Abstract
The safety and optimal dose and schedule of stem cell factor (SCF ) administered in combination with filgrastim for the mobilization of peripheral blood progenitor cells (PBPCs) was determined in 215 patients with high-risk breast cancer. Patients received either filgrastim alone (10 μg/kg/d for 7 days) or the combination of 10 μg/kg/d filgrastim and 5 to 30 μg/kg/d SCF for either 7, 10, or 13 days. SCF patients were premedicated with antiallergy prophylaxis. Leukapheresis was performed on the final 3 days of cytokine therapy and, after high-dose chemotherapy and infusion of PBPCs, patients received 10 μg/kg/d filgrastim until absolute neutrophil count recovery. The median number of CD34+ cells collected was greater for patients receiving the combination of filgrastim and SCF, at doses greater than 10 μg/kg/d, than for those receiving filgrastim alone (7.7 v 3.2 × 106/kg, P < .05). There were significantly (P < .05) more CD34+ cells harvested for the 20 μg/kg/d SCF (median, 7.9 × 106/kg) and 25 μg/kg/d SCF (median, 13.6 × 106/kg) 7-day combination groups than for the filgrastim alone patients (median, 3.2 × 106/kg). The duration of administration of SCF and filgrastim (7, 10, or 13 days) did not significantly affect CD34+ cell yield. Treatment groups mobilized with filgrastim alone or with the cytokine combination had similar hematopoietic engraftment and overall survival after PBPC infusion. In conclusion, the results of this study indicate that SCF therapy enhances CD34+ cell yield and is associated with manageable levels of toxicity when combined with filgrastim for PBPC mobilization. The combination of 20 μg/kg/d SCF and 10 μg/kg/d filgrastim with daily apheresis beginning on day 5 was selected as the optimal dose and schedule for the mobilization of PBPCs.
THE ADMINISTRATION OF high-dose chemotherapy facilitated by autologous progenitor cell support is being more frequently applied to the treatment of cancer. In most centers, mobilized autologous peripheral blood progenitor cells (PBPCs) have replaced autologous marrow as the preferred source of progenitor cell support because of the ease with which PBPCs can be harvested and the more rapid platelet and neutrophil engraftment that has been associated with their use.1-3 Protocols used to mobilize PBPCs vary from center to center and include the use of myeloid growth factors alone or administered during recovery from cytotoxic chemotherapy. Much interest has surrounded the development of protocols for mobilizing increased numbers of PBPCs.
Stem cell factor (SCF ) is a ligand for the receptor encoded by the c-kit protooncogene.4 In humans, SCF exists in two forms: a soluble circulating glycoprotein and a membrane bound molecule that is expressed on stromal cells in the bone marrow microenvironment.5,6c-kit is expressed on a variety of cells, including hematopoietic cells, melanocytes, and mast cells. SCF, in combination with other cytokines, has been shown to induce proliferation, prolong survival, and increase receptiveness to lineage commitment of early hematopoietic progenitors.7,8 Membrane-bound SCF may also facilitate homing of hematopoietic progenitor cells to the marrow.9-11 The addition of SCF synergistically increases the effects of several other hematopoietic cytokines in a variety of in vitro and in vivo models.8 12-15
In animal models, treatment with SCF is associated with the mobilization of PBPCs.16-18 In murine, canine, and primate models, SCF also synergistically increases the number of PBPCs appearing in the peripheral blood during therapy with granulocyte colony-stimulating factor (G-CSF ), which is commonly used in clinical PBPC mobilization and harvesting protocols.19-22 This synergistic increase in PBPC mobilization was seen with concurrent administration of cytokines, but not with sequential administration.20 In addition, mobilization of megakaryocytic progenitors was maximal with 10 to 13 days administration of SCF and G-CSF. In these models, transplantation of PBPCs mobilized with the combination of G-CSF and SCF resulted in sustained long-term engraftment.23 24 These observations suggest that SCF has the potential to increase the number of PBPCs mobilized and harvested during G-CSF therapy, thereby reducing the number of collections required or improving clinical engraftment outcomes associated with high-dose chemotherapy and PBPC rescue.
In phase 1 clinical trials, therapy with recombinant human SCF in doses of 10, 25, and 50 μg/kg/d administered subcutaneously was associated with increases in peripheral blood white blood cell counts (WBC) and bone marrow cellularity.25-27 During SCF therapy, increases in the number of circulating PBPCs were also observed.28 In these phase 1 studies, therapy with SCF was associated with local cutaneous effects at the injection sites, including erythema, swelling, and hyperpigmentation, with the latter apparently due to an effect on melanocytes.29 At SCF doses of 25 and 50 μg/kg/d, some systemic toxicities were observed, including respiratory and cutaneous symptoms. These were attributed to the effects of SCF on tissue mast cells.30
We report here a large phase 2 trial evaluating SCF, administered alone or in combination with filgrastim (G-CSF ), for the mobilization of PBPCs in patients with breast cancer. In this study, patients receiving SCF were premedicated to prevent systemic toxicities mediated by mast cell degranulation. The objectives of the study were to determine the safety and efficacy of SCF administered in combination with filgrastim and to determine the optimal dose and schedule of this combination for the mobilization of PBPCs.
MATERIALS AND METHODS
Patients
Patients with histologically documented infiltrating breast cancer, aged 18 to 65 years, with either high-risk stage II (greater than 10 involved axillary nodes), stage III, or chemotherapy-responsive metastatic cancer were eligible. All patients received at least one prior cycle of cytotoxic chemotherapy, with a maximum of 6 cycles permitted for adjuvant patients and 12 cycles for those with metastatic disease. Previous chemotherapy was to be completed at least 3 weeks before study entry. Patients were required to have an absolute neutrophil count (ANC) of 2 × 109/L or greater, a platelet count of 100 × 109/L or greater, a hemoglobin level of 10 g/dL or greater, serum creatinine and bilirubin levels of less than 2.0 mg/dL, a Karnofsky performance status of 80% or greater, adequate cardiac and pulmonary function, and no known allergy to Escherichia coli-derived products.
Patients were excluded if breast cancer represented more than 10% of the overall cellularity of bilateral bone marrow biopsy specimens, as assessed by standard histologic techniques, or if they had received prior high-dose chemotherapy with autologous progenitor cell support, documented brain metastases, another malignancy within the preceding 5 years, a history of asthma or other significant IgE-mediated hypersensitivities, or if they required concurrent therapy with β adrenergic blocking agents or monoamine oxidase inhibitors. The study protocol was approved by each participating site's Institutional Review Board and written informed consent was obtained.
Hematopoietic Growth Factors
Both filgrastim and SCF were supplied by Amgen Inc (Thousand Oaks, CA). SCF was expressed in E coli as a 166 amino acid nonglycosylated protein and included methionine at the N-terminus (r-metHuSCF ); this was provided as either an aqueous solution or a lyophilized powder. Both the SCF and filgrastim were kept refrigerated at 2°C to 8°C until the time of injection. Lyophilized SCF was reconstituted with sterile water for injection before subcutaneous administration.
Patient Treatment
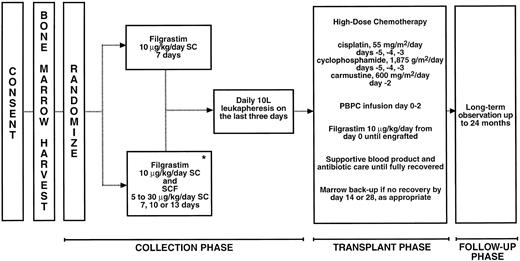
An overview of the treatment of patients for this study is shown in Fig 1. Enrollment was offered to all eligible patients presenting at the three participating institutions. Consenting patients underwent standard bone marrow harvesting with the goal of obtaining 1.5 × 108 mononuclear cells (MNCs)/kg body weight. The pooled marrow aspirate was processed and cryopreserved. Treatment assignment was randomized to either filgrastim alone (control groups) at a dose of 10 μg/kg/d, administered by daily subcutaneous injection for 7 days, or the combination of 10 μg/kg/d filgrastim and SCF administered subcutaneously at doses of 5, 10, 15, 20, 25, or 30 μg/kg/d (experimental groups). The filgrastim and SCF combination was administered for either 7, 10, or 13 days. Different injection sites were used for each cytokine. The dose and schedule of filgrastim selected for the filgrastim alone groups was based on the most common usage of this cytokine. The combination of SCF and filgrastim was initially studied for longer durations, because preclinical studies in baboons had shown that the mobilization of megakaryocytic progenitors was maximal with 10 to 13 days administration of the cytokine combination and that this was associated with improved platelet engraftment.20 Subsequently, we also evaluated 7-day administration. In addition, one group of patients received SCF alone at a dose of 5 μg/kg/d for 13 days. The details of the cytokine dosing and leukapheresis procedures for each treatment group are shown in Table 1. Randomization was performed to maintain enrollment to the control groups throughout the study. The experimental groups were filled sequentially.
Study procedures. *One treatment group (n = 5) received SCF alone at 5 μg/kg/d.
Study Treatment Groups
| Treatment Group . | Dose of SCF (μg/kg/d) . | Dose of Filgrastim (μg/kg/day) . | Duration of Dosing (μg/kg/d) . | Leukapheresis . | No. of Leukapheresis Products Infused* . | No. of Patients . |
|---|---|---|---|---|---|---|
| . | . | . | . | (day of collection phase) . | . | . |
| A | 0 | 10 | 7 | 5-7 | 3 | 35 |
| B | 0 | 10 | 7 | 5-7 | 1 | 7 |
| C | 10 | 10 | 7 | 5-7 | 3 | 25 |
| D | 15 | 10 | 7 | 5-7 | 3 | 15 |
| E | 20 | 10 | 7 | 5-7 | 3 | 27 |
| F | 20 | 10 | 7 | 5-7 | 1 | 8 |
| G | 25 | 10 | 7 | 5-7 | 1 | 9 |
| H | 30 | 10 | 7 | 5-7 | 1 | 3 |
| I | 20 | 10 | 10 | 8-10 | 3 | 15 |
| J | 5 | 10 | 13 | 11-13 | 3 | 6 |
| K | 10 | 10 | 13 | 11-13 | 3 | 11 |
| L | 15 | 10 | 13 | 11-13 | 3 | 12 |
| M | 20 | 10 | 13 | 11-13 | 3 | 11 |
| N | 5 | 0 | 13 | 11-13 | 3 | 5 |
| 189 (Total) |
| Treatment Group . | Dose of SCF (μg/kg/d) . | Dose of Filgrastim (μg/kg/day) . | Duration of Dosing (μg/kg/d) . | Leukapheresis . | No. of Leukapheresis Products Infused* . | No. of Patients . |
|---|---|---|---|---|---|---|
| . | . | . | . | (day of collection phase) . | . | . |
| A | 0 | 10 | 7 | 5-7 | 3 | 35 |
| B | 0 | 10 | 7 | 5-7 | 1 | 7 |
| C | 10 | 10 | 7 | 5-7 | 3 | 25 |
| D | 15 | 10 | 7 | 5-7 | 3 | 15 |
| E | 20 | 10 | 7 | 5-7 | 3 | 27 |
| F | 20 | 10 | 7 | 5-7 | 1 | 8 |
| G | 25 | 10 | 7 | 5-7 | 1 | 9 |
| H | 30 | 10 | 7 | 5-7 | 1 | 3 |
| I | 20 | 10 | 10 | 8-10 | 3 | 15 |
| J | 5 | 10 | 13 | 11-13 | 3 | 6 |
| K | 10 | 10 | 13 | 11-13 | 3 | 11 |
| L | 15 | 10 | 13 | 11-13 | 3 | 12 |
| M | 20 | 10 | 13 | 11-13 | 3 | 11 |
| N | 5 | 0 | 13 | 11-13 | 3 | 5 |
| 189 (Total) |
An additional 26 patients who were mobilized to a target CD34+ cell yield received cytokine for 5 to 9 days; 13 received 15 μg/kg/d SCF and 10 μg/kg/d filgrastim and 13 received 10 μg/kg/d filgrastim alone. In total, 189 patients were evalueated for efficacy and 215 for safety.
Patients receiving only 1 leukapheresis product back were infused the first product collected.
The first dose of SCF was administered on an inpatient basis, with overnight observation after SCF therapy. Subsequent doses were administered on an outpatient basis with at least 4 hours of observation. All patients treated with SCF received prophylaxis against mast cell-mediated adverse effects. Prophylaxis consisted of 50 mg diphenhydramine orally every 6 hours, 150 mg ranitidine orally every 12 hours, two puffs of albuterol metered dose inhaler, and 120 mg pseudoephedrine by sustained release. Administration of diphenhydramine and ranitidine began 12 to 24 hours before the first dose of SCF and was timed so that a dose of all four medications was delivered approximately 1 hour before each injection of SCF. Diphenhydramine and ranitidine therapy was continued until 48 hours after the last SCF injection.
Leukapheresis
Each leukapheresis processed approximately 10 L of blood using a Cobe Spectra (COBE Laboratories, Lakewood, CO) apparatus. Patients were scheduled to undergo leukapheresis on the final 3 days of cytokine therapy, irrespective of the duration of treatment. The required minimum cumulative yield of MNCs was 4.0 × 108 cells/kg actual body weight, which represented an acceptable standard in May 1993 when the study was initiated. If this minimum yield was not achieved, the patient was classified as a mobilization failure and was managed with either further leukapheresis or with supplementation of the PBPCs with the previously harvested bone marrow for progenitor cell support. The leukapheresis product collected on each day was processed and cryopreserved.
Within 1 week of completing the leukapheresis procedure, patients were admitted to hospital and treated with high-dose chemotherapy consisting of 1,875 mg/m2/d cyclophosphamide administered intravenously over 1 hour for 3 days (days −5, −4, and −3), 55 mg/m2/d cisplatin administered by continuous intravenous infusion for 3 days (days −5, −4, and −3), and 600 mg/m2/d carmustine administered intravenously on day −2. After a 48-hour rest period, patients received an intravenous infusion of their PBPCs on days 0 to 2. However, the second and third harvests were not infused to patients in groups B, F, G, and H to test the feasibility of hematopoietic support with only the first day's leukapheresis product (Fig 1 and Table 1).
Progenitor Cell Assays
Progenitor cell assays were performed on samples obtained from each leukapheresis product and from peripheral blood samples taken at baseline (before cytokine administration) and on day 4 through subsequent days of cytokine administration.
All progenitor cell studies were performed at a central laboratory using standardized methodologies.31 Samples were analyzed for expression of CD34 by flow analysis on a FACScan (Becton Dickinson, Mountain View, CA) and labeling with phycoerythrin (PE)-conjugated anti-CD34 (HPCA-2; Becton Dickinson). Cells were labeled with HPCA-2 and analyzed for FL2 and low side scatter, and background labeling was subtracted (PE-conjugated mouse IgG used as a nonspecific control). All samples were gated on a lymphocyte window and 50,000 events were collected in this region, representing a total of 100,000 to 1,000,000 cells depending on the percentage of MNCs in the sample.
A double-layer agarose progenitor cell assay system was used to quantitate granulocyte-macrophage colony-forming cells (GM-CFCs) and burst-forming units-erythroid (BFU-E), as previously described.32 Cultures were established separately to obtain optimal GM-CFC– and BFU-E–derived colony formation. SCF, interleukin-3 (IL-3), IL-6, G-CSF, and granulocyte-macrophage colony-stimulating factor (GM-CSF; all provided by Amgen Inc) were used at optimal doses (100 ng/culture) for GM-CFC–derived colony formation. SCF, IL-3, and IL-6 (100 ng/culture) and erythropoietin (5 U/culture; Amgen Inc) were used at optimal doses for BFU-E–derived colony formation. MNCs were plated at 100,000 and 10,000 cells per 35-mm culture dish. Cultures were incubated for 14 days in a 100% humidified incubator with 5% CO2 in air. Each culture dish was examined on a grid placed on the stage of a dissecting microscope and completely scanned at 20×. Day-14 GM-CFC–derived colonies were defined as any colony containing greater than 50 translucent cells; day-14 BFU-E–derived colonies were defined as any colony containing greater than 50 hemoglobinized cells.
Transplant and Follow-Up
Beginning on day 0 of transplantation, all patients were treated with 10 μg/kg/d filgrastim by either subcutaneous injection or intravenous infusion. Filgrastim therapy was continued until the ANC was ≥5 × 109/L for 3 consecutive days or ≥10 × 109/L on one determination. Antibiotics, blood products, and intravenous fluids were administered as clinically indicated. Complete blood counts were obtained daily until ANC was ≥5 × 109/L and the platelet level was ≥20 × 109/L and were obtained three times per week thereafter until the platelet count was ≥50 × 109/L on two determinations separated by a minimum of 48 hours. Patients were assessed daily during hospitalization and weekly after discharge until platelet recovery had occurred. For treatment groups in which all three leukapheresis products were infused, patients were defined as having failed to engraft if their day 28 ANC was less than 0.2 × 109/L and a bone marrow biopsy showed less than 5% cellularity. These patients then received their previously harvested bone marrow. As a means of ensuring patient safety, for treatment groups in which patients received only 1 day of leukapheresis product, graft failure was defined as an ANC of less than 0.5 × 109/L on day 14 or later. Patients meeting these criteria received the remainder of their previously harvested PBPCs and the previously harvested bone marrow. All patients were discharged from the hospital when the ANC was ≥0.5 × 109/L and intravenous antibiotic therapy was no longer necessary.
Patients were assessed for maintenance of engraftment, defined as ANC ≥0.5 × 109/L and a platelet count of ≥20 × 109/L, and survival at 2, 3, 6, 12, and 24 months after PBPC infusion.
Data Analysis
This study was designed to determine the optimal dose and schedule for the combination of SCF and filgrastim for mobilization and to study the feasibility of using this regimen to harvest PBPCs. No inferential analyses were planned a priori. However, analysis of variance (ANOVA) was used to determine if there were any statistically significant differences between the cytokine combination groups and the filgrastim alone treatment groups with respect to total leukapheresis CD34+ and other progenitor cell yields. A two-sided Dunnett's test was performed on the log-transformed CD34+ and other progenitor cell yields to locate specific differences. The days to platelet recovery for 212 patients were modeled using a Cox proportional hazards analysis with the number of CD34+ cells collected and subsequently infused as the predictor. Results were used to generate predicted probability of engraftment curves. The curve estimates for the following four CD34+ cell levels were calculated: 1.0, 2.0, 5.0, and 10.0 × 106 CD34+ cells/kg. These calculations were performed using the Proportional Hazards Regression procedure in SAS (SAS Institute Inc, Cary, NC). Kaplan-Meier product limit estimates were used to describe overall patient survival.
RESULTS
A total of 215 patients were entered in this study between May 1993 and May 1995. Baseline demographic and disease characteristics for these patients are shown in Table 2. The distribution among the treatment groups of 189 of these patients is shown in Table 1. The remaining 26 patients were enrolled in treatment groups designed to assess the logistics of performing daily leukaphereses to a target CD34+ cell yield; 13 patients received filgrastim 10 μg/kg/d alone and 13 patients received the combination of 15 μg/kg/d SCF and 10 μg/kg/d filgrastim. The leukapheresis and transplant data for these 26 patients have not been included in the efficacy analyses; however, these patients were included in the Cox proportional hazards and in the safety analyses.
Patient Demographic and Baseline Disease Characteristics
| . | Filgrastim . | SCF and Filgrastim . | Total . |
|---|---|---|---|
| N | |||
| 55 | 160 | 215 | |
| Sex (%) | |||
| Male | |||
| 1 (2) | 1 (1) | 2 (1) | |
| Female | |||
| 4 (98) | 159 (99) | 213 (99) | |
| Race (%) | |||
| White | |||
| 48 (87) | 132 (83) | 180 (84) | |
| Black | |||
| 0 (0) | 7 (4) | 7 (3) | |
| Hispanic | |||
| 5 (9) | 13 (8) | 18 (8) | |
| Asian | |||
| 1 (2) | 7 (4) | 8 (4) | |
| Other | |||
| 1 (2) | 1 (1) | 2 (1) | |
| Age (yr) | |||
| Median | |||
| 45 | 44 | 45 | |
| Minimum/maximum | |||
| 29/64 | 25/64 | 25/64 | |
| Weight (kg) | |||
| Median | |||
| 63.6 | 66.5 | 66 | |
| Minimum/maximum | |||
| 47.8/108.2 | 40.8/147.7 | 40.8/147.7 | |
| Stage (%) | |||
| II | |||
| 10 (18) | 32 (20) | 42 (20 | |
| IIIA | |||
| 15 (27) | 39 (24) | 54 (25) | |
| IIIB | |||
| 11 (20) | 22 (14) | 33 (15) | |
| IV | |||
| 19 (35) | 67 (42) | 86 (40) | |
| Previous radiotherapy (%) | |||
| No | |||
| 40 (73) | 124 (79) | 164 (77) | |
| Yes | |||
| 15 (27) | 33 (21) | 48 (23) |
| . | Filgrastim . | SCF and Filgrastim . | Total . |
|---|---|---|---|
| N | |||
| 55 | 160 | 215 | |
| Sex (%) | |||
| Male | |||
| 1 (2) | 1 (1) | 2 (1) | |
| Female | |||
| 4 (98) | 159 (99) | 213 (99) | |
| Race (%) | |||
| White | |||
| 48 (87) | 132 (83) | 180 (84) | |
| Black | |||
| 0 (0) | 7 (4) | 7 (3) | |
| Hispanic | |||
| 5 (9) | 13 (8) | 18 (8) | |
| Asian | |||
| 1 (2) | 7 (4) | 8 (4) | |
| Other | |||
| 1 (2) | 1 (1) | 2 (1) | |
| Age (yr) | |||
| Median | |||
| 45 | 44 | 45 | |
| Minimum/maximum | |||
| 29/64 | 25/64 | 25/64 | |
| Weight (kg) | |||
| Median | |||
| 63.6 | 66.5 | 66 | |
| Minimum/maximum | |||
| 47.8/108.2 | 40.8/147.7 | 40.8/147.7 | |
| Stage (%) | |||
| II | |||
| 10 (18) | 32 (20) | 42 (20 | |
| IIIA | |||
| 15 (27) | 39 (24) | 54 (25) | |
| IIIB | |||
| 11 (20) | 22 (14) | 33 (15) | |
| IV | |||
| 19 (35) | 67 (42) | 86 (40) | |
| Previous radiotherapy (%) | |||
| No | |||
| 40 (73) | 124 (79) | 164 (77) | |
| Yes | |||
| 15 (27) | 33 (21) | 48 (23) |
The 5 patients who received SCF alone are included with the SCF and filgrastim patients. Numbers in parentheses indicate percentages.
Collection Phase
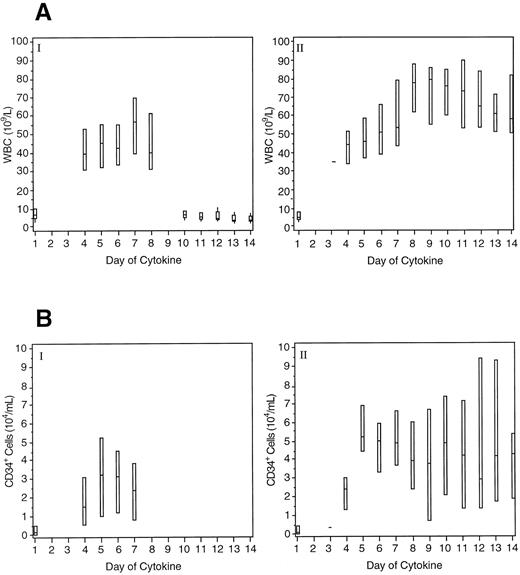
Peripheral blood hematology. As expected, WBC values increased in all treatment groups during the collection phase. Twenty-four patients (11%) had a WBC greater than 100 × 109/L during the collection phase, with a maximum value of 143 × 109/L being recorded. All of these patients received the combination of SCF and filgrastim (15 for 13 days and 9 for 7 days). For 14 of the patients, the leukocytosis was managed by reducing the filgrastim dose to 1 μg/kg/d. For the other 10 patients, this WBC level was reached on the final day or after the cessation of cytokine administration. No patient experienced any clinical sequelae in association with leukocytosis. Figure 2A shows the daily median WBC counts in patients receiving filgrastim alone for 7 days and those receiving 20 μg/kg/d SCF in combination with filgrastim for up to 13 days. The duration of leukocytosis was related to the duration of cytokine administration.
Boxplots of peripheral WBC counts (A) and CD34+ cell levels (B) for patients in the collection phase. Panel I shows the data for patients receiving filgrastim alone 10 μg/kg/d for 7 days (n = 41) and panel II for patients receiving 20 μg/kg/d SCF and 10 μg/kg/d filgrastim for up to 13 days (n = 59). The boxes indicate the range of values and the lines indicate the median value.
Boxplots of peripheral WBC counts (A) and CD34+ cell levels (B) for patients in the collection phase. Panel I shows the data for patients receiving filgrastim alone 10 μg/kg/d for 7 days (n = 41) and panel II for patients receiving 20 μg/kg/d SCF and 10 μg/kg/d filgrastim for up to 13 days (n = 59). The boxes indicate the range of values and the lines indicate the median value.
Figure 2B shows the daily median CD34+ cell levels in the peripheral blood of patients receiving filgrastim alone for 7 days and those receiving 20 μg/kg/d SCF in combination with filgrastim for up to 13 days. CD34+ cell levels were elevated for the duration of cytokine administration.
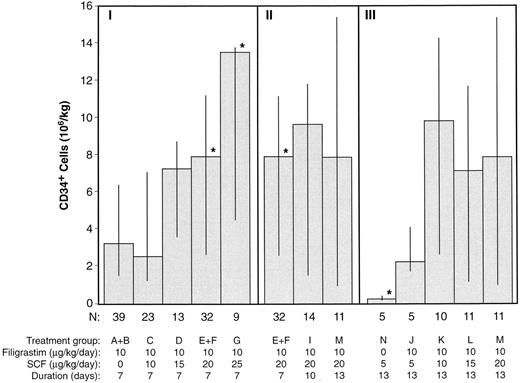
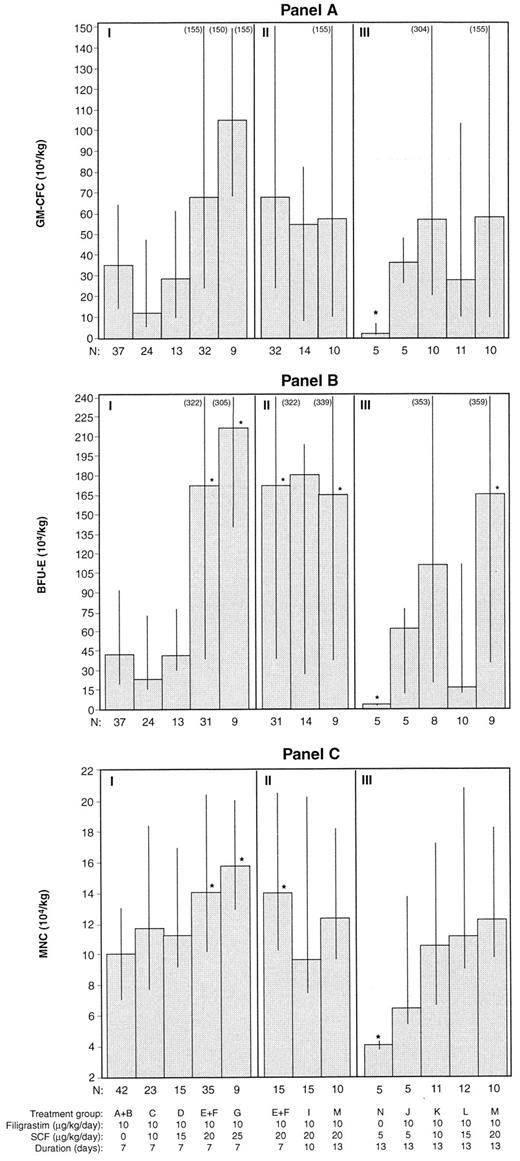
Progenitor cell harvests. The total CD34+ cells obtained from patients in which all 3 days of leukapheresis were performed (n = 172) are shown in Fig 3. Figure 3 shows the 7-day treatment groups, the 20 μg/kg/d SCF in combination with filgrastim combination groups (for 7, 10, and 13 days), and the 13-day treatment groups. The median number of CD34+ cells obtained was greater in treatment groups receiving the combination of filgrastim and SCF, at doses greater than 10 μg/kg/d, than for treatment groups receiving filgrastim alone (7.7 v 3.2 × 106/kg, P < .05). There were significantly (P < .05) more CD34+ cells harvested in the 20 μg/kg/d SCF (median, 7.9 × 106/kg) and 25 μg/kg/d SCF (median, 13.6 × 106/kg) 7-day combination groups compared with the filgrastim alone treatment groups (median, 3.2 × 106/kg) and significantly less for the SCF alone treatment group (median, 0.2 × 106/kg). Duration of administration of SCF and filgrastim (7, 10, or 13 days) did not significantly affect CD34+ cell yield. Similar results are displayed in Fig 4A, B, and C for GM-CFC, BFU-E, and MNC cell yields.
CD34+ cell yields (median, 25th, and 75th percentiles) from 3 days of leukapheresis for each treatment group. Section I includes the 7-day treatment groups, section II the 20 μg/kg/d SCF and filgrastim groups, and section III the 13-day treatment groups. Only 3 patients received 30 μg/kg/d SCF and filgrastim (H); their individual CD34+ cell yields were 1.43, 5.20, and 8.52 × 106/kg. *P < .05, comparing all other treatment groups with the filgrastim alone groups (A and B).
CD34+ cell yields (median, 25th, and 75th percentiles) from 3 days of leukapheresis for each treatment group. Section I includes the 7-day treatment groups, section II the 20 μg/kg/d SCF and filgrastim groups, and section III the 13-day treatment groups. Only 3 patients received 30 μg/kg/d SCF and filgrastim (H); their individual CD34+ cell yields were 1.43, 5.20, and 8.52 × 106/kg. *P < .05, comparing all other treatment groups with the filgrastim alone groups (A and B).
Progenitor cell yields (median, 25th, and 75th percentiles) from 3 days of leukapheresis for each treatment group are shown in (A) (GM-CFC), (B) (BFU-E), and (C) (MNC). Section I includes the 7-day treatment groups, section II includes the 20 μg/kg/d SCF and filgrastim groups, and section III the 13-day treatment groups. Only 3 patients received 30 μg/kg/d SCF and filgrastim (H); their individual progenitor cell yields were 56.20, unknown, and 149.70 × 104/kg (GM-CFC); 68.91, unknown, and 424.67 × 104/kg (BFU-E); and 13.48, 8.59, and 29.99 × 104/kg (MNC). *P < .05, comparing all other treatment groups with the filgrastim alone groups (A and B).
Progenitor cell yields (median, 25th, and 75th percentiles) from 3 days of leukapheresis for each treatment group are shown in (A) (GM-CFC), (B) (BFU-E), and (C) (MNC). Section I includes the 7-day treatment groups, section II includes the 20 μg/kg/d SCF and filgrastim groups, and section III the 13-day treatment groups. Only 3 patients received 30 μg/kg/d SCF and filgrastim (H); their individual progenitor cell yields were 56.20, unknown, and 149.70 × 104/kg (GM-CFC); 68.91, unknown, and 424.67 × 104/kg (BFU-E); and 13.48, 8.59, and 29.99 × 104/kg (MNC). *P < .05, comparing all other treatment groups with the filgrastim alone groups (A and B).
Seven (of 172) patients (4.1%) did not meet the minimum target yield of 4.0 × 108 MNCs/kg after 3 leukaphereses. Three of these patients received filgrastim alone (7.6% of the 39 filgrastim alone patients), 1 received the cytokine combination (1.0% of the 129 cytokine combination patients), and 3 received SCF alone (75% of the 4 SCF alone patients).
Although no minimum CD34+ cell yield was stipulated in the protocol, standards have changed and the yield of CD34+ cells is now considered more appropriate with respect to PBPC transplantation. The proportion of patients who did not achieve a yield ≥1 × 106 CD34+ cells/kg was 15% (26 of 172). Seven of these patients received filgrastim alone (18% of the filgrastim alone patients), 16 received the cytokine combination (12% of the cytokine combination patients), and 3 received SCF alone (75% of the SCF alone patients). The proportion of patients who did not achieve a yield ≥2 × 106 CD34+ cells/kg was 26% (28% of the filgrastim alone patients, 23% of the cytokine combination patients, and 75% of the SCF alone patients), and the proportion of patients who did not achieve a yield ≥5 × 106 CD34+ cells/kg was 48% (67% of the filgrastim alone patients, 40% of the cytokine combination patients, and 100% of the SCF alone patients).
Safety. SCF administration was associated with manageable levels of toxicity. Adverse events, considered possibly, probably, or definitely related to cytokine administration, occurring in greater than 3% of patients during the collection phase of the study are listed in Table 3. The overall frequency of these events, regardless of relationship to cytokine administration, is also shown. Adverse events, considered at least possibly related to cytokine administration, which were observed more frequently in patients receiving SCF and filgrastim, compared with filgrastim alone included mild injection site reactions, primarily erythema, and pruritus, which were seen in 88% of the combination patients. Four patients (3%) had mild to moderate rashes and 6 patients (4%) had mild to moderate urticaria, distant from the injection site. Most of these patients were able to continue SCF therapy without treatment; however, 2 patients received additional diphenhydramine. There was also an increased incidence of headache (15%) and tachycardia (3%) in patients receiving the cytokine combination, which was possibly related to the antiallergy medication. Two patients (2%) had systemic allergic-like reactions related to SCF. One patient, who received 30 μg/kg/d SCF, developed urticaria on the legs and arms, pruritus, and nausea after the second dose of cytokine. These symptoms recurred after the next dose. SCF was discontinued and the patient received filgrastim alone for the remainder of the collection phase. The second patient, who received SCF at 15 μg/kg/d, developed an allergic-like reaction within 10 to 15 minutes after the fifth dose of cytokine. She experienced abdominal cramping, respiratory symptoms (throat tightness, dyspnea, dysphagia, and rhinitis), and chest discomfort and had repeated episodes of vomiting. These symptoms resolved with additional diphenhydramine, ranitidine, and discontinuation of SCF.
Adverse Events During Collection Phase
| Adverse Event . | Filgrastim Alone . | SCF and Filgrastim . | ||
|---|---|---|---|---|
| . | (n = 55, 10 μg/kg/d) . | (n = 160, 5-30 μg/kg/d) . | ||
| . | Related . | Overall . | Related . | Overall . |
| Injection site reactions | 2% | 5% | 88% | 88% |
| Musculoskeletal symptoms | 29% | 45% | 19% | 43% |
| Headache | 5% | 24% | 15% | 31% |
| Fever | 4% | 18% | 5% | 24% |
| Pruritus | 0% | 2% | 3% | 3% |
| Urticaria | 0% | 0% | 4% | 4% |
| Tachycardia | 0% | 16% | 3% | 31% |
| Chest pain | 2% | 7% | 4% | 6% |
| Ecchymosis | 2% | 4% | 3% | 8% |
| Dyspnea | 2% | 7% | 3% | 6% |
| Pain | 0% | 18% | 3% | 18% |
| Fatigue | 4% | 13% | 2% | 14% |
| Rash | 0% | 0% | 3% | 10% |
| Adverse Event . | Filgrastim Alone . | SCF and Filgrastim . | ||
|---|---|---|---|---|
| . | (n = 55, 10 μg/kg/d) . | (n = 160, 5-30 μg/kg/d) . | ||
| . | Related . | Overall . | Related . | Overall . |
| Injection site reactions | 2% | 5% | 88% | 88% |
| Musculoskeletal symptoms | 29% | 45% | 19% | 43% |
| Headache | 5% | 24% | 15% | 31% |
| Fever | 4% | 18% | 5% | 24% |
| Pruritus | 0% | 2% | 3% | 3% |
| Urticaria | 0% | 0% | 4% | 4% |
| Tachycardia | 0% | 16% | 3% | 31% |
| Chest pain | 2% | 7% | 4% | 6% |
| Ecchymosis | 2% | 4% | 3% | 8% |
| Dyspnea | 2% | 7% | 3% | 6% |
| Pain | 0% | 18% | 3% | 18% |
| Fatigue | 4% | 13% | 2% | 14% |
| Rash | 0% | 0% | 3% | 10% |
Adverse events, considered at least possibly related to cytokine administration, occurring in greater than 3% of patients during the collection phase; these are shown in descending order of event frequency in the combination cytokine group. The overall frequency of these events, regardless of relationship to cytokine, is also shown. The SCF alone patients (n = 5) are included with the combination cytokine patients.
There were no hematologic toxicities attributable to SCF in the collection phase. The median baseline hemoglobin values in patients receiving the cytokine combination or filgrastim alone were 112 and 111 g/L, respectively. At the end of the collection phase, these values both decreased to 98 g/L. The leukapheresis procedure was associated with decreases in platelet levels; however, these were not of clinical significance. The median baseline platelet counts in the patients receiving the cytokine combination or filgrastim alone were 220 × 109/L and 210 × 109/L, respectively. At the cessation of the collection phase, these values decreased to 138 × 109/L and 110 × 109/L, respectively. The lowest platelet count recorded was 35 × 109/L on day 10 of 13 days of administration of 15 μg/kg/d SCF and 10 μg/kg/d filgrastim.
Transplant Phase
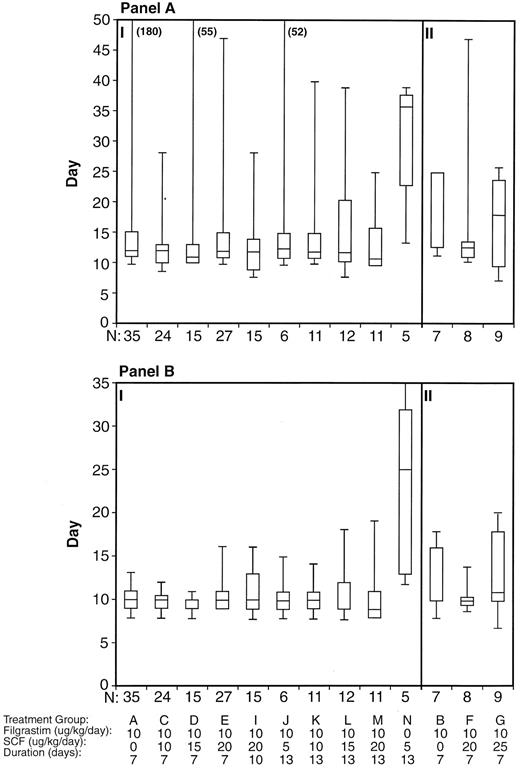
Engraftment. Boxplots of the neutrophil and platelet engraftment data for the various treatment groups are shown in Fig 5. For all patients, the median time to ANC ≥0.5 × 109/L was 10 days, with a minimum of 7 days and a maximum of 35 days. The median time to platelets ≥20 × 109/L was 12 days, with a minimum of 7 days and a maximum of greater than 180 days. ANC and platelet recovery was similar for groups treated with filgrastim alone or with the cytokine combination before leukapheresis. Of the 143 patients undergoing 3 leukaphereses and receiving all three products back, 32% (38% of the filgrastim alone patients and 31% of the cytokine combination patients) experienced platelet engraftment at ≥day 14. Nine percent of patients (12.5% of the filgrastim alone patients and 8% of the cytokine combination patients) experienced platelet engraftment at ≥ day 28. Overall, only 1 patient failed to engraft by day 90 posttransplantation. There was a trend towards slightly delayed engraftment, particularly for platelets, for those groups (B, F, and G) receiving only 1 day of leukapheresis product as the sole source of hematopoietic support.
Days to engraftment, for all treatment groups, for platelets ≥20 × 109/L (A) and for ANC ≥0.5 × 109/L (B). Censored patients have been included and the median, 25th, and 75th percentiles and range are shown. Section I includes those treatment groups in which 3 PBPC harvests were infused and section II includes those in which only 1 harvest was infused. Data from 185 patients have been included; this does not include 1 patient who withdrew from the study before PBPC infusion and treatment group H, which only enrolled 3 patients.
Days to engraftment, for all treatment groups, for platelets ≥20 × 109/L (A) and for ANC ≥0.5 × 109/L (B). Censored patients have been included and the median, 25th, and 75th percentiles and range are shown. Section I includes those treatment groups in which 3 PBPC harvests were infused and section II includes those in which only 1 harvest was infused. Data from 185 patients have been included; this does not include 1 patient who withdrew from the study before PBPC infusion and treatment group H, which only enrolled 3 patients.
Of the 5 patients who received SCF alone during the collection phase, 4 received their previously cryopreserved marrow due to engraftment failure. Recovery to ANC ≥0.5 × 109/L and platelet count ≥20 × 109/L was delayed compared with the other treatment groups. No further patients were treated with SCF alone. Five additional patients, 4 of whom only received the leukapheresis product collected on the first day, received back-up bone marrow infusion to facilitate engraftment.
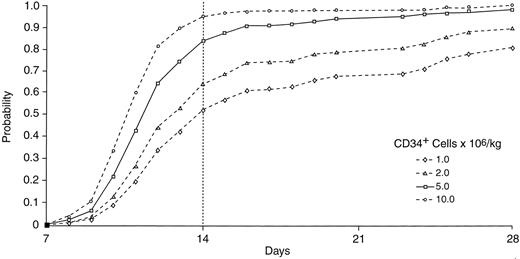
The relationship between the number of CD34+ cells infused and days to platelet recovery was modeled using a Cox proportional hazards analysis. Two hundred twelve patients from both treatment groups were included, because there was no treatment effect observed in the model. As shown in Fig 6, infusion of 5 × 106 CD34+ cells/kg provides about an 85% probability of platelet engraftment to 20 × 109/L by day 14 posttransplantation and a very low incidence of delayed platelet recovery beyond 28 days; this constitutes rapid hematopoietic reconstitution because ANC recovery to ≥0.5 × 109/L consistently occurs before platelet recovery. With the infusion of less than 5 × 106 CD34+ cells/kg, there is a lower probability of a patient achieving rapid engraftment. Infusion of 2 × 106 CD34+ cells/kg provides a probability of platelet engraftment by day 14 of about 65%, with approximately 10% of patients having delayed platelet recovery beyond day 28.
Cox proportional hazards analysis for probability of engraftment to platelets ≥20 × 109/L, with the yield of CD34+ cells as the predictor (n = 212).
Cox proportional hazards analysis for probability of engraftment to platelets ≥20 × 109/L, with the yield of CD34+ cells as the predictor (n = 212).
The median follow-up duration posttransplantation has been 23 months (range, 8 days to 37 months). One patient, who received cells mobilized by the cytokine combination, experienced delayed graft failure of ANC less than 0.5 × 109/L and a platelet count of less than 20 × 109/L 70 days posttransplantation, subsequent to earlier successful reconstitution. In addition, 4 patients were diagnosed with chemotherapy-related hemolytic uremic syndrome/thrombotic thrombocytopenic purpura and experienced transient decreases in their platelet counts.
All patients required platelet transfusions within 90 days posttransplantation and all except 1 filgrastim alone patient required red blood cell transfusions. Twelve percent of the filgrastim alone patients and 9% of those receiving SCF were rehospitalized within 90 days posttransplantation.
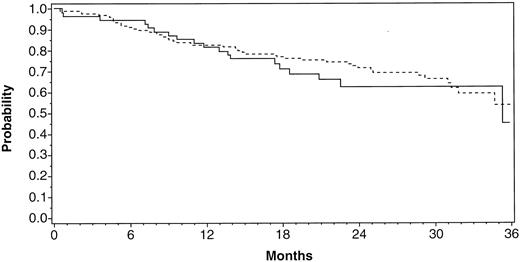
Safety. Adverse events occurring during the transplant phase of the study were consistent with those generally observed after high-dose chemotherapy. There were no adverse events reported to be related to prior SCF administration. Five of 215 patients (2.3%) died within 30 days after PBPC infusion from chemotherapy-related complications. Three patients had received the cytokine combination and 2 had received filgrastim alone for PBPC mobilization. Overall survival (Fig 7) was not influenced by the addition of SCF during mobilization and at 23 months was 64% for the filgrastim alone patients and 74% for the SCF and filgrastim patients. The treatment groups were evenly matched for stage IV patients and those with high-risk localized disease (Table 2).
Overall survival for patients post-PBPC infusion. The patients either received filgrastim alone ( — ; n = 55) or SCF with/without filgrastim (- - - -; n = 158) for mobilization of PBPCs.
Overall survival for patients post-PBPC infusion. The patients either received filgrastim alone ( — ; n = 55) or SCF with/without filgrastim (- - - -; n = 158) for mobilization of PBPCs.
DISCUSSION
In this large, controlled clinical trial, therapy with SCF and filgrastim compared with filgrastim alone was associated with substantially greater numbers of PBPCs obtained during leukapheresis. This result is consistent with the findings in animal models19-22 as well as with the preliminary results of other clinical trials.33-35 Higher CD34+ cell yields were obtained when SCF doses of greater than 10 μg/kg/d were administered in combination with filgrastim for 7, 10, or 13 days. On the basis of the mobilization results from this study and the overall safety profile of SCF, the combination of 20 μg/kg/d SCF and 10 μg/kg/d filgrastim with daily apheresis beginning on day 5 was selected as the optimal dose and schedule for the mobilization of PBPCs. Treatment with SCF alone mobilized significantly less CD34+ cells than did filgrastim alone. In this study, only a dose of 5 μg/kg/d was studied; however, in phase 1 studies, doses of up to 50 μg/kg/d resulted in only modest PBPC mobilization.26 28
In this study of moderately pretreated breast cancer patients, treatment groups mobilized with filgrastim alone or with SCF and filgrastim showed similar hematopoietic engraftment after the infusion of PBPCs. However, somewhat fewer patients receiving the cytokine combination (all doses of SCF combined) experienced platelet engraftment beyond days 14 and 28 compared with the patients receiving filgrastim alone.
Recent developments in the field have increased the importance of strategies aimed at increasing the number of hematopoietic progenitor cells obtained in the leukapheresis product. If fewer leukaphereses are required to obtain sufficient numbers of cells to ensure a high probability of rapid engraftment, the cost of these treatments should decrease and patients' inconvenience should be lessened. Moreover, increased numbers of PBPCs would facilitate the development of important innovations in the field, including the administration of multiple cycles of high-dose chemotherapy and ex vivo manipulation of PBPCs, including tumor cell purging, gene therapy, and progenitor cell expansion.
Alternative approaches to increasing PBPCs yields have included other combinations of cytokines, including GM-CSF and G-CSF,36 IL-3 and G-CSF or GM-CSF37 and PIXY-321, the IL-3/GM-CSF fusion molecule.38 These combinations have not been studied in large, randomized, controlled clinical trials involving patients receiving standardized high-dose therapy with centralized CD34+ cell enumeration. An alternative and widely used approach has been to use chemotherapy followed by cytokines to mobilize PBPCs.39 This approach has the potential advantage of providing additional anticancer therapy and the disadvantages of increased toxicity, increased costs due to the treatment of febrile neutropenia, a lower efficiency of pheresis bed use, and the inability to adopt this approach to the mobilization of normal donors in the allogeneic transplant setting. A recent study has shown that the addition of SCF to mobilization regimens using filgrastim after chemotherapy is also associated with further increases in the number of PBPC harvested40 of similar magnitude to that in the present study.
Some questions with respect to PBPC mobilization with the SCF and filgrastim combination remain to be studied further in future clinical trials. Although studies to date have not established the ability of filgrastim alone administered at higher doses to enhance PBPC collection compared with the dose and schedule used in this study,41 clinical trials are in progress exploring this possibility. The effect, if any, of SCF on tumor cell contamination of the PBPC product is also under study. However, in the present study, no differences were observed in overall survival in the patients treated with SCF and filgrastim compared with those receiving filgrastim alone, suggesting that SCF administration has no clinically relevant effect on outcome of high-dose chemotherapy treatment for breast cancer. Finally, although this study and other studies have shown that the addition of SCF enhances the quantity of CD34+ cells obtained, further studies will be required to characterize any qualitative differences between CD34+ cells harvested with SCF and filgrastim and those harvested with filgrastim alone. Our data demonstrate that, in this clinical model, hematopoietic support with SCF and filgrastim mobilized cells is associated with durable, long-term engraftment.
It is probable that SCF will be particularly useful in the treatment of subsets of patients, such as those who have received extensive prior chemotherapy, from whom it is expected that inadequate numbers of PBPCs would be obtained.42-44 In this context, it is important to note that our data show that sustained increases in circulating CD34+ cell numbers and in the number of CD34+ cells obtained by leukapheresis occur in patients receiving the SCF and filgrastim combination and persist for up to 13 days. This has not been observed with filgrastim alone45 and would make continued daily leukapheresis to an adequate CD34+ cell harvest feasible in patients in whom PBPCs are difficult to mobilize.
We conclude from this randomized phase 2 study that (1) SCF administered at doses of 5 to 30 μg/kg/d with antiallergy prophylaxis is associated with manageable levels of toxicity and is feasible in the mobilization of PBPCs; (2) the addition of SCF to filgrastim is associated with the mobilization and harvesting of larger numbers of CD34+ cells; (3) this increased CD34+ cell mobilization is sustained for up to 13 days; and (4) 20 μg/kg/d SCF and 10 μg/kg/d filgrastim, with daily apheresis beginning on day 5, is the optimal dose and schedule for PBPC mobilization. Further studies, including a randomized phase 3 study, exploring the clinical benefits of this regimen are currently underway.
ACKNOWLEDGMENT
The authors thank John Costa, MD, Lisa Hami, Jane Hobbs, Anna Moore, Linda Norton, Anne Sharpe, Sharon Taffs, Marianne Zblyski, Judith Barnard, Mark Davis, Eric Guempel, Jerome Hill, Kathy Jelaca-Maxwell, Hillary O'Kelly, and William Parker for their contribution towards this research.
Supported in part by National Institutes of Health (NIH) Grant No. M01-RR00865 (J.A.G.), The Revlon/UCLA Women's Cancer Research Program, NIH/National Cancer Institute Grant No. R01-CA61508 (E.J.S.), and Amgen Inc.
Address reprint requests to J.A. Glaspy, MD, UCLA School of Medicine, 200 Medical Plaza, Room 120-64, Los Angeles, CA 90095-6956.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal