HEMATOPOIESIS IS A continuous developmental process in which pluripotent stem cells and their progeny make sequential cell fate decisions, producing mature blood cells of the various lineages. The constant generation of appropriate numbers and types of mature cells, as well as the maintenance of multipotent progenitors, requires a complex regulatory network, many aspects of which remain incompletely understood.1-7 Members of theNotch family play critical roles in the determination of cell fates and maintenance of progenitors in many developmental systems.8-10 Given the extensive evolutionary and phylogenetic conservation of Notch function, it is not surprising that signaling through the Notch pathway has been implicated in the regulation of hematopoiesis. Since the initial demonstration thatNotch1 is expressed in normal bone marrow (BM) hematopoietic precursors,11 considerable evidence has emerged to support a conserved role for Notch in the mediation of cell fate decisions and self-renewal of progenitors during hematopoiesis. This review presents an overview of the Notch signaling pathway, evidence regarding Notch function, interactions of the Notch pathway with other signal transduction pathways, and a model for Notch function in hematopoiesis.
NOTCH AS A MEDIATOR OF CELL FATE DECISIONS: GENERAL CONSIDERATIONS
During development, multipotent progenitors undergo lineage commitment and maturation in a strict temporal and spatial pattern that reflects the expression of different genes among originally equipotent cells.2,3,6,12 Although many factors contribute to differential gene expression, signaling between cells is one of the key components of gene regulation and consequent appropriate cell fate specification.6,13-15 The Notch family comprises a group of highly conserved proteins that function both as cell surface receptors and direct regulators of gene transcription.9 16-18 As such, these molecules represent a unique conduit for signal transduction from the cell surface to the nucleus, permitting cells to directly influence gene expression in their neighbors. In general, Notch activation leads to transcriptional suppression of lineage-specific genes, inhibiting differentiation in response to inductive signals. Notch signaling limits the number of cells that adopt a particular fate and leaves some progenitors uncommitted but competent to adopt alternative fates.
The requirement for Notch activation through appropriate cell-cell interactions, combined with continuous changes in gene expression during development, permits Notch to influence cell fate decisions in a wide variety of tissues and cell types. In Drosophila, Notch is essential for the appropriate specification of many different cell fates during oogenesis, neurogenesis, myogenesis, and wing and eye development.9,10,19 The four known mammalian Notchgenes, Notch1-4, are widely expressed during embryogenesis and also play crucial developmental roles.20-27 New evidence extending the role of Notch as a general mediator of cell fate determination in mammalian systems is constantly emerging.10,28 29
Conservation of Notch Structure
The evolutionary conservation of Notch function is reflected in the high degree of structural conservation of Notch proteins and other molecules that mediate signal transduction through the Notch pathway. Figure 1 depicts the general structure of Notch, showing conserved regions of known functional significance. Molecules that directly interact with different Notch domains are noted in Fig 1 and are discussed in subsequent sections. The Notch extracellular domain contains a variable number of tandem epidermal growth factor (EGF)-like repeats and three Lin/Notch repeats (LNR), which function in ligand binding and Notch activation.30-32The conserved cysteines between the LNR and the transmembrane domain (TM) are likely involved in disulfide bonding of the heterodimeric receptor (Fig 1, inset). The putative cleavage sites involved in generation of the functional Notch receptor and release of intracellular Notch upon activation are indicated by dark and light arrows, respectively (see below, Notch Activation).
General structure of Notch proteins, showing conserved functional domains and proteins that interact with different regions. Inset shows the Notch heterodimeric receptor, which is generated by proteolytic processing and reassociation of the extracellular and intracellular fragments before reaching the cell surface. The putative cleavage site is indicated in the main figure by the black arrow. The extracellular domain consists of 29-36 tandem EGF-like repeats and 3 Lin/Notch repeats (LNR) involved in DSL ligand binding and Notch activation. The ligand-induced proteolytic cleavage site is indicated by the white arrow. The intracellular domain includes 6 cdc10 repeats, which mediate protein interactions essential for Notch function; the RAM domain, which binds CSL effector molecules; and the NCR region associated with cytokine-specific effects of Notch1 and 2. See text for discussion of the various proteins that interact with Notch.
General structure of Notch proteins, showing conserved functional domains and proteins that interact with different regions. Inset shows the Notch heterodimeric receptor, which is generated by proteolytic processing and reassociation of the extracellular and intracellular fragments before reaching the cell surface. The putative cleavage site is indicated in the main figure by the black arrow. The extracellular domain consists of 29-36 tandem EGF-like repeats and 3 Lin/Notch repeats (LNR) involved in DSL ligand binding and Notch activation. The ligand-induced proteolytic cleavage site is indicated by the white arrow. The intracellular domain includes 6 cdc10 repeats, which mediate protein interactions essential for Notch function; the RAM domain, which binds CSL effector molecules; and the NCR region associated with cytokine-specific effects of Notch1 and 2. See text for discussion of the various proteins that interact with Notch.
The Notch intracellular domain includes six cdc10/ankyrin repeats (hereafter referred to as cdc10 repeats), motifs characteristic of molecules involved in protein-protein interactions; this is the most highly conserved region and is essential for Notch signal transduction.31-35 The Notch protein does not have any known enzymatic activity, but rather transmits signals through direct molecular interactions. The region of Notch C-terminal to the cdc10 repeats has been associated with distinct protein interactions and transactivation,36-38 and the PEST domain is thought to regulate protein turnover. Drosophila Notch and the mammalian Notch1, 2, and 3 molecules also contain conserved nuclear localization signals (nls) and OPA sequences. More recently described regions include the RAM domain, which binds CSL (CBF1/Suppressor of Hairless/Lag-1) effectors of Notch signal transduction,35,39-41 and the Notch Cytokine Response (NCR) region associated with distinct effects of Notch1 and 2 on myeloid differentiation.42
Conservation of the Notch Signaling Pathway
In addition to Notch receptors, the primary components of the Notch signal transduction pathway include ligands homologous to Drosophila Delta and Serrate andCaenorhabditis elegans Lag-2 (DSL proteins) and intracellular effector molecules homologous toSuppressor of Hairless (CSL proteins) and Enhancer of split (E[spl]). Other effectors, targets, and modulators of Notch have also been evolutionarily conserved. Table 1summarizes the corresponding components of Notch signaling in flies, worms, and mammals. For the sake of clarity, this review focuses on mammalian systems, using Drosophila Notch as the prototype. Studies of lin-12 and glp-1 function during worm development have also contributed major insights into the mechanisms of Notch signaling. Comprehensive discussions of Notch/Lin-12/Glp-1 signaling in flies and worms can be found in a number of recent reviews.19,43 44
Conserved Components of the Notch Signaling Pathway
| . | C elegans . | Drosophila . | Mammals . | . |
|---|---|---|---|---|
| NOTCH receptors | Lin-12 Glp-1 | Notch | Notch1 Notch3 Notch2 Notch4 | |
| Extracellular ligands (DSL proteins) | Lag-2 Apx-1 | Delta | Delta-1 Delta-1 Delta-like 1 (DII-1) Delta-like 3 (DII-3) | |
| Serrate | Jagged1 (Serrate1) Jagged2 (Serrate2) | |||
| Intracellular effectors | Lag-1 | Suppressor of Hairless [Su(H)] | CBF-1/RBP-Jκ | |
| Deltex | Deltex NFκB | |||
| Target genes | Enhancer of split [E(spl)] | HES (Hairy/Enhancer of split) | ||
| bHLH | bHLH | |||
| Groucho | TLE | |||
| Processing molecules | SUP-17 | Kuzbanian | Kuzbanian | |
| Modifiers | Fringe | Lunatic Fringe | ||
| Manic Fringe | ||||
| Radical Fringe | ||||
| Numb | Numb | |||
| Numb-like | ||||
| Dishevelled | Dishevelled 1,2,3 |
| . | C elegans . | Drosophila . | Mammals . | . |
|---|---|---|---|---|
| NOTCH receptors | Lin-12 Glp-1 | Notch | Notch1 Notch3 Notch2 Notch4 | |
| Extracellular ligands (DSL proteins) | Lag-2 Apx-1 | Delta | Delta-1 Delta-1 Delta-like 1 (DII-1) Delta-like 3 (DII-3) | |
| Serrate | Jagged1 (Serrate1) Jagged2 (Serrate2) | |||
| Intracellular effectors | Lag-1 | Suppressor of Hairless [Su(H)] | CBF-1/RBP-Jκ | |
| Deltex | Deltex NFκB | |||
| Target genes | Enhancer of split [E(spl)] | HES (Hairy/Enhancer of split) | ||
| bHLH | bHLH | |||
| Groucho | TLE | |||
| Processing molecules | SUP-17 | Kuzbanian | Kuzbanian | |
| Modifiers | Fringe | Lunatic Fringe | ||
| Manic Fringe | ||||
| Radical Fringe | ||||
| Numb | Numb | |||
| Numb-like | ||||
| Dishevelled | Dishevelled 1,2,3 |
In the prevailing model for Notch signal transduction (Fig 2), Notch activation through binding to DSL ligands on adjacent cells results in proteolytic cleavage, with release and nuclear translocation of the Notch intracellular domain (Notch-IC). Notch-IC interacts with a number of cytoplasmic and nuclear proteins, permitting signal transduction through at least two pathways, one involving CSL proteins and one independent of CSL. The interaction of Notch-IC with CSL proteins results in transcriptional activation ofE(spl)/HES genes, which function as negative regulators of lineage-specific gene expression. CSL-independent signaling also results in transcriptional regulation, but is mediated by different effector molecules, such as Deltex, and may regulate distinct target genes.
Signal transduction through the Notch pathway. In the presence of a specific differentiation signal, activation of Notch through ligand binding results in proteolytic cleavage and release of the intracellular domain. Activated intracellular Notch (Notch-IC) and/or CSL proteins translocate to the nucleus, where they activate transcription of E(spl)/HES. The transcription factors encoded by E(spl)/HES in turn suppress transcription of lineage-specific genes, thereby inhibiting cellular differentiation. An equivalent cell, in the absence of Notch activation (right), will respond to the differentiation signal by activating transcription of lineage-specific genes, permitting differentiation along the induced pathway.
Signal transduction through the Notch pathway. In the presence of a specific differentiation signal, activation of Notch through ligand binding results in proteolytic cleavage and release of the intracellular domain. Activated intracellular Notch (Notch-IC) and/or CSL proteins translocate to the nucleus, where they activate transcription of E(spl)/HES. The transcription factors encoded by E(spl)/HES in turn suppress transcription of lineage-specific genes, thereby inhibiting cellular differentiation. An equivalent cell, in the absence of Notch activation (right), will respond to the differentiation signal by activating transcription of lineage-specific genes, permitting differentiation along the induced pathway.
Homotypic and Heterotypic Cell-Cell Interactions
Intercellular signaling through the Notch receptor permits equipotent cells in the same environment to respond differently to developmental signals. Although Notch and its ligands are often expressed on the same cell, Notch is activated primarily through binding to its ligand on adjacent cells.45,46 Notch signaling can occur either among a group of equivalent cells (homotypic interactions) or between nonequivalent cells (heterotypic interactions), both of which are essential during development.16,19,43 44
Homotypic interactions: lateral inhibition.
Notch activation through homotypic interactions results in what has been termed lateral inhibition: among a group of equipotent cells exposed to a specific differentiation signal, a limited number will adopt the specific cell fate, whereas adjacent cells (that express more Notch) are inhibited from differentiating (Fig 3A). These uncommitted cells remain competent to respond to subsequent signals, but the adoption of alternative fates may again be regulated by Notch. The dosage-dependent effects of Notch during lateral signaling have been demonstrated in chimeric flies and mice, in which cells expressing different amounts of Notch are juxtaposed. In the prototype of lateral signaling, the neural/epidermal cell fate decision during fly central nervous system (CNS) development, cells expressing less Notch adopt the primary (neuronal) fate and adjacent cells, expressing more Notch, adopt the alternative (epidermal) fate.45Similarly, during T-cell development, thymocytes expressing less Notch adopt the primary γδ cell fate, whereas those expressing more fail to adopt the primary fate and thus adopt the alternative αβ T cell fate.28 47 Figure 3A shows how sequential lateral signaling among progenitors facilitates the generation of distinct cell types.
Relative levels of Notch expression determine sequential cell fates. (A) Lateral signaling among a group of equipotent progenitors permits the generation of cells of distinct lineages in response to inductive signals. When exposed to signal A, cells expressing less Notch and more ligand (L) respond by adopting the primary cell fate A; adjacent cells expressing more Notch (N) are inhibited from adopting fate A, but remain competent to respond to subsequent signals. Among these remaining progenitors, differential expression of Notch again determines which cells will respond to inductive signal B: those expressing less Notch adopt fate B, whereas those expressing more are again inhibited from differentiating. Differential Notch and DSL ligand expression among the remaining progenitors at each subsequent step similarly restricts the number of cells responding to signals C and D. Thus, from a group of originally equipotent progenitors, cells of multiple distinct lineages are established, and some uncommitted progenitors are maintained. (B) Notch functions through successive cell divisions to influence the numbers and types of cells generated from a multipotent progenitor. Normal Notch expression (left panel) allows the A/B progenitor to give rise to cells of four distinct lineages; at each cell division, the daughter cell expressing less Notch adopts the primary fate, whereas the cell expressing more adopts the alternative secondary fate. The A/B progenitor gives rise to A (primary) and B (secondary) cells; progeny of type A cells expressing less Notch subsequently adopt the primary fate A1, whereas those expressing more Notch adopt the secondary fate A2; the same occurs for type B cells. The result is balanced production of cells of all four lineages. When Notch activity is dysregulated, the result is overproduction of one cell type at the expense of another. With loss of Notch function (middle panel), all cells adopt the primary fates resulting in production of only A1 cells. With increased Notch activity (right panel), daughter cells adopt the secondary fates, generating only B2 cells.
Relative levels of Notch expression determine sequential cell fates. (A) Lateral signaling among a group of equipotent progenitors permits the generation of cells of distinct lineages in response to inductive signals. When exposed to signal A, cells expressing less Notch and more ligand (L) respond by adopting the primary cell fate A; adjacent cells expressing more Notch (N) are inhibited from adopting fate A, but remain competent to respond to subsequent signals. Among these remaining progenitors, differential expression of Notch again determines which cells will respond to inductive signal B: those expressing less Notch adopt fate B, whereas those expressing more are again inhibited from differentiating. Differential Notch and DSL ligand expression among the remaining progenitors at each subsequent step similarly restricts the number of cells responding to signals C and D. Thus, from a group of originally equipotent progenitors, cells of multiple distinct lineages are established, and some uncommitted progenitors are maintained. (B) Notch functions through successive cell divisions to influence the numbers and types of cells generated from a multipotent progenitor. Normal Notch expression (left panel) allows the A/B progenitor to give rise to cells of four distinct lineages; at each cell division, the daughter cell expressing less Notch adopts the primary fate, whereas the cell expressing more adopts the alternative secondary fate. The A/B progenitor gives rise to A (primary) and B (secondary) cells; progeny of type A cells expressing less Notch subsequently adopt the primary fate A1, whereas those expressing more Notch adopt the secondary fate A2; the same occurs for type B cells. The result is balanced production of cells of all four lineages. When Notch activity is dysregulated, the result is overproduction of one cell type at the expense of another. With loss of Notch function (middle panel), all cells adopt the primary fates resulting in production of only A1 cells. With increased Notch activity (right panel), daughter cells adopt the secondary fates, generating only B2 cells.
Although the mechanisms responsible for initial differences in Notch expression among equivalent progenitors have not been clearly established, recent studies suggest that, rather than being strictly stochastic, specific intrinsic and extrinsic factors dictate which cells will express more or less Notch.43,44,48 Subsequent signaling is then biased by a feedback loop that reinforces either Notch or DSL ligand expression. Many downstream components of the Notch pathway participate in this feedback regulation, which permits the amplification of very minor differences in Notch expression.19 44
Heterotypic interactions: inductive signaling.
Notch signaling also occurs between Notch and DSL ligand expressed on different cell types. Inductive signaling through these heterotypic interactions is regulated primarily by ligand expression, limiting Notch activation to those cells in direct contact with ligand-expressing cells. Interactions between Notch and DSL ligands can also be modulated by other molecules, such as Fringe and Wingless,49,50 and further regulated by a feedback loop.51,52 Inductive signaling thus permits the establishment of finely demarcated boundaries between cell types, exemplified by dorsoventral boundary formation during fly wing margin specification and vertebrate limb development.52-54
In the hematopoietic system, Notch interactions may be either homotypic or heterotypic, and both lateral and inductive signaling mechanisms are likely to be important. The different extrinsic factors and cell types involved may provide distinct regulatory mechanisms and increase the diversity of Notch function. However, despite important differences, in some respects the effects of Notch activation through either type of signaling are similar, ie, the generation of distinct cell lineages from initially equivalent cells. Figure 3B shows the influence of Notch on the normal production of different cell types from a single progenitor (left panel) and the effects of loss of Notch activity (middle panel) or increased Notch activity (right panel). Normally, differences in Notch expression permit daughter cells to adopt distinct cell fates, resulting in a balanced distribution of four cell types after two generations. Dysregulated Notch activity results in cells adopting only primary fates (loss of Notch activity) or secondary fates (increased Notch activity), resulting in overproduction of one cell type at the expense of the others. In the case of increased Notch activity, adoption of the secondary fate depends on the capacity of the cell to respond to the secondary signal. If Notch expression is constitutive, cells may fail to differentiate in response to any signal, resulting in the lack of production of any mature cells.
Diversification of Notch Function in Mammals
The critical role of Notch signaling in mammalian development is apparent from Notch1 and 2, Jagged1 and 2, andDelta1 knockout mice, which have severe defects resulting in embryonic or perinatal lethality.55-59 However, these mice also display distinct phenotypic defects, showing the lack of complete functional redundancy of the different mammalian Notch molecules and ligands. It seems likely that the evolution of multiple genes encoding Notch receptors and ligands in mammals reflects a need for diversification of Notch function in these more complex developmental systems.
Functional diversity of Notch signaling in vivo most likely results from a combination of intrinsic and extrinsic mechanisms. In some cases, distinct functions may be dictated by differential tissue expression or by expression levels of different Notch molecules and ligands. For example, Notch4 expression is largely restricted to endothelial cells,27 and although Notch1 and2 are widely expressed, within the lymphoid system they are preferentially expressed in thymus or spleen, respectively.23,24 Similarly, Jagged1 and 2are preferentially expressed in BM or thymus.60-62Restricted expression of DSL ligands may serve an important regulatory role by limiting Notch activity to a subset of expressing cells and further regulating Notch expression through a feedback loop.62 The timing and pattern of expression of differentNotch molecules and ligands may also permit distinct functions within the same tissue, as suggested by Notch1, 2, and3 expression in the developing tooth63 and byNotch, Jagged, and Delta expression during nervous system development.64
It has been suggested that the mammalian Notch orthologues are biochemically redundant, ie, that they are capable of participating in the same molecular interactions and thus, in principle, can functionally compensate for each other and that their specific roles in vivo reflect differential expression. However, it is also possible that intrinsic differences in the Notch proteins permit distinct interactions that define functional differences. Specific interactions with DSL ligands and modulators may primarily be determined by ligand,29 but may also reflect differences in the Notch extracellular domains. Structural differences in the intracellular domain may also define distinct activities, even within the same cell, as shown by the correlation of physical properties with specific effects of Notch1 and 2 on 32D differentiation.42
NOTCH IN HEMATOPOIESIS
Defining the cellular and molecular mechanisms responsible for differentiation and self-renewal of hematopoietic progenitors is central to achieving a complete understanding of hematopoiesis. Hematopoietic stem cells and multipotent progenitors must continuously undergo lineage commitment, differentiation, and proliferation, while also maintaining a pool of uncommitted progenitors to support the production of new blood cells. Despite considerable progress, the molecular processes that mediate cell fate specification and self-renewal of progenitors remain incompletely understood and controversial.2-7,12 65 Notch is a general regulator of cell fate determination and interacts with a host of factors that are of known significance in hematopoiesis. The integration of Notch signaling with other cell-cell interactions, cytokine pathways and transcriptional regulation may be a key to understanding the regulation of hematopoiesis.
A considerable amount of indirect evidence supporting a role for Notch in hematopoiesis has emerged over the past several years. Our initial observation that human Notch1 is expressed in normal BM hematopoietic precursors provided the foundation for the hypothesis that Notch functions in hematopoiesis.11 These studies demonstrated that Notch1 is expressed in marrow CD34+ progenitors, including the immature subset that lacks expression of lineage-associated antigens (CD34+lin−). Interestingly,Notch1 is also expressed in the CD34+lin+ subset and, at lower levels, in more mature CD34− cells. We subsequently found thatNotch1 is also expressed in lymphoid, myeloid, and erythroid precursor populations, as well as in peripheral blood T and B lymphocytes, monocytes, and neutrophils (L.A.M., unpublished data), suggesting that Notch functions in multiple lineages and at various stages of maturation. The observations that DSL ligands are expressed in BM, fetal liver, and thymus60,61,66,67 and that Notch2, 3, and 4 are also expressed by hematopoietic progenitors11 68 (and L.A.M., unpublished data) provide further evidence that Notch signaling plays a significant role in hematopoiesis.
Myeloid Differentiation
The first evidence for Notch function in myelopoiesis came from studies in 32D cells, a progenitor cell line frequently used as a model system for myeloid differentiation. 32D cells proliferate as undifferentiated blasts in the presence of interleukin-3 (IL-3), but can be induced to differentiate in response to other cytokines, including granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin.69In initial studies, we found that expression of an activated intracellular form of Notch1 inhibits differentiation of 32D cells in response to G-CSF and permits the expansion of undifferentiated cells, findings consistent with the effects of constitutive Notch activity in other systems.68 In subsequent studies we confirmed that activation of full-length Notch1 by the ligand, Jagged1, results in comparable phenotypic effects.60 These studies further validate the use of intracellular forms of Notch as a model for Notch activity and provide the important demonstration that a complete Notch signaling pathway is intact in 32D cells.
Cytokine-specific effects of Notch1 and 2: The NotchCytokine Response (NCR) region.
Notch1 and 2 are both expressed in myeloid progenitors, raising the possibility that they have distinct functions in these cells. In studies to address this question, we found that, whereas either Notch1 or Notch2 is capable of inhibiting myeloid differentiation, they do so in a cytokine-specific manner: Notch1 in response to G-CSF, and Notch2 in response to GM-CSF.42 Furthermore, this cytokine specificity is associated with a previously uncharacterized region of Notch, which we have termed the Notch CytokineResponse (NCR) region (Fig 1). The Notch1 and 2 NCR regions also confer differences in subcellular localization and electrophoretic mobility, suggesting that differences in posttranslational modification of the NCR region may define specific molecular interactions. These studies provide the first evidence that different Notch orthologues may have distinct functions in the same cell type and indicate a molecular basis for those differences.
The finding that Notch1 and 2 are active only in the context of specific cytokines elucidates a potentially important link between Notch and cytokine signaling pathways in hematopoietic regulation. The use of truncated intracellular Notch1 and 2 molecules in these experiments suggests that the Notch1 and 2 intracellular domains interact specifically with components of the G-CSF or GM-CSF intracellular signal transduction pathways. (For a model, see Fig 4B.) The involvement of distinct JAK/STAT molecules in G-CSF and GM-CSF signaling raises the possibility that these factors are involved in the cytokine specificity of Notch. It is also possible that interactions involving the extracellular domain of Notch and cytokines or cytokine receptors influence the effects of Notch on hematopoietic differentiation. When reagents are available, it will be important to confirm cytokine specificity and define molecular interactions using full-length Notch1 and 2 molecules activated by DSL ligands.
A model for Notch function in hematopoiesis, showing its role in mediating cell fate decisions through cell-cell interactions and transcriptional regulation. (A) Cellular interactions and effects of Notch signaling in different hematopoietic microenvironments, showing the influence of Notch on hematopoietic cells of different lineages and at different stages of maturation. Each compartment is used to emphasize particular features of Notch signaling that are also applicable to the other compartments. In the progenitor compartment (top panel), Notch signaling occurs between stromal cells and hematopoietic progenitors and between equivalent or nonequivalent hematopoietic cells. Hematopoietic progenitors express multiple Notch molecules (depicted as Notch1 and 2) and DSL ligands. Stromal cells express DSL ligands, including Jagged and Delta. In the context of various cytokines, progenitors are induced to differentiate. Notch signaling regulates the response of progenitors to cytokine stimulation, permitting some to differentiate and others to self-renew. Cells expressing more Notch are inhibited from differentiating and thus maintain a pool of uncommitted progenitors. Cells expressing less escape from the Notch signal and undergo the next step in differentiation. Commitment to the lymphoid or myeloid lineage depends on specific cytokines and the relative activities of Notch1 and 2. Increased Notch1 activity inhibits myeloid differentiation and thus favors the lymphoid pathway; however, for lymphoid commitment, progenitors must also express less Notch2 than their neighbors (increased Notch1 and 2 results in self-renewal). Myeloid differentiation is similarly favored by increased Notch2 expression (which inhibits lymphoid differentiation) and permitted by relatively low levels of Notch1. At the next step, lymphoid and myeloid precursors again either differentiate or self-renew: those expressing less Notch continue to differentiate, whereas those expressing more self-renew at this stage of maturation. In the myeloid compartment (lower left), precursors express both Notch1 and 2 and the effects on differentiation are cytokine-specific, as shown by granulocytic differentiation in response to G-CSF and GM-CSF. Either activation of Notch1 in the presence of G-CSF or activation of Notch2 in the presence of GM-CSF results in inhibition of differentiation and self-renewal of progenitors. These progenitors remain competent to adopt alternative fates in response to subsequent signals. In the absence of Notch1 or 2 activity or in the context of different cytokines (eg, GM-CSF for Notch1 or G-CSF for Notch2), myeloid progenitors differentiate to produce mature granulocytes. In the lymphoid compartment (lower right), Notch signaling involves interactions of thymocytes with each other and with thymic epithelial cells. When induced to differentiate, immature CD4−CD8− thymocytes expressing more Notch1 self-renew, whereas those expressing less undergo the next step in T-cell maturation. At this next step, in the context of a productive TCR rearrangement, CD4−CD8− precursors expressing less Notch adopt the primary γδ T-cell fate; those expressing more Notch fail to adopt the γδ cell fate, subsequently express both CD4 and CD8, and adopt the alternative β T-cell fate. These CD4+CD8+ β precursors, in turn, can either develop either as mature CD4 or CD8 T cells. Cells expressing less Notch adopt the primary CD4 cell fate, normally in association with class II MCH molecules. Development of CD8 T cells generally requires MHC class I ligation, and Notch expression in this context permits cells to adopt the CD8 cell fate. However, expression of high levels of Notch in the presence of MHC class II molecules will also permit CD8 development, while preventing cells from adopting the usual CD4 fate in this context. (B) Distinct intracellular interactions result in cytokine-specific effects of Notch1 and 2 on myeloid differentiation. The activated intracellular Notch molecule includes the cdc10 repeats, which are necessary for Notch function, and the NCR region, which confers cytokine specificity on the Notch1 and 2 molecules. In an inactive conformation, the cdc10 domain is masked and therefore unable to participate in molecular interactions required for Notch activity. Stimulation by G-CSF induces signal transduction through a pathway that includes molecule X, which can interact with the NCR domain of Notch1, but not Notch2. The interaction of X with Notch1 results in unmasking of the cdc10 repeats and facilitates the interaction of Notch1 with nuclear factors. The result is transcriptional suppression of genes that would otherwise be activated in response to G-CSF. Because the Notch2 NCR cannot interact with X, the cdc10 domain remains masked, Notch2 remains inactive, and transcriptional activation of G-CSF–induced genes results in cellular differentiation. GM-CSF signals through a different pathway, inducing molecule Y, which can interact with the NCR domain of Notch2, but not Notch1. Thus, in the context of GM-CSF stimulation, Notch2 is active (the cdc10 domain is unmasked) and inhibits transcription of GM-CSF–induced genes. In contrast, in the presence of GM-CSF, Notch1 remains inactive, lineage-specific gene transcription is permitted, and cells differentiate.
A model for Notch function in hematopoiesis, showing its role in mediating cell fate decisions through cell-cell interactions and transcriptional regulation. (A) Cellular interactions and effects of Notch signaling in different hematopoietic microenvironments, showing the influence of Notch on hematopoietic cells of different lineages and at different stages of maturation. Each compartment is used to emphasize particular features of Notch signaling that are also applicable to the other compartments. In the progenitor compartment (top panel), Notch signaling occurs between stromal cells and hematopoietic progenitors and between equivalent or nonequivalent hematopoietic cells. Hematopoietic progenitors express multiple Notch molecules (depicted as Notch1 and 2) and DSL ligands. Stromal cells express DSL ligands, including Jagged and Delta. In the context of various cytokines, progenitors are induced to differentiate. Notch signaling regulates the response of progenitors to cytokine stimulation, permitting some to differentiate and others to self-renew. Cells expressing more Notch are inhibited from differentiating and thus maintain a pool of uncommitted progenitors. Cells expressing less escape from the Notch signal and undergo the next step in differentiation. Commitment to the lymphoid or myeloid lineage depends on specific cytokines and the relative activities of Notch1 and 2. Increased Notch1 activity inhibits myeloid differentiation and thus favors the lymphoid pathway; however, for lymphoid commitment, progenitors must also express less Notch2 than their neighbors (increased Notch1 and 2 results in self-renewal). Myeloid differentiation is similarly favored by increased Notch2 expression (which inhibits lymphoid differentiation) and permitted by relatively low levels of Notch1. At the next step, lymphoid and myeloid precursors again either differentiate or self-renew: those expressing less Notch continue to differentiate, whereas those expressing more self-renew at this stage of maturation. In the myeloid compartment (lower left), precursors express both Notch1 and 2 and the effects on differentiation are cytokine-specific, as shown by granulocytic differentiation in response to G-CSF and GM-CSF. Either activation of Notch1 in the presence of G-CSF or activation of Notch2 in the presence of GM-CSF results in inhibition of differentiation and self-renewal of progenitors. These progenitors remain competent to adopt alternative fates in response to subsequent signals. In the absence of Notch1 or 2 activity or in the context of different cytokines (eg, GM-CSF for Notch1 or G-CSF for Notch2), myeloid progenitors differentiate to produce mature granulocytes. In the lymphoid compartment (lower right), Notch signaling involves interactions of thymocytes with each other and with thymic epithelial cells. When induced to differentiate, immature CD4−CD8− thymocytes expressing more Notch1 self-renew, whereas those expressing less undergo the next step in T-cell maturation. At this next step, in the context of a productive TCR rearrangement, CD4−CD8− precursors expressing less Notch adopt the primary γδ T-cell fate; those expressing more Notch fail to adopt the γδ cell fate, subsequently express both CD4 and CD8, and adopt the alternative β T-cell fate. These CD4+CD8+ β precursors, in turn, can either develop either as mature CD4 or CD8 T cells. Cells expressing less Notch adopt the primary CD4 cell fate, normally in association with class II MCH molecules. Development of CD8 T cells generally requires MHC class I ligation, and Notch expression in this context permits cells to adopt the CD8 cell fate. However, expression of high levels of Notch in the presence of MHC class II molecules will also permit CD8 development, while preventing cells from adopting the usual CD4 fate in this context. (B) Distinct intracellular interactions result in cytokine-specific effects of Notch1 and 2 on myeloid differentiation. The activated intracellular Notch molecule includes the cdc10 repeats, which are necessary for Notch function, and the NCR region, which confers cytokine specificity on the Notch1 and 2 molecules. In an inactive conformation, the cdc10 domain is masked and therefore unable to participate in molecular interactions required for Notch activity. Stimulation by G-CSF induces signal transduction through a pathway that includes molecule X, which can interact with the NCR domain of Notch1, but not Notch2. The interaction of X with Notch1 results in unmasking of the cdc10 repeats and facilitates the interaction of Notch1 with nuclear factors. The result is transcriptional suppression of genes that would otherwise be activated in response to G-CSF. Because the Notch2 NCR cannot interact with X, the cdc10 domain remains masked, Notch2 remains inactive, and transcriptional activation of G-CSF–induced genes results in cellular differentiation. GM-CSF signals through a different pathway, inducing molecule Y, which can interact with the NCR domain of Notch2, but not Notch1. Thus, in the context of GM-CSF stimulation, Notch2 is active (the cdc10 domain is unmasked) and inhibits transcription of GM-CSF–induced genes. In contrast, in the presence of GM-CSF, Notch1 remains inactive, lineage-specific gene transcription is permitted, and cells differentiate.
Lymphoid Differentiation: T-Cell Development
A role for Notch in lymphoid development was first proposed when the human Notch homologue, TAN-1(hNotch1), was cloned from T-cell leukemias containing translocations involving the Notch1 gene.23Convincing evidence that Notch1 plays a role in normal T-cell development has since been provided by expression analyses and functional studies.28,70 In the developing mouse thymus,Notch1 is expressed at relatively high levels in the least mature (CD4−CD8−) thymocytes and at very low levels in mature CD4+CD8− and CD4−CD8+ cells, an expression pattern consistent with a role for Notch in maintaining cells in a less differentiated state.71,72 The expression ofJagged2 further indicates that Notch signaling occurs in the developing thymus.61,62 Elegant studies by Robey et al47 72 have demonstrated that Notch1 can influence both the CD4/CD8 and αβ/γδ T-cell fate decisions during T-lymphocyte development. The general conclusions from these studies are that increased Notch activity favors the CD8 and αβ T-cell fate decisions over the CD4 and γδ cell fates, respectively. However, the effect of Notch on developing thymocytes is modulated by other factors, including the productive rearrangement and expression of T-cell receptors (TCR) and ligation with major histocompatability complex (MHC) molecules.
The CD4/CD8 lineage decision.
Transgenic mice generated by Robey et al72carry an activated intracellular form of Notch1 under control of the proximal Lck promoter, which permits expression of the transgene early in thymocyte development. Evaluation of thymic T-cell subsets from these mice revealed that expression of activated Notch1results in an increase in mature CD8+ T cells and a corresponding decrease in CD4+ T cells.72Expression of the Notch1 transgene in MHC class I-deficient mice demonstrated that Notch1 activity permits the development of CD8+ cells even in the absence of class I MHC molecules, which are normally required for differentiation to this lineage. However, expression of Notch1 was not sufficient to promote the generation of CD8+ cells in the absence of both MHC class I and II molecules, suggesting that MHC ligation is required for the developing thymocyte to receive the Notch signal at this maturational stage. Alternatively, MHC ligation may initiate a developmental program that can be modulated, but not initiated, by Notch signaling.
The αβ/γδ lineage decision.
At an earlier stage of thymocyte development, Notch1 cooperates with the TCR in the specification of γδ and αβ cell fates. At this developmental branch point, immature CD4−CD8− cells either adopt the γδ T-cell fate or further differentiate to express both CD4 and CD8 as well as the αβ TCR, eventually becoming mature αβ CD4 or CD8 T cells. By analyzing transgenic and chimeric mice having thymocytes expressing different amounts of Notch1, Washburn et al47concluded that decreased Notch1 expression permits cells to adopt the γδ fate, whereas increased expression favors the αβ lineage. In chimeric mice having thymocytes containing one or two copies of a functional Notch1 gene, cells heterozygous forNotch1 were more likely to develop as γδ T cells, whereas wild-type cells containing two copies were more likely to become αβ T cells. Although thymocytes having productive γδ TCR gene rearrangements normally adopt the γδ fate, increased Notch activity permitted γδ TCR+ cells to develop along the αβ lineage. However, Notch activity in the absence of either the γδ or αβ TCR was not sufficient to drive the development of αβ T cells. Thus, in the CD4/CD8 and αβ/γδ cell fate decisions, excess Notch activity can override the MCH II or γδ TCR signals, but cannot dictate cell fates in the complete absence of MHC ligation or TCR signaling, respectively.
The observations from Notch1 chimeric mice provide a classic illustration of lateral signaling and the dosage effects ofNotch among developing thymocytes. (See above Homotypic Interactions: Lateral Inhibition and Fig 3A.) During thymic T-cell development, cells expressing less Notch than their neighbors adopt the primary γδ fate, regardless of whether the absolute amount of Notch reflects one or two copies of theNotch1 gene. Thymocytes expressing more Notch are inhibited from adopting the γδ fate, but remain competent to adopt the alternative αβ fate. The αβ (CD4+CD8+) T-cell precursors, in turn, may be subject to lateral signaling through differential expression ofNotch; those expressing less Notch adopt the primary CD4 fate, whereas those expressing more Notch adopt the alternative CD8 fate.
B-Cell Development
A number of observations provide circumstantial evidence thatNotch also influences B-lymphocyte development. NF-κB and CBF1/RBP-Jκ, which regulate expression of B-cell–specific genes, also physically interact with Notch and participate in Notch signaling.39-41,73-75 Transcriptional regulation by NF-κB is essential for normal B-cell development and activation.76-79 CBF1 binds to promoters of several B-cell genes and acts as a transcriptional regulator in B-cell immortalization induced by Epstein-Barr virus (EBV).40,80 The recent demonstration that Notch1 and 2 inhibit the bHLH transcription factor E47 also supports a role for Notch in the regulation of B-cell–specific genes.81 E47 is essential for early B-lymphocyte development, activation of the Ig heavy chain locus, and initiation of Ig gene rearrangement. The conserved role of Notch in the regulation of bHLH activity makes this finding particularly intriguing.
Lymphoid Malignancies
Given the broad developmental role for Notch and its general function in regulating differentiation of immature cells, it is not surprising that both unregulated and ectopic expression ofNotch have been implicated in oncogenesis. Although theNotch1-4 genes are located on different chromosomes, all have been mapped to regions of neoplasia-associated translocation or oncogenic viral insertion, and three have been directly associated with malignant transformation. Notch1 and 2 have been implicated in the development of T-lymphoid malignancies23,82,83 and contribute to neoplastic transformation in vitro.84 The Notch homologue now known as Notch4 was first identified as the int-3oncogene associated with primary mouse mammary tumors.27,85In all of these cases, the aberrations in Notch involve expression of truncated molecules lacking most or all of the extracellular domain. Similar truncated molecules have been shown to behave as constitutively active forms of Notch,31-34 86-89 suggesting that unregulated intracellular Notch activity might contribute to malignant transformation by inhibiting normal differentiation and permitting the continued proliferation of undifferentiated cells.
T-cell malignancies.
In a subset of T-cell acute lymphoblastic leukemias, breakpoint translocations involving the Notch1 gene predict expression of truncated intracellular Notch1 proteins that likely function as constitutively activated forms of Notch1.23 A direct association between expression of such truncated Notch1 proteins and the development of T-cell malignancies has been confirmed by Pear et al90 using a mouse transplantation model. These investigators found that mice transplanted with BM cells transduced with activated forms of Notch developed T-cell malignancies at a high frequency. Interestingly, equivalent tumorigenesis was observed for Notch constructs containing only the intracellular domain and those including the transmembrane domain, suggesting that either membrane-bound or free intracellular Notch molecules are oncogenic. This is in contrast to other reports associating malignant transformation primarily with nuclear forms of Notch.
Truncated Notch2 molecules have also been associated with T-cell malignancies. Rohen et al82 described transduction ofNotch2 sequences in thymic lymphomas from cats infected with feline leukemia virus. The transduced region of Notch2 included the conserved extracellular cysteines, the transmembrane domain, and portions of the intracellular domain, including the cdc10 repeats. In contrast to the corresponding Notch1 protein in mice (discussed above), the truncated Notch2 protein localized to the nucleus, indicating it was not tethered to the membrane. These investigators have proposed that nuclear Notch2 is generated through internal translation initiation at a site immediately downstream of the transmembrane domain and thus would not be membrane-bound. If correct, this observation suggests a nonproteolytic mechanism for generating activated intracellular forms of Notch.
B-cell malignancies.
Notch has also been linked to B-cell malignancies induced by EBV. Immortalization of B lymphocytes by EBV requires EBNA2, a virally encoded transcriptional activator. EBNA2 transactivates cellular genes through its association with the CSL protein CBF1/RBP-Jκ,80 a primary component of the Notch pathway. EBV-induced immortalization through EBNA2 involves a mechanism that mimics intracellular Notch activity,40 73 implicating dysregulation of Notch/CBF1 signaling in the development of EBV-associated malignancies.
The normal role of Notch includes mediating cell fate decisions such that appropriate numbers of different cell types are produced. Aberrations in Notch signaling disrupt this regulation, with either excess or insufficient Notch activity resulting in expansion of one cell type at the expense of another (Fig 3B). Thus, any process disrupting Notch signaling could potentially contribute to malignant transformation by permitting inappropriate expansion of a single cell type. Evidence supporting this possibility includes the association of other components of the Notch signaling pathway with various malignancies.91 92 Dysregulated Notch signaling may prove to be a frequent occurrence in malignant transformation. The contribution of Notch, if any, to the development of hematopoietic malignancies other than lymphomas should become apparent as the role of Notch in hematopoiesis and its integration with other signaling pathways become more clearly elucidated.
NOTCH LIGANDS IN HEMATOPOIESIS
Within the hematopoietic microenvironment a complex signaling network involving soluble and cell-bound cytokines, as well as interactions among hematopoietic cells and stromal elements, regulates differentiation and proliferation of hematopoietic progenitors.1,2,4,5,7,12,93 Although the importance of signaling through cytokine production has been established, the influence of direct cell-cell interactions among equivalent or different hematopoietic cells remains largely undefined. Notch, a molecule that mediates intercellular interactions and directly influences cell fate decisions may provide an important adjunct to other regulatory mechanisms. Expression of Notch ligands by BM and fetal liver stromal cells, thymic epithelial cells, and hematopoietic cells60,61,66 67 (and L.A.M., unpublished data) supports a role for Notch signaling through homotypic and heterotypic interactions in the hematopoietic and lymphopoietic microenvironments.
Notch Ligands: DSL Proteins
In Drosophila, the two Notch ligands Delta and Serratehave both distinct and overlapping functions.10,94Multiple ligands corresponding to each of these two general classes have been identified in vertebrates, leading to a somewhat confusing nomenclature. In mammals, ligands having high homology to Deltaare referred to as Delta or Delta-like (Dll), and those homologous to Serrate are called Serrate orJagged (Table 1). DSL ligands, like Notch, are transmembrane proteins having an extracellular domain containing a variable number of EGF-like repeats. The extracellular domain also contains a conserved region unique to this family of molecules: a DSL (Delta/Serrate/Lag-2) domain that is required for Notch binding and activation.46,95 Serrate and Jagged also contain a conserved cysteine-rich region that is not present in Delta homologues. The intracellular domains of DSL proteins consist of short, diverse sequences of unknown function, but may be involved in multimerization.46 96
Jagged1 and 2
Human Jagged1 was cloned from a normal BM cDNA library and is expressed by a subset of marrow stromal cells,60 indicating that it functions in the hematopoietic microenvironment. The coexpression of Jagged2 and Notch1 in the developing thymus suggests that Jagged2 is a ligand for the Notch1 receptor in this tissue.61,62 Mutant mice lacking a functionalJagged2 gene further illustrate the significance of Jagged-Notch signaling in T lymphopoiesis: these mice, in addition to other severe defects, have abnormal thymic morphology and impaired differentiation of γδ T cells.58
Expression of Jagged1 by BM stromal cells and Notch1 by hematopoietic progenitors suggests that, within the marrow microenvironment, interactions between stromal and hematopoietic cells include Jagged1-Notch1 signaling. The expression of Jagged1 by the HS-27a stromal cell line is intriguing in this regard. HS-27a supports the maintenance and proliferation of hematopoietic progenitors and promotes cobblestone area formation in long-term marrow cultures,97 properties that could be attributed to Jagged-Notch signaling. The demonstration that both HS-27a and a purified Jagged1 protein inhibit G-CSF–induced differentiation of Notch1-expressing 32D cells and permit proliferation of undifferentiated progenitors supports this hypothesis.60The requirement for both Notch1 expression by 32D cells and presence of ligand strongly suggests that Jagged1-Notch1 signaling is responsible for these effects. Although Jagged1 was most effective when endogenously expressed as a membrane-bound protein by the HS-27a cell line, two soluble forms of Jagged1 produced similar effects. Of particular interest was the finding that a small peptide corresponding to the unique DSL domain could activate Notch in this system, raising the possibility that DSL peptides might be useful for stem cell expansion.
Two recent reports provide further evidence that Jagged-Notch signaling promotes the maintenance and expansion of normal hematopoietic progenitors. Varnum-Finney et al66 found that the addition of Jagged1 to primary cultures of mouse lin−sca-1+c-kit+ BM progenitors resulted in a twofold to threefold increase in the subsequent generation of high proliferative potential (HPP)-mix colonies. Jones et al67 reported a similar effect of Jagged1 on mouse CD34+c-kit+ AGM and fetal liver hematopoietic progenitors: primary culture on a stromal cell line expressing Jagged1 resulted in a fourfold (AGM cells) or greater (fetal liver cells) increase in HPP-mix colonies generated in subsequent methylcellulose cultures. Although these studies suggest that Notch ligands may be useful for in vitro expansion of hematopoietic stem/progenitor cells, the complex interactions involving various Notch molecules, ligands, and cytokines may present a considerable challenge.
Delta-Like Molecules
At least five distinct vertebrate orthologues of DrosophilaDelta have been identified, including Delta-like (Dll) 1 and 3 in mice and humans.98,99,Dll1 and 3 have both overlapping and distinct patterns of expression, suggesting cooperative and specific signaling functions in several developmental processes. A complete analysis of Dllexpression in hematopoietic tissues has not yet been reported, butDll1 is expressed in BM stroma67 and spleen,62 suggesting possible roles in hematopoiesis and B-cell regulation.
The delta-like (dlk) molecule expressed by the fetal liver stromal cell line AFT024 is another potential Notch ligand. AFT024 maintains transplantable hematopoietic progenitors in vitro100 and like HS-27a (which expresses Jagged1) supports the formation of cobblestone areas characteristic of proliferation of primitive hematopoietic cells.101 Moore et al101 have provided convincing evidence that dlk contributes to these properties by demonstrating that a soluble dlk protein stimulates cobblestone area formation from fetal liver and adult BM stem cells and that cell lines transfected with dlkincrease the short-term repopulating ability of cultured hematopoietic progenitors. Although these effects are consistent with Notch signaling, the precise relationship of dlk to other Notch ligands and its role in Notch signaling remain to be elucidated; it is noteworthy that dlk lacks the DSL domain characteristic of established Notch ligands, and activation of a Notch receptor by dlk binding has not yet been demonstrated.
INTRACELLULAR NOTCH SIGNAL TRANSDUCTION
Evidence regarding signal transduction after activation of Notch is derived mostly from nonhematopoietic systems. However, the fact that Notch, its ligands, and the intracellular factors that transmit Notch signals are all present in hematopoietic cells strongly implies that these signaling pathways function in hematopoiesis. The following section is provided as a guide to the known pathways with the expectation that this will prove to be the case.
Notch Activation
Signal transduction through the Notch pathway is initiated when the extracellular domain of Notch binds to its ligand on adjacent cells, resulting in activation of the intracellular domain (Fig 2). Several recent studies suggest a model for Notch processing and activation that involves two distinct proteolytic events: the first to generate a functional Notch receptor and the second to activate Notch in response to ligand binding. Blaumueller et al102 have demonstrated that functional Notch receptors are present on the cell surface as heterodimers, generated by proteolytic cleavage of the full-length Notch protein and reassociation of the extracellular and intracellular cleavage products through disulfide bonds. The metalloprotease-disintegrin Kuzbanian plays a role in Notch signaling103,104 and has been implicated in the processing of Drosophila Notch and mammalian Notch2.104 However, Logeat et al105 have found that a furin-like convertase is responsible for the processing of Notch1, suggesting that Kuzbanian is not an invariant part of Notch signaling. Different mechanisms may be involved in processing the different Notch orthologues, or different cell types may use distinct mechanisms, variables that could contribute to specificity of Notch signaling in mammals.
The initial proteolytic processing of Notch generates a functional receptor, but does not result in Notch activation. Studies by Kopan et al106,107 indicate that a second, ligand-dependent, cleavage is required for Notch activation. In these studies, ligand binding to membrane-bound Notch1 induced proteolytic cleavage to release Notch-IC, which could then translocate to the nucleus. Together, these studies provide the basis for an appealing model of Notch processing and activation. However, it remains possible that other proposed mechanisms for Notch signaling will also prove to be important.82 103
Nuclear Functions for Notch
Activation of the Notch receptor results in signal transduction through an intracellular pathway that culminates in transcriptional regulation. The molecular mechanisms involved have been a focus of intense research and remain controversial. Although there is increasing evidence that Notch-IC translocates to the nucleus and directly participates in transcriptional regulation, it is unclear whether nuclear translocation is required for Notch function in all systems. The observations that intracellular forms of Notch localize to the nucleus31,32,34,86,87 and physically interact with nuclear factors39,74,108 provide circumstantial evidence for Notch function in the nucleus. Using a model of mammalian myogenesis, Kopan et al34,107,109 have produced considerable substantiating evidence. Initial studies showed that activated Notch1 (Notch-IC) inhibits myogenesis by suppressing Myo-D–induced transcription of muscle-specific genes and that, whereas Notch-IC localizes to the nucleus, deletion of the nuclear localization sequences results in both loss of nuclear localization and function.34 Subsequent studies suggest the molecular mechanism involves association of Notch-IC with CBF1/RBP-Jκ,109 translocation to the nucleus,107 and transcriptional activation of HES (a negative regulator of Myo-D) through binding of Notch/CBF1 to the HES-1 promoter.109 Studies by Struhl and Adachi110and Lecourtois and Schweisguth111 provide convincing evidence that Drosophila Notch is also cleaved upon ligand binding, resulting in translocation and activity of Notch-IC in the nucleus. In these experiments, transcriptional regulatory domains (either activators or repressors) were inserted into the intracellular domain of full-length Notch receptors. Activation of Notch by ligand binding resulted in transcriptional activation or repression of target reporter genes, indicating the nuclear translocation of Notch-IC.
Despite the evidence for Notch function in the nucleus, physical evidence for nuclear localization of endogenous Notch protein has remained elusive. The recent studies demonstrating nuclear activity of Notch, in the absence of visualization of Notch protein by immunostaining, have led to speculation that only minute amounts of Notch are necessary for nuclear activity. However, studies showing that membrane-bound forms of Notch-IC are active suggest that nuclear localization is not always required for Notch function.38,86 90 Although it is possible that some spontaneous activation occurs with these truncated molecules, it is also possible that alternative pathways for Notch signaling exist, only some of which involve nuclear translocation of Notch itself.
Signal Transduction Through CSL Proteins
The CSL (CBF1/RBP-Jκ, Su(H), Lag-1) family of transcriptional regulators represent a highly conserved component of the Notch signaling pathway.9,10,16,17,112,113These proteins physically interact with Notch-IC and mediate cytoplasmic to nuclear signal transduction upon Notch activation.35,39,109,114 Whereas it has been proposed that CSL proteins sequestered by Notch are released and translocated to the nucleus upon Notch activation,9,112 this model is not entirely consistent with CSL expression patterns.113,115 As discussed above, a model incorporating recent data involves nuclear translocation of Notch-IC and its cooperation with CSL proteins in transcriptional regulation. CSL proteins bind specific DNA sequences to regulate gene expression.40,41,73,80 The primary target genes for Su(H) in the fly are those of the Enhancer of split (E[spl]) complex that encode basic helix-loop-helix (bHLH) transcription factors.116,117,E(spl) genes have vertebrate counterparts, such as mammalian Hairy/Enhancer of split (HES), that are similarly activated by the CSL proteins CBF1/RBP-Jκ.73,109,118 E(spl)/HES proteins inhibit activity of other bHLH proteins, thereby suppressing transcription of lineage-specific genes. In Drosophila neurogenesis, Notch signal transduction through Su(H) and E(spl)inhibits neuronal-specific genes of the achaete-scutecomplex.119,120 This pathway is highly conserved in mammalian neurogenesis, with signaling through Notch andRBP-Jκ resulting in the regulation of HES-5 and the neuronal-specific genes Mash-1 andNeuroD.113
The general role of CSL proteins in Notch signaling has been well established. However, the interactions between Notch and CSL proteins appear to be more complex, and the effects more diverse, than originally described. CSL proteins bind to the Notch RAM domain with high affinity,35,39 but also interact with the cdc10 domain, possibly having distinct effects.35,38,41 CSL binding to specific promoters can lead to either transcriptional activation or suppression,112,116,117 and Notch may act either synergistically or antagonistically to regulate gene expression. In the absence of Notch, CSL proteins often function as transcriptional repressors121-123; the effect of Notch in this case is release of transcriptional suppression.73,75 Notch and CSL proteins may also cooperate in transcriptional regulation by binding as a complex to DNA regulatory sequences109,114,124 or through other synergistic mechanisms.125 The variety of effects resulting from Notch/CSL interactions suggests a very complex regulatory structure that will undoubtedly be the subject of many further studies.
CSL-Independent Signaling
In addition to the Notch → Su(H)/CSL → E(spl)/HES → bHLH pathway, Notch signaling occurs through other pathways that may be equally important. In the fly, only a subset of processes mediated by Notch-Su(H) signaling require E(spl), implying divergent pathways for transcriptional activation and repression after CSL activation.126 Notch signaling also occurs through pathways that do not require CSL.81,127,128 It is particularly interesting that Notch-mediated inhibition of myogenesis in C2C12 myoblasts occurs through a mechanism that does not require either CSL or HES,127 but in 3T3 fibroblasts and 10T1/2 cells occurs through the CSL-HES pathway,34 118 indicating that both CSL-dependent and CSL-independent signaling may occur in the same developmental process.
One important CSL-independent pathway involves the cytoplasmic protein Deltex, which physically interacts with the cdc10 domain of Notch.128-130 Notch signaling through Deltex is conserved in vertebrates, and this pathway is involved in Notch-mediated inhibition of the bHLH protein E47.81 These studies are particularly relevant to hematopoiesis in that they suggest a role for Notch in B-cell development. In addition, they illustrate the capacity of Notch to influence bHLH activity through more than one pathway and provide evidence for Notch inhibition of Ras as a mechanism for transcriptional regulation. Members of the bHLH family of transcriptional regulators are also frequent targets of Notch signaling through CSL proteins and are important factors in the regulation of hematopoiesis.3 131 It is possible that regulation of bHLH molecules such as SCL/tal-1, Id, and E2 will prove to be an important component of Notch signaling in hematopoietic cells.
Notch Interactions With NF-κB
Interactions of Notch-IC with members of the NF-κB/Rel and IκB families of transcriptional regulators may also have particular relevance to hematopoiesis. In cooperation with other transcription factors, NF-κB regulates transcription of numerous genes involved in the differentiation and activation of hematopoietic cells, including cytokines, cytokine receptors, acute-phase reactants, and adhesion molecules.76,78,79 The NF-κB regulatory network is particularly important in the immune response, but also plays a role in proliferation and differentiation of hematopoietic cells, apoptosis, and embryogenesis.77-79 132
NF-κB factors are widely expressed and subject to a number of regulatory mechanisms that permit a rapid response to environmental stimuli. A major component of NF-κB regulation is the association with specific inhibitory molecules, proteins of the IκB family, that influence subcellular localization and DNA binding of NF-κB.76,133,134 Like Notch, the IκB molecules have a conserved cdc10 repeat domain, and it is this domain that physically interacts with NF-κB.135 Interaction of the Notch cdc10 domain with NF-κB may mimic IκB function, permitting Notch to act as an inhibitor of NF-κB. The demonstration by Guan et al74 that Notch-IC physically interacts with the p50 subunit of NF-κB, interferes with its DNA binding capacity, and inhibits transcriptional activation by NF-κB is consistent with such a function. By interacting preferentially with specific NF-κB proteins, Notch may affect the relative levels of different subunits, a variable known to influence NF-κB activity. In addition, Notch may interact with IκB proteins such as Bcl-3,108 potentially either inhibiting or augmenting regulation by IκB.
Recent studies suggest that Notch also modulates NF-κB activity through transcriptional regulation. Oswald et al75 have shown that binding of CBF/RBP-Jκ to the NF-κB2 promoter represses transcription and that Notch releases this repression, indicating that NF-κB2 is a direct target of Notch signaling. A similar mechanism may permit Notch to influence the expression of genes controlled by NF-κB, as suggested by the finding that CBF/RBP-Jκ represses IL-6 transcription by binding to the NF-κB site in the IL-6 promoter.123 The influence of Notch on NF-κB-mediated gene expression, through both protein-protein interactions and transcriptional regulation, could add a new level of complexity and specificity to an already complex regulatory network.
Notch Interactions With Other Nuclear Factors
Notch interactions in the nucleus are not limited to transcription factors, but also include several proteins that regulate chromatin structure. In C elegans, EMB-5 interacts with the cdc10 repeats of Glp-1 and Lin-12 and is required for signal transduction through these Notch receptors.136 EMB-5 is thought to influence gene transcription by facilitating changes in chromatin structure in response to Notch activation. Mammalian homologues of EMB-5 have been identified, but their role in Notch signaling remains to be determined.
In Drosophila, the nuclear proteins Mastermind and Groucho contribute to the regulation of gene expression resulting from Notch activation.137,138 Mastermind associates with specific chromatin regions and can either enhance or suppress transcription, but has little homology to known transcriptional regulators.139An unusual protein with unique properties, mastermind may play a unique role in determining chromatin structure. Groucho is a non-bHLH protein product of the E(spl) complex and has multiple mammalian relatives, the transducin-like Enhancer of split (TLE) proteins.91,140 Groucho/TLE proteins physically interact with the DNA-binding bHLH E(spl)/HES proteins and function as corepressors of transcription.141,142 Recent studies indicate that transcriptional silencing by Groucho/TLE may be due to interactions with Histone H3 and alterations in chromatin structure.143 The variety of Notch interactions with transcription factors and chromatin proteins suggests that Notch may help organize multicomponent transcriptional regulatory complexes.
MODULATORS OF NOTCH FUNCTION
In general, Notch activation inhibits differentiation, leaving cells competent to adopt alternative fates. However, it has become increasingly evident that superimposed on this general function are subtleties in Notch signaling that permit diverse effects, depending on the developmental stage of an individual cell and the microenvironmental context. Some of these effects are imparted by molecules that are not integral components of the Notch pathway, but that modulate Notch signal transduction (Table 1).10 These modulators include molecules that influence Notch-DSL ligand interactions (Fringe), intracellular signal transduction (Dishevelled), and intrinsic Notch signaling (Numb), as well as molecules that modulate Notch function through unknown mechanisms such as SEL-12/Presenilins.144
Fringe
Drosophila Fringe145 and the mammalianLunatic, Manic, and Radical fringegenes146 encode secreted molecules that modulate interactions of Notch with distinct ligands. For example, during dorsal-ventral boundary formation in the fly wing, Fringe specifically inhibits Serrate-Notch signaling and facilitates Delta-Notch signaling in the dorsal compartment.49,147,Lunatic fringeplays an essential role in Notch signaling and boundary formation during mammalian somitogenesis, as shown by the phenotype of knockout mice.148,149 The coincident expression of mammalianFringe, Dll, and Jagged along boundaries of other Notch-dependent processes indicates a generally conserved role for Fringe in Notch signaling and pattern formation.146,150,151The expression of Lunatic fringe by hematopoietic cells in the fetal liver and along the boundary between immature and mature thymocytes in the thymus further suggests that this role is conserved in the hematopoietic system.151
Numb
Progeny of a single cell may adopt distinct cell fates as a consequence of both extrinsic and intrinsic factors. Asymmetric cell division is a fundamental mechanism that contributes to the generation of diverse cell types.48,152 During neurogenesis in flies and mice, the asymmetric distribution of Numb inhibits Notch activity in one daughter cell, thus biasing Notch-mediated signaling.36,48,153-155 In the mouse, some cell divisions are symmetric, giving rise to equivalent progeny; in this case, Numb is equally distributed to daughter cells. Whether the cell division, and the distribution of Numb, is asymmetric or symmetric depends on whether the orientation of the mitotic spindle is horizontal or vertical. Interestingly, Notch is also asymmetrically distributed in horizontal cell divisions during mammalian neurogenesis.156 Together, these observations suggest that asymmetric distribution of Numb and/or Notch in stem cell progeny may result in differential Notch activity that establishes an initial signaling bias between otherwise equivalent progenitors.
INTERSECTIONS BETWEEN NOTCH SIGNALING AND OTHER DEVELOPMENTAL PATHWAYS: IMPLICATIONS FOR HEMATOPOIESIS
Over the past few years the interactions of Notch with other signaling pathways have come to be recognized as an important aspect of developmental regulation.10,44,157 158 These interactions create a regulatory network that influences Notch activity as well as the effects of various inductive signals. Although the precise molecular mechanisms involved have yet to be established, recent studies have contributed important insights and have shown the complex nature of Notch interactions with other pathways. The reciprocal interactions between Notch and Ras and Notch and Wingless (Wg/Wnt) signaling reflect this complexity and have potentially important implications for Notch interactions with other regulatory pathways in hematopoiesis.
Integration of Notch and Receptor Tyrosine Kinase-Ras Signaling Pathways
Receptor tyrosine kinases (RTK) comprise a large family of cell surface proteins that function extensively in intercellular signaling and play an important role in hematopoiesis.159,160 Although there is considerable temporal, spatial, and functional overlap between RTK/Ras and Notch signaling in a number of developmental systems, until recently these pathways were thought to function independently. However, it is now evident that cross-talk between the two pathways occurs at a number of levels, resulting in significant interdependence and reciprocal regulation.157,161,162 In invertebrate systems, such as the Drosophila eye, appropriate specification of sequential cell fates and establishment of precise pattern formation require both inductive signals provided by RTKs and regulatory signals from Notch.10,157 162-164
Although RTK/Ras signals generally induce differentiation and Notch serves to restrict the number of responding cells, interactions between the two pathways can result in reinforcement or antagonism of either pathway by the other. The highly conserved RTK/Ras pathway involves a cascade of mitogen-activated protein kinases (MAPK) that culminates in phosphorylation of transcriptional regulators.165,166Phosphorylation may increase or decrease activity of individual transcription factors and may result in activation or suppression of target genes. The effects of RTK/Ras activity on Notch signal transduction may be due in part to phosphorylation of Notch or effector molecules such as CSL, bHLH, and Groucho/TLE proteins.10Another mechanism that contributes to mutual regulation includes physical interactions between components of the Notch and RTK/Ras pathways, which may alter signal transduction through either pathway.162,167 168
Most of the evidence for integration of RTK/Ras and Notch signaling comes from invertebrate systems. However, the conservation of both pathways and the observation that Notch inhibits E47 (which is crucial for B-cell development) by inhibiting Ras signaling81 imply that similar interactions occur in the hematopoietic system. Receptor tyrosine kinases such as c-Kit (SCF receptor), c-Fms (CSF-1 receptor), and Flk/Flt play major roles in hematopoiesis and the integration of these signaling pathways with Notch would have important implications for hematopoietic regulation. Notch may also interact with cytokine pathways that use receptors other than RTKs, some of which also signal through Ras.160,169 In addition, it is possible that Notch interacts with the many pathways that use members of the cytokine receptor family and signal through Janus kinases (Jaks) and Stat factors, such as G-CSF and GM-CSF.42,160 169 Interactions of Notch with various cytokine pathways could account for some of the diverse responses of hematopoietic cells to cytokine stimulation.
Integration of Notch and Wnt Signaling Pathways
Members of the Wnt family, including Drosophila Wingless (Wg), C elegans lin-44, and numerous mammalian Wntgenes, encode secreted glycoproteins important for establishment of cell polarity, axis formation, and specification of cell fates during development.170,171 In mammals, Wnt signaling is emerging as an important component of a variety of developmental processes, many of which also involve Notch. Recent studies indicate that Wnt signaling plays a role in hematopoiesis,172 173 making it tempting to speculate that the two pathways interact in the hematopoietic system.
The frequent coincident signaling through Notch and Wnt during development suggests that these two pathways function cooperatively in developmental regulation. It is now clear that the Notch and Wnt signaling pathways are not simply parallel pathways that regulate the same downstream target genes, but that reciprocal modulation by the two pathways results in synergistic or antagonistic effects depending on the developmental context.50,52,53,174,175 The result is a complex regulatory feedback loop that maintains a strict pattern of gene expression, particularly notable along developmental boundaries.50,52,54 176
In addition to transcriptional feedback regulation, the Notch and Wnt pathways directly interact through the cytoplasmic protein Dishevelled (Dsh). Dsh is an integral component of Wnt signal transduction177,178 and also physically interacts with Notch-IC.37 The association of Dsh with Notch interferes with Wg signal transduction and at the same time inhibits Notch activity, resulting in mutual inhibition of the two pathways. Thus, Dsh provides a direct molecular link between Notch and Wnt signaling in which cell fate specification depends on the level of Notchexpression and strength of the Wnt signal.
Notch, Wingless, and control of cell proliferation.
Interactions between Notch and Wg in the fly wing have elucidated another role for Notch in developmental regulation: the control of cell proliferation. In the wing imaginal disc, Notch induces mitotic activity and Wg acts synergistically with Notch to promote cell proliferation.179,180 In contrast, at the wing margin, Notch and Wg induce cell-cycle arrest to establish a zone of nonproliferating cells.181 These findings have important implications for the role of Notch in developmental processes, extending its role to include both differentiation and proliferation and again showing how the effects of Notch depend on the precise developmental context.
Wnt signaling in hematopoiesis.
A role for Wnt signaling in hematopoiesis has recently been proposed, raising the possibility that Notch and Wnt also cooperate in hematopoietic regulation. Austin et al172 have demonstrated that Wnt-5a and Wnt-10b, as well as severalfrizzled genes (which encode Wnt receptors171), are expressed in the murine embryonic yolk sac, fetal liver, and fetal liver AA4+ hematopoietic progenitors. Wnt-5awas specifically expressed in fetal liver stromal cells, andWnt-10b was specifically expressed in fetal liver AA4+Sca+kit+ cells, a population enriched for hematopoietic stem/progenitor cells. Furthermore, culture of fetal liver AA4+Sca+kit+ cells in the presence of Wnt proteins promoted the expansion of multilineage progenitors, and AA4+Sca+kit+ cells transduced with Wnt-5a showed increased proliferation in vitro. Although Wnt expression was not detected in linloSca+ BM progenitors, these cells expressedfrizzled genes and showed increased survival and proliferation in cultures containing Wnt proteins.
Van Den Berg et al173 have reported similar findings in human hematopoietic progenitors: expression of three Wnt genes and several frizzled genes by fetal BM stromal cells, expression of Wnt-5a by CD34+lin−progenitors, and expansion of multilineage progenitors (CFU-MIX) from CD34+lin− cells cultured in the presence of Wnt proteins. Together, these studies provide substantial evidence for Wnt function in hematopoiesis and suggest that Wnt-5a andWnt-10b may be particularly important. Whether differences inWnt expression by progenitors from mouse fetal liver, mouse BM, and human BM reflect differences in the progenitor populations, the distinct microenvironments, or differences between mouse and human progenitors is an interesting question. Further studies to define the role of Wnt proteins and to explore potential interactions between Notch and Wnt signaling in the regulation of hematopoiesis should be of considerable interest.
Integration of Notch With Other Signaling Pathways: Coordination of Inductive Signals
In many developmental systems, the integration of inductive signals imparted by members of the EGF receptor (RTK), Wnt, transforming growth factor-β (TGF-β), and Hedgehog families is crucial for pattern formation and tissue organization.158,162,176,182,183 These signals may also cooperate to form microenvironmental niches that permit the maintenance and proliferation of stem cells.184The influence of Notch on signaling through these pathways, together with its role as a mediator of progenitor self-renewal and cell fate determination in the same processes, suggests that coordination of various inductive signals by Notch may be a common theme in developmental regulation. The integration of Fringe with Wnt, Hedgehog, and TGF-β/BMP signaling in wing and limb development further supports a central role for Notch in these processes.54,185 TGF-β and other members of this family, including the Bone Morphogenic Proteins BMP2 and 4, are crucial for blood formation during embryogenesis186-189; thus, it is intriguing to speculate that Notch may function at this earliest stage of hematopoiesis to regulate cell fate decisions induced by TGF-β, BMP, and Wnt signals.
A MODEL FOR NOTCH FUNCTION IN HEMATOPOIESIS
Considering the specific evidence for Notch function in hematopoiesis, as well as the interactions of Notch with factors that are of known importance in hematopoiesis, it seems very likely that Notch plays a key role in many aspects of hematopoiesis. The hematopoietic microenvironment represents a complex network of inductive signals, regulatory molecules, and cell-cell interactions that permit the simultaneous determination of various hematopoietic cell fates. The interaction of Notch with multiple components of this regulatory network may allow it to function as a master regulator, integrating various signaling pathways to limit the number of cells that respond to diverse signals. The experimental evidence confirming this role is likely to emerge over time; a model incorporating the current data might facilitate this work.
The model in Fig 4 depicts selected interactions showing how Notch might function in hematopoiesis to mediate specification of different cell fates and to maintain multipotent progenitors at various stages of maturation. Figure 4A shows cell-cell interactions and the influence of other signals on Notch activity in different hematopoietic microenvironments. Figure 4B presents a mechanism for the specificity of different Notch orthologues through distinct intracellular interactions, using the cytokine-specific effects of Notch1 and 2 on myeloid differentiation as an example. This model is speculative, but we have attempted to include features of Notch signaling that have been demonstrated in studies to date. Salient features of the model include the following: (1) Multiple different Notch molecules are expressed by hematopoietic progenitors (depicted as Notch1 and 2 for simplicity, but also likely to include Notch3 and 4), and multiple DSL ligands are expressed by both stromal and hematopoietic cells. (2) Activation of a specific Notch molecule depends on the relative levels of Notch and DSL ligand expressed on adjacent cells and on specific inductive (cytokine) signals. (3) The specific effects of Notch activity in a given cell depend on environmental signals, the maturational state and lineage of neighboring cells, as well as its own precise stage of maturation. (4) Activation of Notch suppresses transcription of genes required for the next step in differentiation of a particular cell. (5) The ability of Notch to influence expression of diverse genes reflects its capacity to act in concert with a wide variety of signaling pathways and transcriptional regulators.
The central role of Notch requires that the model incorporate many aspects of hematopoiesis. We encourage the reader to focus first on his/her area of interest to establish a base from which to view other aspects. Figure 4A depicts effects of Notch interactions in three different hematopoietic microenvironments: progenitor, myeloid, and lymphoid compartments. Each compartment in the figure focuses on particular features of Notch signaling that are also pertinent (but not depicted in detail) in the other compartments. The progenitor compartment (top) illustrates the effects of cell-cell interactions on the differentiation and self-renewal of progenitors. Important factors include the relative expression levels of different Notch molecules and ligands on neighboring cells and the influence of various cytokines. The myeloid compartment (lower left) uses the cytokine-specific effects of Notch1 and 2 as an example of Notch integration with cytokine pathways. The potential for diverse effects becomes obvious when additional cytokines and Notch molecules are also considered. The lymphoid (T-cell) compartment (lower right) shows the role of Notch in sequential cell-fate decisions within a single lineage, the importance of cell-cell interactions and relative levels of Notch expression, and the requirement for additional signaling molecules (in this case, the TCR and MHC molecules) in defining Notch function. The lymphoid portion of this model is derived largely from studies by Robey et al.47 72
The unique role of Notch as a general mediator of cell fate determination stems from its function as both a cell surface receptor and a transcriptional regulator. The ability of Notch to regulate specific genes in response to different signals may reflect its capacity to engage in a variety of molecular interactions. The different mammalian Notch orthologues may also engage in specific interactions that permit distinct functions. Figure 4B shows how Notch1 and 2 might function as specific transcriptional regulators in the context of different cytokine pathways, using myeloid differentiation as an example. In this case, the NCR regions of Notch1 and 2 interact specifically with molecules induced by G-CSF or GM-CSF, respectively. The result is transcriptional suppression of G-CSF–specific genes only in the context of both G-CSF stimulation and Notch1 activation and of GM-CSF–specific genes only in the context of both GM-CSF stimulation and Notch2 activation.
CONCLUSIONS
If this review makes anything clear, it should be the awesome array of interactions between Notch and other molecules that have important roles in hematopoiesis. Although the evidence allows us to speculate broadly on the role of Notch in hematopoiesis, specific information is lacking for all but a few aspects of the problem. This review raises many questions; we hope it will inspire research leading to some answers. Which of the Notch molecules, ligands, and modulators function in different hematopoietic cells at various stages of hematopoiesis? How does the Notch pathway interact with cytokine pathways, what is the molecular basis of those interactions, and what specific interactions and effects can be attributed to the different Notch molecules? How does Notch interact with other developmental pathways, especially during embryonic hematopoiesis? What is the effect of Notch on transcriptional regulation, how does Notch interact with specific transcription factors and chromatin proteins, and what genes are regulated through Notch signaling? What are the clinical implications for Notch in hematopoiesis? What role does Notch play in hematopoietic malignancies, proliferative disorders, and cytopenias? Can Notch signaling be exploited for stem cell expansion and gene therapy? Deciphering the complex interactions involving multiple Notch molecules in hematopoiesis presents a considerable challenge and should provide fertile ground for investigation for some time to come.
ACKNOWLEDGMENT
The authors have attempted to cite the most pertinent references. However, due to space constraints and the expanse of current literature, we could not include every appropriate reference. We apologize to any investigators who believe their work was not adequately credited and refer readers to the cited reviews for primary references. We express our appreciation to David Martin and Matt Lorincz for helpful comments on the manuscript and to the James S. McDonnell Foundation for laboratory support (L.A.M.).
REFERENCES
Author notes
Address reprint requests to Laurie A. Milner, MD, The Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, MS C3-168, PO Box 19024, Seattle, WA 98109-1024; e-mail: lmilner@fhcrc.org.

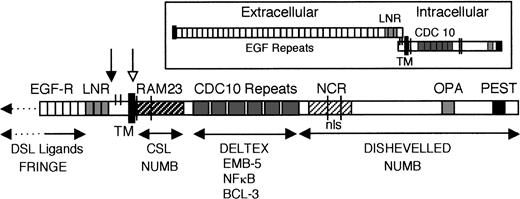



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal