 (20) |
| IIIA | 42 (76) | 33 (70) | 75 (74) |
| IIIB | 2 (4) | 1 (2) | 3 (3) |
| ECOG score (%) | |||
| 0 | 19 (35) | 21 (45) | 40 (39) |
| 1 | 32 (58) | 23 (49) | 55 (54) |
| 2 | 4 (7) | 3 (6) | 7 (7) |
| Prior cycles of chemotherapy | |||
| Median | 3 | 3 | 3 |
| Range | 1-38 | 3-19 | 1-38 |
| No. of patients receiving prior radiotherapy (%) | 12 (22) | 13 (28) | 25 (25) |
| . | SCF + Filgrastim . | Filgrastim . | Total . |
|---|---|---|---|
| n | 55 | 47 | 102 |
| Sex (%) | |||
| Male | 36 (65) | 24 (51) | 60 (59) |
| Female | 19 (35) | 23 (49) | 42 (41) |
| Age (yrs) | |||
| Median | 60 | 59 | 59 |
| Range | 38-66 | 37-65 | 37-66 |
| Stage (DS) at diagnosis (%) | |||
| I | 1 (2) | 3 (6) | 4 (4) |
| II | 10 (18) | 10 (21) | 20 (20) |
| IIIA | 42 (76) | 33 (70) | 75 (74) |
| IIIB | 2 (4) | 1 (2) | 3 (3) |
| ECOG score (%) | |||
| 0 | 19 (35) | 21 (45) | 40 (39) |
| 1 | 32 (58) | 23 (49) | 55 (54) |
| 2 | 4 (7) | 3 (6) | 7 (7) |
| Prior cycles of chemotherapy | |||
| Median | 3 | 3 | 3 |
| Range | 1-38 | 3-19 | 1-38 |
| No. of patients receiving prior radiotherapy (%) | 12 (22) | 13 (28) | 25 (25) |
Number of Leukaphereses to Achieve the Target Stem Cell Yield (Primary Endpoint)
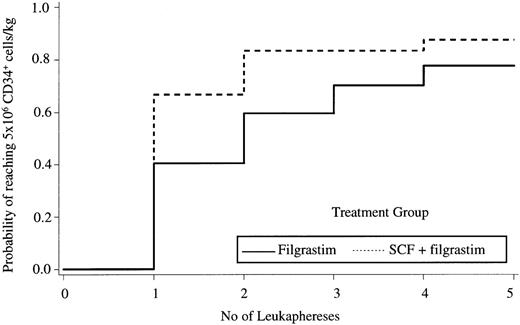
The median number of leukaphereses to reach the target yield of 5 × 106 CD34+ cells/kg was 1 in the SCF group versus 2 in the filgrastim group (P = .008, Gehan-Wilcoxon test; Fig 2). The proportion of patients reaching the target yield of 5 × 106CD34+ cells/kg after a single leukapheresis was 65% (36/55) versus 40% (19/45) in the SCF and filgrastim alone groups, respectively (P = .011; odds ratio, 2.79; 95% confidence interval [CI], 1.25 to 6.25; Table 2). Although not an endpoint of the study, we also analyzed the proportion of patients reaching a yield of 2 × 106CD34+ cells/kg in a single leukapheresis. This cell yield was reached in a single leukapheresis in 80% (44/55) versus 62% (29/47) of patients in the SCF and filgrastim groups, respectively (P = .041; odds ratio, 2.48; 95% CI, 1.04 to 6.16).
Kaplan Meier plot of the probability of reaching the target yield of 5 × 106 CD34+ cells/kg according to the treatment group.
Kaplan Meier plot of the probability of reaching the target yield of 5 × 106 CD34+ cells/kg according to the treatment group.
Cumulative Number and Proportion of Patients Reaching a CD34+ Cell Yield of 5.0 × 106/kg According to Day of Leukapheresis
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| SCF + filgrastim (n = 55) | 36 (65.4) | 45 (81.8) | 45 (81.8) | 47 (85.4) |
| Filgrastim (n = 47) | 19 (40.4) | 28 (59.6) | 33 (70.2) | 36 (76.6) |
| Odds ratio | 2.79 | 3.05 | 1.91 | 1.80 |
| 95% CI | 1.25-6.25 | 1.24-7.51 | 0.76-4.83 | 0.65-4.92 |
| . | Day 1 . | Day 2 . | Day 3 . | Day 4 . |
|---|---|---|---|---|
| SCF + filgrastim (n = 55) | 36 (65.4) | 45 (81.8) | 45 (81.8) | 47 (85.4) |
| Filgrastim (n = 47) | 19 (40.4) | 28 (59.6) | 33 (70.2) | 36 (76.6) |
| Odds ratio | 2.79 | 3.05 | 1.91 | 1.80 |
| 95% CI | 1.25-6.25 | 1.24-7.51 | 0.76-4.83 | 0.65-4.92 |
Values are the number of patients, with percentages in parentheses.
PBPC Yields
The median number of CD34+ cells collected in the first leukapheresis was significantly higher in the SCF group (11.3 × 106 CD34+ cells/kg) than in the filgrastim group (4.0 × 106 CD34+ cells/kg;P = .003). Numbers of CFU-GM and MNC were also significantly higher (Table 3). Over all leukaphereses, because collections were preplanned on the basis of reaching a target yield of 5.0 × 106 CD34+ cells/kg and because patients exposed to SCF underwent fewer leukaphereses, the difference in yields was smaller, but was also significantly improved with SCF. The increase in the number of CD34+ cells/kg collected after exposure to the combined cytokines was also observed in the subgroups of patients with and without prior treatment with melphalan (Table 4). The analysis of variance on log CD34+ cell yields obtained from the first leukapheresis showed no significant interaction between SCF and melphalan (F1,98 = 1.33, P = .25), indicating that the effect of SCF was the effectively same in both melphalan groups (and the effect of melphalan was effectively the same in both SCF groups). The effects of SCF and melphalan were both statistically significant (melphalan: F1,98 = 26.91, P = .0001; SCF: F1,98 = 7.20, P = .009). The CD34+cell yields were reduced by 84% in the melphalan group (ratio of geometric means = 0.16; 95% CI, 0.08 to 0.31) and increased by 137% by 2.4-fold in the SCF group (ratio of geometric means = 2.37; 95% CI, 1.25 to 4.48; Table 5).
Median CD34+ Cells, CFU-GM, and MNC Contents in the Product of the First Leukapheresis and All Leukaphereses
| . | SCF + Filgrastim (n = 55) . | Filgrastim (n = 47) . | P Value3-150 . |
|---|---|---|---|
| First leukapheresis | |||
| CD34+cells (×106/kg) | 11.3 | 4.0 | .003 |
| Range | 0.0-90.4 | 0.0-65.9 | |
| CFU-GM (×104/kg) | 45.4 | 10.3 | .011 |
| Range | 0.0-625.0 | 0.3-174.2 | |
| MNC count (×108/kg) | 114.8 | 34.2 | .0008 |
| Range | 2.42-1719.7 | 2.39-491.9 | |
| All leukaphereses | |||
| CD34+ cells (×106/kg) | 12.4 | 8.2 | .007 |
| Range | 0.0-90.4 | 0.3-65.9 | |
| CFU-GM (×104/kg) | 45.2 | 23.1 | .018 |
| Range | 0.0-625.0 | 0.2-174.2 | |
| MNC count (×108/kg) | 177.5 | 75.2 | .043 |
| Range | 5.2-3658.1 | 3.7-2183.6 |
| . | SCF + Filgrastim (n = 55) . | Filgrastim (n = 47) . | P Value3-150 . |
|---|---|---|---|
| First leukapheresis | |||
| CD34+cells (×106/kg) | 11.3 | 4.0 | .003 |
| Range | 0.0-90.4 | 0.0-65.9 | |
| CFU-GM (×104/kg) | 45.4 | 10.3 | .011 |
| Range | 0.0-625.0 | 0.3-174.2 | |
| MNC count (×108/kg) | 114.8 | 34.2 | .0008 |
| Range | 2.42-1719.7 | 2.39-491.9 | |
| All leukaphereses | |||
| CD34+ cells (×106/kg) | 12.4 | 8.2 | .007 |
| Range | 0.0-90.4 | 0.3-65.9 | |
| CFU-GM (×104/kg) | 45.2 | 23.1 | .018 |
| Range | 0.0-625.0 | 0.2-174.2 | |
| MNC count (×108/kg) | 177.5 | 75.2 | .043 |
| Range | 5.2-3658.1 | 3.7-2183.6 |
Wilcoxon rank-sum test.
CD34+ Cell Yields (×106/kg) in Patients With or Without Prior Treatment With Melphalan
| . | Without Melphalan . | With Melphalan . | ||
|---|---|---|---|---|
| SCF + Filgrastim (n = 36) . | Filgrastim (n = 34) . | SCF + Filgrastim (n = 19) . | Filgrastim (n = 13) . | |
| CD34+ cells (×106/kg) | ||||
| Mean | 24.6 | 14.1 | 8.8 | 5.0 |
| All leukaphereses | ||||
| Median | 21.3 | 9.3 | 7.1 | 5.3 |
| Range | 0.1-90.4 | 1.1-65.9 | 0.0-47.1 | 0.3-9.7 |
| . | Without Melphalan . | With Melphalan . | ||
|---|---|---|---|---|
| SCF + Filgrastim (n = 36) . | Filgrastim (n = 34) . | SCF + Filgrastim (n = 19) . | Filgrastim (n = 13) . | |
| CD34+ cells (×106/kg) | ||||
| Mean | 24.6 | 14.1 | 8.8 | 5.0 |
| All leukaphereses | ||||
| Median | 21.3 | 9.3 | 7.1 | 5.3 |
| Range | 0.1-90.4 | 1.1-65.9 | 0.0-47.1 | 0.3-9.7 |
Geometric Means of the CD34+ Cell Yields (×106/kg) From the First Leukapheresis According to the Treatment Effects of Prior Melphalan and SCF (Analysis of Variance)
| Group . | n . | Mean CD34+ Cells (×106/kg) (first leukapheresis) . | 95% CI . |
|---|---|---|---|
| Melphalan | 32 | 1.32 | 0.75-2.34 |
| Without melphalan | 70 | 8.45 | 5.76-12.39 |
| Ratio of melphalan/without melphalan | 0.16 | 0.08-0.31 | |
| SCF + filgrastim | 55 | 5.14 | 3.30-8.03 |
| Filgrastim | 47 | 2.17 | 1.33-3.55 |
| Ratio of SCF + filgrastim/filgrastim | 2.37 | 1.25-4.48 |
| Group . | n . | Mean CD34+ Cells (×106/kg) (first leukapheresis) . | 95% CI . |
|---|---|---|---|
| Melphalan | 32 | 1.32 | 0.75-2.34 |
| Without melphalan | 70 | 8.45 | 5.76-12.39 |
| Ratio of melphalan/without melphalan | 0.16 | 0.08-0.31 | |
| SCF + filgrastim | 55 | 5.14 | 3.30-8.03 |
| Filgrastim | 47 | 2.17 | 1.33-3.55 |
| Ratio of SCF + filgrastim/filgrastim | 2.37 | 1.25-4.48 |
Median time to first leukapheresis from the first day of mobilization was the same in both treatment groups (median of 12 days in both groups; ranges, 9 to 22 days v 10 to 21 days for the SCF and filgrastim groups, respectively).
Engraftment and Transfusions
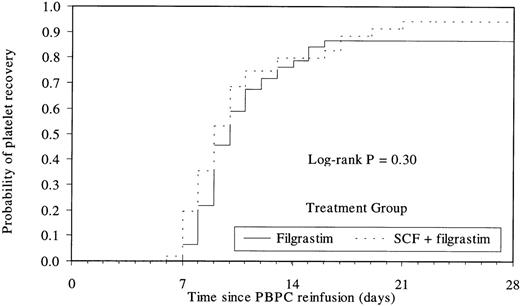
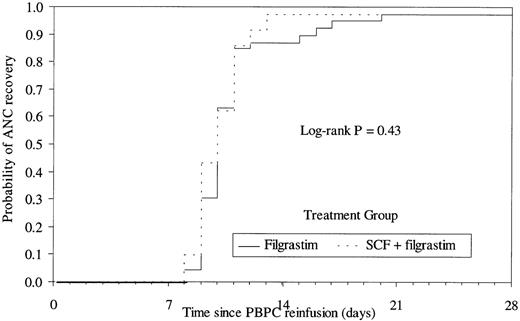
The median number of cells reinfused to the patient was 14.3 × 106 CD34+ cells/kg (range, 1.6 to 90.4 × 106) in the SCF group and 8.4 × 106 CD34+ cells/kg (range, 0.6 to 65.9 × 106) in the filgrastim group. Time to platelet recovery (median of 9 days and 10 days for the SCF and filgrastim groups, respectively) or neutrophil recovery (median of 10 days in both groups) was similar in patients having received PBPC collected after mobilization by the combination or by filgrastim alone (Figs 3 and 4). The time to platelet and RBC transfusion independence was also similar in the 2 groups (platelets, median of 8 days; RBC, median of 10 days). The median number of days of platelet or RBC transfusions was 1 in both groups (platelet transfusions: range, 0 to 19 in the SCF group and 0 to 7 in the filgrastim group; RBC transfusions: range, 0 to 9 in the SCF group and 0 to 4 in the filgrastim group).
Kaplan Meier plot of the time to platelet recovery (≥20 × 109/L) according to treatment group. Median of 9 days versus 10 days for SCF and filgrastim groups, respectively.
Kaplan Meier plot of the time to platelet recovery (≥20 × 109/L) according to treatment group. Median of 9 days versus 10 days for SCF and filgrastim groups, respectively.
Kaplan Meier plot of the time to neutrophil recovery (≥0.5 × 109/L) according to treatment group. Median of 10 days for both groups.
Kaplan Meier plot of the time to neutrophil recovery (≥0.5 × 109/L) according to treatment group. Median of 10 days for both groups.
Three-Month Follow-Up
Eighty-five patients were assessed for engraftment and survival at 3 months. All patients had platelet and neutrophil count recovery greater than 20 × 109/L and 0.5 × 109/L, respectively. No death and no fulminant progression of multiple myeloma occurred during this 3-month follow-up.
Safety
The number of days of exposure to cytokines during the collection phase was similar between treatment (12 v 13 days for SCF and filgrastim groups, respectively; Table 6). The cumulative dose of cyclophosphamide was also similar (data not shown).
Exposure to Treatment and Incidence of Treatment-Related Adverse Events During Collection Phase
| . | SCF + Filgrastim . | Filgrastim . |
|---|---|---|
| No. of patients | 55 | 47 |
| Median duration of cytokine exposure (d) | 12 | 13 |
| Range | 2-26 | 9-29 |
| Cumulative SCF dose (μg/kg) | 239.2 | — |
| Range | 39.3-520.0 | — |
| Cumulative filgrastim dose (μg/kg) | 59.8 | 65.2 |
| Range | 15.7-131.4 | 45.0-143.4 |
| No. (%) with treatment-related adverse event | 44 (80%) | 8 (17%) |
| Application site | 24 (44%) | 0 (0%) |
| Injection site erythema | 12 (22%) | 0 (0%) |
| Injection site reaction | 8 (15%) | 0 (0%) |
| Skin and appendages | 14 (25%) | 1 (2%) |
| Rash | 7 (13%) | 0 (0%) |
| Erythema | 3 (5%) | 1 (2%) |
| Fever | 6 (11%) | 1 (2%) |
| Metabolic/nutrition | 7 (13%) | 0 (0%) |
| LDH increased | 7 (13%) | 0 (0%) |
| Musculo-skeletal | 7 (13%) | 5 (11%) |
| Pain skeletal | 4 (7%) | 1 (2%) |
| Pain back | 3 (5%) | 2 (4%) |
| Myalgia | 0 (0%) | 2 (4%) |
| . | SCF + Filgrastim . | Filgrastim . |
|---|---|---|
| No. of patients | 55 | 47 |
| Median duration of cytokine exposure (d) | 12 | 13 |
| Range | 2-26 | 9-29 |
| Cumulative SCF dose (μg/kg) | 239.2 | — |
| Range | 39.3-520.0 | — |
| Cumulative filgrastim dose (μg/kg) | 59.8 | 65.2 |
| Range | 15.7-131.4 | 45.0-143.4 |
| No. (%) with treatment-related adverse event | 44 (80%) | 8 (17%) |
| Application site | 24 (44%) | 0 (0%) |
| Injection site erythema | 12 (22%) | 0 (0%) |
| Injection site reaction | 8 (15%) | 0 (0%) |
| Skin and appendages | 14 (25%) | 1 (2%) |
| Rash | 7 (13%) | 0 (0%) |
| Erythema | 3 (5%) | 1 (2%) |
| Fever | 6 (11%) | 1 (2%) |
| Metabolic/nutrition | 7 (13%) | 0 (0%) |
| LDH increased | 7 (13%) | 0 (0%) |
| Musculo-skeletal | 7 (13%) | 5 (11%) |
| Pain skeletal | 4 (7%) | 1 (2%) |
| Pain back | 3 (5%) | 2 (4%) |
| Myalgia | 0 (0%) | 2 (4%) |
Exposure to SCF was associated with manageable levels of toxicity. It is noteworthy that there were no reports of serious allergic-like reactions. The most commonly reported severe adverse events were gastrointestinal events (nausea, vomiting, and diarrhea) related to the associated chemotherapy, which occurred in 8 patients (15%) in the SCF group versus 4 (9%) in the filgrastim group.
Adverse events were reported as possibly, probably, or definitely related to the experimental cytokine treatment in 44 (80%) SCF recipients and 8 (17%) filgrastim recipients (Table 6). Application site reactions were the most frequent adverse reactions reported as at least possibly related to cytokine and were observed in 24 (44%) SCF recipients but in none of the filgrastim alone recipients. They were mild to moderate in severity and consisted primarily of injection site erythema. Nonserious skin reactions distant from the injection site, also reported as at least possibly related to cytokine, were also observed mostly in the SCF group. They consisted of rash, maculo-papular rash, erythema, pruritus, abnormal pigmentation, or urticaria and occurred in 25% of patients exposed to SCF overall (2% to 13% for any individual reaction). Consistent with the reported side effects of filgrastim, musculo-skeletal pain was reported as at least possibly related to cytokines in 7 (13%) patients exposed to the combination of SCF and filgrastim and in 5 (11%) patients exposed to filgrastim alone. Seven patients (13%) in the SCF group and none in the filgrastim group experienced treatment-related asymptomatic elevations in lactate dehydrogenase (LDH) levels. Cytokine-related fever was reported in 6 patients (11%) versus 1 patient (2%) in the SCF and filgrastim groups, respectively. Overall, 39 SCF patients (71%) versus 29 filgrastim patients (62%) experienced fever of any origin during the mobilization phase.
No clinically meaningful differences were observed between treatment groups regarding hematologic variables, although platelet and neutrophil counts were higher in the SCF group (data not shown).
DISCUSSION
In this controlled randomized study conducted in a large number of myeloma patients, the addition of SCF to a stem cell mobilization regimen consisting of cyclophosphamide and filgrastim resulted in a 3-fold enhancement of the number of PBPC collected in the first leukapheresis and a related decrease in the number of leukaphereses required to collect 5.0 × 106 CD34+cells/kg. These results are in line with those observed in other trials of the combination of SCF and filgrastim to improve PBPC collection.18-25
The use of SCF and filgrastim significantly reduced the number of leukaphereses procedures needed. This is important, because leukaphereses can be associated with adverse experiences related to anticoagulant therapy and central venous catheter complications, such as infection or thrombosis.26 Even in the absence of severe complications, the presence and maintenance of a central venous catheter is a source of discomfort and alters the quality of life of patients undergoing high-dose therapy programs.26Leukapheresis procedures are also associated with significant costs.27
In the future, use of a combination of SCF and filgrastim could reduce the length of the first leukapheresis required to collect a target number of CD34+ in a substantial proportion of patients. Indeed, in this study, cell yields reached a median of 11.3 × 106 CD34+ cells/kg in the first leukapheresis in patients exposed to this combination, potentially allowing a 50% reduction in apheresis blood volume in most patients.
A sufficient number of progenitor cells might also be collected in a limited volume of whole blood. This would avoid leukapheresis-associated morbidity altogether and would significantly reduce the costs of PBPC collections. Infusion of 1 L of whole blood after filgrastim priming has already been reported as capable of supporting high-dose melphalan therapy in patients with lymphoma.28 In patients with ovarian cancer, mobilization of progenitor cells into the peripheral blood using a combination of chemotherapy and cytokines equivalent to the present one (3 g/m2 cyclophosphamide, 20 μg/kg/d SCF, and 5 μg/kg/d filgrastim) resulted in a theoretical collection of 2 × 106/kg CD34+ cells in a median volume of 512 mL of whole blood.23 In a subset of patients from the present study, we have quantified the number of circulating CD34+cells in the peripheral blood before harvesting. In 43% of the 30 patients tested who had been exposed to the combination of SCF and filgrastim, a blood volume of 500 mL would have contained at least 2 × 106 CD34+ cells/kg. Such a yield would have been obtained in only 15% of the 25 patients tested in the filgrastim group.28
Combining SCF with filgrastim could also be of benefit for patients with prior exposure to melphalan, who are known to be at risk of failure to mobilize sufficient progenitor cells.29 Exposure to melphalan was also a predictor of lower PBPC yields in the present study. As demonstrated in the ANOVA analysis (Tables 4 and 5), the effect of SCF is consistent both in patients without prior melphalan and in the subset of patients at risk of insufficient mobilization due to prior melphalan.
PBPC collected after exposure to the combination of cytokines were equivalent to those obtained after mobilization with filgrastim as far as short-term engraftment is concerned. Consistent published data have shown a clear dose-response relationship between the number of CD34+ cells reinfused and the speed of platelet and granulocyte recovery.9 Although 1 to 2 × 106 CD34+ cells/kg can still be considered adequate to prevent graft failure, available data show that cell doses ≥5 × 106 CD34+ cells/kg are now considered as optimal, because they are associated with rapid engraftment in a higher proportion of patients. This translates in lower mean costs after the high-dose chemotherapy.30 The present study was not designed to observe differences in engraftment, because only the leukaphereses totaling the target of 5 × 106 CD34+ cells/kg were to be reinfused to the patient.
In this study, administration of the combination of SCF and filgrastim was safe and well-tolerated. The adverse event profile of the cytokine combination regimen was consistent with prior observations, although no serious allergic-like reactions were observed. Absence of such events in the present study might be related to careful screening for allergy history, systematic premedication, or relatively small patient numbers for detecting low frequency events. In other large randomized studies, such severe events were reported in 3% to 10% of patients.18 25
It would now be of interest to compare the combination of SCF and filgrastim, without cyclophosphamide, with the cyclophosphamide and filgrastim regimen used as a control in the present study. If equivalent or superior CD34+ cell collections could be achieved with cytokines alone, the morbidity associated with cyclophosphamide use would be avoided.
The present study also suggests that, because high numbers of CD34+ cells can be collected in a vast majority of patients, several cycles of high-dose chemotherapy with autologous stem cell support could be explored. Indeed, tandem autologous transplantation has been reported as an encouraging therapeutic option, at least for some young patients with myeloma.31 However, this option remains to be evaluated for the vast majority of myeloma patients and some patients still have a very poor outcome, even with tandem transplants.
The combination of SCF with filgrastim could also facilitate exploration of the medical benefits of ex vivo manipulations of progenitor cell products such as tumor cell purging32 or expansion and maturation of progenitor cells in culture and, in the future, gene therapy.
APPENDIX
SCF multiple myeloma study group.
Prof Jean-Pierre Jouet, Dr Florence Villard, Dr Marie-Odile Pétillon, and Dr Philippe Cabre (Department of Haematology, Hôpital Claude Huriez, Lille, France); Dr Claire de Cervens and Dr Chantal Adjou (Department of Haematology, Hôtel Dieu Hospital, Nantes, France); Dr Alain Bohbot (Department of Haematology, Strasbourg, France); Dr Anne Huynh, Dr Catherine Payen, Dr Jean-Pierre Calot, and Dr Cécile Demur (Department of Haematology, Toulouse, France); Dr Pierre Feugier, Dr François Schooneman, and Dr Catherine Claise (Vandoeuvre-les-Nancy, France); Dr Alain Sadoun and Dr Christine Giraud (Poitiers, France); Dr Bouzgarrou (Department of Haematology, Pessac, France); Heleen Denecker (Amgen, Brussels, Belgium); Gemma Hernandez (Amgen, Barcelona, Spain); Mireille Mur and Anne-Marie Sainte-Beuve (Amgen, Paris, France).
Supported by a clinical grant (950114 study) from Amgen Inc (Thousand Oaks, CA).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Thierry Facon, MD, Service des Maladies du Sang, Hôpital Claude Huriez, 59037 Lille Cedex, France; e-mail:tfacon.lille@invivo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal