Abstract
In The Netherlands from July 1988 to October 1991, children (0 to 16 years of age) with de novo acute lymphoblastic leukemia (ALL) were treated according to protocol ALL-7 of the Dutch Childhood Leukemia Study Group (DCLSG). In this protocol, chemotherapy and treatment stratification were identical to the ALL-BFM-86 protocol (Reiter et al, Blood 84:3122, 1994), but cranial irradiation was restricted to patients with initial central nervous system (CNS) involvement. Patients were stratified into 3 risk groups, based on leukemia cell mass and response to initial treatment: standard-risk group (SRG), risk group (RG), and experimental group (EG). As in ALL-BFM-86, a randomized study on late intensification (protocol S) was performed in RG patients, and during the study (since October 1990), early reinduction treatment (protocol II) was introduced for SRG patients. Treatment duration for all patients was 18 months. Two hundred eighteen children entered the study: 74 SRG, 127 RG, and 17 EG patients. The overall complete remission (CR) rate was 98%. The 5-year event-free survival (EFS) for all DCLSG ALL-7 patients was 65.3% (standard error [SE] 3.2%), which was significantly different from the 73% (SE 1%) 5-year EFS achieved in the ALL-BFM-86 study (P = .02, Z-test). However, restricting the analysis to SRG patients receiving protocol II with a total duration of treatment of 18 months, the 5-year EFS rates were 64.6% (SE 4.0%) and 67% (SE 4%), respectively, and no significant difference could be established (P = .67, Z-test). The 5-year EFS rates for SRG, RG, and EG patients were 63.5% (SE 5.6%), 66.6% (SE 4.2%), and 63.3% (SE 12.0%), respectively. SRG patients receiving protocol II fared better than patients not receiving protocol II (5-year EFS 76.7% [SE 7.7] and 54.5% [SE 7.5], respectively). No difference in 5-year EFS was observed in RG patients randomized to receive or not to receive late intensification with protocol S. The overall CNS relapse rate at 5 years was 5.5%. The incidence rate at 5 years was 11.4% in SRG patients not receiving protocol II, whereas no CNS relapses occurred in SRG patients receiving protocol II. Six children died in first complete remission and 2 children developed a second malignancy (thyroid carcinoma and acute nonlymphoblastic leukemia). Systemic high-dose methotrexate (MTX) and intrathecal chemotherapy is a safe and effective method of CNS prophylaxis in the context of BFM-oriented treatment for all children with ALL, regardless of the risk group (with the possible exception of T-ALL patients with high white blood cell counts). The results of the DCLSG ALL-7 study confirm those of the ALL-BFM-86 study showing that early reinduction with protocol II is essential in the treatment of SRG patients and that late intensification with protocol S does not improve the prognosis for RG patients.
FROM 1988 TO 1991, CHILDREN with newly diagnosed acute lymphoblastic leukemia (ALL) in The Netherlands were treated using the Dutch Childhood Leukemia Study Group (DCLSG) ALL-7 protocol. This protocol was similar to the ALL-BFM-86 protocol,1 with the exception that no prophylactic cranial radiation was applied. Before 1988, non–high-risk (NHR) ALL patients were entered into the nationwide DCLSG-ALL-6 protocol that also did not include prophylactic cranial radiotherapy; children with high-risk (HR) ALL were treated according to institutional guidelines. In 1988, when the results of DCLSG-ALL-62 were still unknown, cooperation was sought with the Berlin-Frankfurt-Münster (BFM) group to adopt their very effective treatment strategy3-5 to further improve the treatment results of NHR-ALL patients achieved with the preceding DCLSG ALL-5 study.6
The data presented here represent the treatment results for children with ALL in The Netherlands diagnosed in the period from 1988 to 1991.
PATIENTS AND METHODS
Patients.
The population-based DCLSG protocol ALL-7 was open to children (0 through 15 years of age) with de novo ALL diagnosed from July 1988 through September 1991. Patients with mature B-cell ALL, either morphologic (French-American-British [FAB] type L3) or immunophenotypic (surface IgM positive), were excluded as well as patients treated with corticosteroids and/or cytostatic drugs less than 4 weeks before diagnosis. Informed consent was obtained according to institutional guidelines before treatment and before randomization for late intensification with protocol S in risk group (RG) patients (see below).
Diagnosis.
The diagnosis of ALL was made by cytomorphological and cytochemical examination of blood and bone marrow smears at the local institution, followed by confirmation and classification according to FAB criteria7,8 by the DCLSG laboratory. For the diagnosis of ALL, ≥25% blasts in the bone marrow was mandatory. A sample of cerebrospinal fluid (CSF), mixed 1:1 with a transport medium, was sent to the DCLSG laboratory at diagnosis and when a relapse was suspected.9,10 Central nervous system (CNS) involvement was defined as the presence of ≥5/μL cells in the CSF with leukemic blasts (cytomorphological) without major blood contamination (<50/3 erythrocytes) or as leukemic infiltration of the brain, assessed by cranial computed tomography. The leukemic cell mass estimate, the BFM risk factor RF, was calculated by the equation: RF = 0.2 × log (number of peripheral blood blasts/μL + 1) + 0.06 × liver centimeters below costal margin + 0.04 × spleen centimeters below costal margin.11,12 Immunophenotyping was performed by the DCLSG laboratory if cytospin bone marrow smears contained ≥60% blasts.13 Immunological markers were judged positive if expressed in ≥20% of the malignant cells. A leukemia was classified as precursor B-ALL if the malignant cells were positive for TdT, CD19, and HLA-DR (pro-B ALL); for TdT, CD10, CD19, and HLA-DR (common ALL); or for TdT, CD10, CD19, HLA-DR, and CyIgμ (pre-B-ALL). A T-ALL was defined by positivity for TdT, CD2, cytoplasmic CD3 (CyCD3), and/or CD7. Acute undifferentiated leukemia (AUL) was defined if common ALL, pre-B, T-cell characteristics, and also myeloid markers were negative. Cytogenetic analysis was performed by members of the Dutch Working Party on Cancer Genetics and Cytogenetics at the various Clinical Genetics Centers in The Netherlands.14 All findings were peer-reviewed before submission to the database. An abnormal clone was defined as a minimum of 2 metaphases with the same structural abnormality or same additional chromosome or 3 metaphases with the same missing chromosome. Cytogenetic analysis was considered a failure if less than 20 metaphases with an apparently normal karyotype from an unstimulated or unsynchronized culture had been analyzed. Chromosome structural abnormality included all structural abnormalities (not just translocations). Cell ploidy was based on cytogenetic findings. The DNA index was measured by the Department of Experimental Therapy of The Netherlands Cancer Institute (Amsterdam, The Netherlands).15
Treatment and treatment stratification.
The treatment scheme is summarized in Table1, while details of the protocol ALL-7 are given in Appendix II. Patients were stratified into 3 groups; standard-risk group (SRG), RG, and experimental group (EG), which is identical to the protocol ALL-BFM-86.1
Treatment Scheme for 3 Stratification Treatment Groups According to DCLSG Protocol ALL-7
| Treatment Stratification . | Induction Protocol . | Consolidation Protocol . | Reinduction Protocol . | Maintenance Treatment . |
|---|---|---|---|---|
| SRG | I | M | no, till Oct.90 SRG II, from Oct.90 SRT | Maintenance treatment |
| RG* | I | M | II + CRT if initial CNS involvement* | Maintenance treatment versus maintenance + protocol S |
| EG* | I | E in CR Allogeneic BMT optional | II + CRT if initial CNS involvement* | Maintenance treatment |
| Months | 0 | 3 | 6 | 818 |
| Treatment Stratification . | Induction Protocol . | Consolidation Protocol . | Reinduction Protocol . | Maintenance Treatment . |
|---|---|---|---|---|
| SRG | I | M | no, till Oct.90 SRG II, from Oct.90 SRT | Maintenance treatment |
| RG* | I | M | II + CRT if initial CNS involvement* | Maintenance treatment versus maintenance + protocol S |
| EG* | I | E in CR Allogeneic BMT optional | II + CRT if initial CNS involvement* | Maintenance treatment |
| Months | 0 | 3 | 6 | 818 |
The chemotherapy applied in the protocols I, M, E, II, maintenance treatment, and protocol S are given in Appendix II. Treatment stratification was defined as follows: SRG: RF <0.8, without mediastinal mass, CNS involvement or EG characteristics; RG: as RF ≥0.8 and/or mediastinal mass and/or CNS involvement, without EG characteristics; and EG: independant of RF, immunophenotypically acute undifferentiated leukemia, and/or ≥1,000/μL blood blasts on day 8 after 7 days of monotherapy with prednisone (poor steroid response), and/or no remission after (the first part of) protocol I (day 42), and/or leukemic blasts karyotype t(9;22) or t(4;11).
Patients with initial CNS disease: 12 to 18 Gy of CRT in 15 days after protocol II.
RG patients who were in continuous complete remission (CCR) after 1 year were randomized to receive or not to receive late intensification with protocol S if informed consent was obtained. EG patients in first CR with a matched sibling donor were offered allogeneic bone marrow transplantation (BMT) after protocol E. Following an earlier amendment of the study ALL-BFM-86, protocol ALL-7 was amended (in October 1990) with the introduction of protocol II for SRG patients (group SRT). The total duration of chemotherapy for all patients was 18 months. In contrast to the ALL-BFM-86 study, in which cranial irradiation (12 to 18 Gy, depending on age) was applied to all RG and EG patients, in protocol DCLSG ALL-7 cranial radiotherapy (CRT; 12 to 18 Gy for 15 days after protocol II) was restricted to patients with initial CNS involvement.
Evaluation criteria.
CR was defined as less than 5% blasts in the bone marrow and recovery of normal hematopoiesis, absence of peripheral blood blasts, and no evidence of disease at any other site. Relapse was defined as ≥20% blasts cells in the bone marrow and/or blasts cells in the peripheral blood, CNS involvement, and/or leukemic infiltrations elsewhere.
Results of treatment were evaluated by bone marrow examination on day 42, before the start of protocol M, E, II, and S, and every 12 to 14 weeks during maintenance treatment. All smears were examined at the DCLSG laboratory. Registration forms with data on dosage, toxicity, and results of treatment for each patient were sent to the DCLSG operations office after protocol I, M, E, II, and S; every 3 months during maintenance treatment; and up to 5 years after cessation of treatment.
Statistical methods.
For comparison of patient and disease characteristics, the Student’st-test, the Mann-Whitney U test, and the χ2 test were applied. Event-free survival (EFS) was defined as the time from diagnosis to induction failure, relapse, death in remission, or the occurrence of a second tumor. For patients event-free and alive at latest follow-up (censored observations), EFS was calculated until this latest follow-up. Patients who did not achieve remission were included in the analysis and considered as treatment failures on day 0. The duration of survival was calculated from diagnosis to time of death; the time from diagnosis to latest follow-up evaluation was calculated as survival for patients alive, the so-called censored observations. All analyses were based on intention to treat and on data for all patients who entered the study; no patients have been excluded for whatever reason (treatment refusal, toxicity, etc). Survival curves and standard errors (SEs) were calculated according to the Kaplan-Meier method. The statistical significance of differences between curves was determined by the 2-sided log-rank test.16 17 Comparison between DCLSG and BFM 5-year EFS results was performed using a Z-score.
RESULTS
Patients.
From July 1988 to October 1991, 267 consecutive children with ALL were diagnosed; 259 of them were eligible for protocol ALL-7. Two hundred eighteen (84%) were registered onto the ALL-7 study. Forty-one (16%) did not enter the study because of institutional choice (39 patients), missing data (1 patient), or patient’s refusal (1 patient). Of the 218 patients on the study, 182 (83%) were registered by pediatricians in university hospitals and 36 (17%) were registered by pediatricians in general hospitals. The patient and disease characteristics of the 259 eligible patients are summarized in Table 2. No significant differences were observed between the 218 protocol and 41 nonprotocol patients, except for white blood cell counts (WBC), which were lower in the nonprotocol patients (P = .012). Of the 218 protocol patients, 74 (34%) were stratified into the SRG, 127 (58%) into the RG, and 17 (8%) in the EG (Table3). Of the 74 SRG patients, 30 did receive protocol II (SRT patients) and 44 did not. Seventeen patients were stratified into the EG because of an acute undifferentiated leukemia (AUL; CD10− and TdT−; 1 patient), t(9:22) (1 patient), t(4;11) (1 patient), poor steroid response (12 patients), no complete remission on day 42 (1 patient), and progressive disease (1 patient).
Patient Characteristics
| . | Eligible Patients . | Protocol Patients . | Nonprotocol Patients . | |||
|---|---|---|---|---|---|---|
| N (259) . | %3-150 . | N (218) . | %3-150 . | N (41) . | %3-150 . | |
| Sex | ||||||
| Boys | 144 | 56 | 123 | 56 | 21 | 51 |
| Girls | 115 | 44 | 95 | 44 | 20 | 49 |
| Age (yrs) | ||||||
| <1 | 3 | 1 | 3 | 1 | 0 | 0 |
| 1-9 | 213 | 82 | 177 | 81 | 36 | 88 |
| ≥10 | 43 | 17 | 38 | 17 | 5 | 12 |
| Risk factor | ||||||
| <0.8 | 101 | 40 | 82 | 38 | 19 | 58 |
| 0.8-1.2 | 84 | 33 | 76 | 35 | 8 | 24 |
| 1.2-1.7 | 58 | 23 | 52 | 24 | 6 | 18 |
| ≥1.7 | 8 | 3 | 8 | 4 | 0 | 0 |
| Unknown | 8 | 0 | 8 | |||
| Hb (mmol/L) | ||||||
| <5 | 140 | 54 | 118 | 54 | 22 | 54 |
| ≥5 | 118 | 46 | 99 | 45 | 19 | 46 |
| Unknown | 1 | 1 | 0 | |||
| WBC (×109/L) | ||||||
| <10 | 121 | 47 | 95 | 44 | 26 | 63 |
| 10-<50 | 89 | 34 | 77 | 35 | 12 | 29 |
| 50-<100 | 23 | 9 | 21 | 10 | 2 | 5 |
| ≥100 | 26 | 10 | 25 | 11 | 1 | 2 |
| Platelets (×109/L) | ||||||
| <50 | 122 | 47 | 103 | 47 | 19 | 46 |
| 50-<100 | 61 | 24 | 54 | 25 | 7 | 17 |
| ≥100 | 75 | 29 | 60 | 28 | 15 | 37 |
| Unknown | 1 | 1 | 0 | |||
| CNS disease | 5 | 2 | 5 | 2 | 0 | 0 |
| Mediastinal mass | 21 | 8 | 19 | 9 | 2 | 5 |
| FAB | ||||||
| L1 | 196 | 76 | 166 | 76 | 30 | 73 |
| L2 | 57 | 22 | 47 | 22 | 10 | 24 |
| L3 | 0 | 0 | 0 | 0 | 0 | 0 |
| AUL | 4 | 2 | 3 | 2 | 1 | 2 |
| Unknown | 2 | 2 | 0 | |||
| Immunophenotype | ||||||
| AUL | 0 | 0 | 0 | 0 | 0 | 0 |
| Pro-B-ALL | 10 | 4 | 10 | 5 | 0 | 0 |
| C-ALL | 141 | 58 | 115 | 55 | 26 | 70 |
| Pre-B-ALL | 57 | 23 | 48 | 23 | 9 | 25 |
| T-ALL | 36 | 15 | 34 | 16 | 2 | 5 |
| Other | 1 | 0 | 1 | 0 | 0 | 0 |
| Not determined | 14 | 10 | 4 | |||
| Cellploidy3-150 | ||||||
| Diploid | 35 | 20 | 30 | 21 | 5 | 15 |
| Hypodiploid <46 | 7 | 4 | 5 | 4 | 2 | 6 |
| Hyperdiploid 47-50 | 23 | 13 | 17 | 12 | 6 | 18 |
| Hyperdiploid >50 chr | 55 | 32 | 44 | 31 | 11 | 33 |
| Pseudodiploid | 49 | 28 | 41 | 29 | 8 | 24 |
| Polyploid | 3 | 2 | 2 | 1 | 1 | 3 |
| Not evaluable3-151 | 57 | 55 | 2 | |||
| Not determined | 30 | 24 | 6 | |||
| DNA index3-150 | ||||||
| <1.16 | 118 | 75 | 114 | 75 | 4 | 67 |
| ≥1.16 | 40 | 25 | 38 | 25 | 2 | 33 |
| Not determined | 101 | 66 | 35 | |||
| Chromosome structural anomalies3-150 | ||||||
| Absent | 54 | 35 | 45 | 36 | 9 | 30 |
| Present | 100 | 65 | 79 | 64 | 21 | 70 |
| Not evaluable | 75 | 70 | 5 | |||
| Not determined | 30 | 24 | 6 | |||
| Down’s syndrome | 6 | 2 | 4 | 2 | 2 | 5 |
| Prednison response on day 83-150 | ||||||
| <1,000 blasts/μL | 198 | 94 | ||||
| ≥1,000 blasts/μL | 12 | 6 | ||||
| Unknown | 8 | |||||
| . | Eligible Patients . | Protocol Patients . | Nonprotocol Patients . | |||
|---|---|---|---|---|---|---|
| N (259) . | %3-150 . | N (218) . | %3-150 . | N (41) . | %3-150 . | |
| Sex | ||||||
| Boys | 144 | 56 | 123 | 56 | 21 | 51 |
| Girls | 115 | 44 | 95 | 44 | 20 | 49 |
| Age (yrs) | ||||||
| <1 | 3 | 1 | 3 | 1 | 0 | 0 |
| 1-9 | 213 | 82 | 177 | 81 | 36 | 88 |
| ≥10 | 43 | 17 | 38 | 17 | 5 | 12 |
| Risk factor | ||||||
| <0.8 | 101 | 40 | 82 | 38 | 19 | 58 |
| 0.8-1.2 | 84 | 33 | 76 | 35 | 8 | 24 |
| 1.2-1.7 | 58 | 23 | 52 | 24 | 6 | 18 |
| ≥1.7 | 8 | 3 | 8 | 4 | 0 | 0 |
| Unknown | 8 | 0 | 8 | |||
| Hb (mmol/L) | ||||||
| <5 | 140 | 54 | 118 | 54 | 22 | 54 |
| ≥5 | 118 | 46 | 99 | 45 | 19 | 46 |
| Unknown | 1 | 1 | 0 | |||
| WBC (×109/L) | ||||||
| <10 | 121 | 47 | 95 | 44 | 26 | 63 |
| 10-<50 | 89 | 34 | 77 | 35 | 12 | 29 |
| 50-<100 | 23 | 9 | 21 | 10 | 2 | 5 |
| ≥100 | 26 | 10 | 25 | 11 | 1 | 2 |
| Platelets (×109/L) | ||||||
| <50 | 122 | 47 | 103 | 47 | 19 | 46 |
| 50-<100 | 61 | 24 | 54 | 25 | 7 | 17 |
| ≥100 | 75 | 29 | 60 | 28 | 15 | 37 |
| Unknown | 1 | 1 | 0 | |||
| CNS disease | 5 | 2 | 5 | 2 | 0 | 0 |
| Mediastinal mass | 21 | 8 | 19 | 9 | 2 | 5 |
| FAB | ||||||
| L1 | 196 | 76 | 166 | 76 | 30 | 73 |
| L2 | 57 | 22 | 47 | 22 | 10 | 24 |
| L3 | 0 | 0 | 0 | 0 | 0 | 0 |
| AUL | 4 | 2 | 3 | 2 | 1 | 2 |
| Unknown | 2 | 2 | 0 | |||
| Immunophenotype | ||||||
| AUL | 0 | 0 | 0 | 0 | 0 | 0 |
| Pro-B-ALL | 10 | 4 | 10 | 5 | 0 | 0 |
| C-ALL | 141 | 58 | 115 | 55 | 26 | 70 |
| Pre-B-ALL | 57 | 23 | 48 | 23 | 9 | 25 |
| T-ALL | 36 | 15 | 34 | 16 | 2 | 5 |
| Other | 1 | 0 | 1 | 0 | 0 | 0 |
| Not determined | 14 | 10 | 4 | |||
| Cellploidy3-150 | ||||||
| Diploid | 35 | 20 | 30 | 21 | 5 | 15 |
| Hypodiploid <46 | 7 | 4 | 5 | 4 | 2 | 6 |
| Hyperdiploid 47-50 | 23 | 13 | 17 | 12 | 6 | 18 |
| Hyperdiploid >50 chr | 55 | 32 | 44 | 31 | 11 | 33 |
| Pseudodiploid | 49 | 28 | 41 | 29 | 8 | 24 |
| Polyploid | 3 | 2 | 2 | 1 | 1 | 3 |
| Not evaluable3-151 | 57 | 55 | 2 | |||
| Not determined | 30 | 24 | 6 | |||
| DNA index3-150 | ||||||
| <1.16 | 118 | 75 | 114 | 75 | 4 | 67 |
| ≥1.16 | 40 | 25 | 38 | 25 | 2 | 33 |
| Not determined | 101 | 66 | 35 | |||
| Chromosome structural anomalies3-150 | ||||||
| Absent | 54 | 35 | 45 | 36 | 9 | 30 |
| Present | 100 | 65 | 79 | 64 | 21 | 70 |
| Not evaluable | 75 | 70 | 5 | |||
| Not determined | 30 | 24 | 6 | |||
| Down’s syndrome | 6 | 2 | 4 | 2 | 2 | 5 |
| Prednison response on day 83-150 | ||||||
| <1,000 blasts/μL | 198 | 94 | ||||
| ≥1,000 blasts/μL | 12 | 6 | ||||
| Unknown | 8 | |||||
Percentage of patients with available information.
Including Down’s syndrome.
Patient Characteristics According to Risk Group
| . | SRG Without Protocol II . | SRG With Protocol II (SRT) . | RG . | EG . | ||||
|---|---|---|---|---|---|---|---|---|
| N (44) . | %4-150 . | N (30) . | %4-150 . | N (127) . | %4-150 . | N (17) . | %4-150 . | |
| Stratification criteria | ||||||||
| Risk factor | ||||||||
| <0.80 | 44 | 30 | 7 | 1 | ||||
| 0.8-1.19 | 0 | 0 | 69 | 7 | ||||
| 1.2-1.69 | 0 | 0 | 44 | 8 | ||||
| ≥1.70 | 0 | 0 | 7 | 1 | ||||
| CNS disease | 0 | 0 | 5 | 0 | ||||
| Mediastinal mass | 0 | 0 | 16 | 3 | ||||
| Blast cells on day 8 | ||||||||
| <1,000/μL | 42 | 30 | 121 | 5 | ||||
| ≥1,000/μL | 0 | 0 | 0 | 12 | ||||
| Unknown | 2 | 0 | 0 | 0 | ||||
| Day 42 BM >5% blasts | 0 | 0 | 0 | 2 | ||||
| Other characteristics | ||||||||
| Age (yrs) | ||||||||
| <1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 12 |
| 1-9 | 41 | 93 | 24 | 80 | 101 | 80 | 11 | 65 |
| ≥10 | 3 | 7 | 6 | 20 | 25 | 19 | 4 | 24 |
| WBC (109/L) | ||||||||
| <10 | 44 | 100 | 26 | 87 | 25 | 20 | 0 | 0 |
| 10-<50 | 0 | 0 | 4 | 3 | 68 | 54 | 5 | 30 |
| ≥50 | 0 | 0 | 0 | 0 | 34 | 27 | 12 | 70 |
| Hb (mmol/L) | ||||||||
| <5 | 27 | 61 | 13 | 43 | 70 | 55 | 8 | 47 |
| ≥5 | 17 | 39 | 17 | 57 | 56 | 45 | 9 | 53 |
| Unknown | 0 | 0 | 1 | 0 | ||||
| Immunophenotype4-150 | ||||||||
| Pro-B-ALL | 0 | 0 | 1 | 3 | 5 | 4 | 4 | 24 |
| c-ALL | 29 | 73 | 22 | 76 | 60 | 49 | 4 | 24 |
| Pre-B-ALL | 10 | 25 | 6 | 20 | 30 | 25 | 2 | 12 |
| T-ALL | 0 | 0 | 0 | 0 | 27 | 22 | 7 | 41 |
| AUL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 4 | 1 | 5 | 0 | ||||
| DNA index4-150 | ||||||||
| <1.16 | 19 | 76 | 12 | 63 | 73 | 77 | 10 | 77 |
| ≥1.16 | 6 | 29 | 7 | 37 | 22 | 23 | 3 | 21 |
| Unknown | 19 | 11 | 32 | 4 | ||||
| Cellploidy4-150 | ||||||||
| Diploid | 5 | 25 | 4 | 22 | 21 | 23 | 0 | 0 |
| Hypodiploid <46 | 0 | 0 | 1 | 6 | 4 | 4 | 0 | 0 |
| Hyperdiploid 47-50 | 4 | 20 | 1 | 6 | 11 | 12 | 1 | 9 |
| Hyperdiploid >50 | 10 | 50 | 8 | 44 | 21 | 23 | 5 | 45.5 |
| Pseudodiploid | 1 | 5 | 4 | 22 | 31 | 34 | 5 | 45.5 |
| Polyploid | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| Not evaluable4-151 | 16 | 10 | 24 | 5 | ||||
| Not determined | 8 | 2 | 13 | 1 | ||||
| Chromosome structural anomalies4-150 | ||||||||
| Absent | 8 | 57 | 7 | 41 | 28 | 34 | 2 | 18 |
| Present | 6 | 43 | 10 | 59 | 54 | 66 | 9 | 82 |
| Not evaluable | 22 | 11 | 32 | 5 | ||||
| Not determined | 8 | 2 | 13 | 1 | ||||
| . | SRG Without Protocol II . | SRG With Protocol II (SRT) . | RG . | EG . | ||||
|---|---|---|---|---|---|---|---|---|
| N (44) . | %4-150 . | N (30) . | %4-150 . | N (127) . | %4-150 . | N (17) . | %4-150 . | |
| Stratification criteria | ||||||||
| Risk factor | ||||||||
| <0.80 | 44 | 30 | 7 | 1 | ||||
| 0.8-1.19 | 0 | 0 | 69 | 7 | ||||
| 1.2-1.69 | 0 | 0 | 44 | 8 | ||||
| ≥1.70 | 0 | 0 | 7 | 1 | ||||
| CNS disease | 0 | 0 | 5 | 0 | ||||
| Mediastinal mass | 0 | 0 | 16 | 3 | ||||
| Blast cells on day 8 | ||||||||
| <1,000/μL | 42 | 30 | 121 | 5 | ||||
| ≥1,000/μL | 0 | 0 | 0 | 12 | ||||
| Unknown | 2 | 0 | 0 | 0 | ||||
| Day 42 BM >5% blasts | 0 | 0 | 0 | 2 | ||||
| Other characteristics | ||||||||
| Age (yrs) | ||||||||
| <1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 12 |
| 1-9 | 41 | 93 | 24 | 80 | 101 | 80 | 11 | 65 |
| ≥10 | 3 | 7 | 6 | 20 | 25 | 19 | 4 | 24 |
| WBC (109/L) | ||||||||
| <10 | 44 | 100 | 26 | 87 | 25 | 20 | 0 | 0 |
| 10-<50 | 0 | 0 | 4 | 3 | 68 | 54 | 5 | 30 |
| ≥50 | 0 | 0 | 0 | 0 | 34 | 27 | 12 | 70 |
| Hb (mmol/L) | ||||||||
| <5 | 27 | 61 | 13 | 43 | 70 | 55 | 8 | 47 |
| ≥5 | 17 | 39 | 17 | 57 | 56 | 45 | 9 | 53 |
| Unknown | 0 | 0 | 1 | 0 | ||||
| Immunophenotype4-150 | ||||||||
| Pro-B-ALL | 0 | 0 | 1 | 3 | 5 | 4 | 4 | 24 |
| c-ALL | 29 | 73 | 22 | 76 | 60 | 49 | 4 | 24 |
| Pre-B-ALL | 10 | 25 | 6 | 20 | 30 | 25 | 2 | 12 |
| T-ALL | 0 | 0 | 0 | 0 | 27 | 22 | 7 | 41 |
| AUL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 4 | 1 | 5 | 0 | ||||
| DNA index4-150 | ||||||||
| <1.16 | 19 | 76 | 12 | 63 | 73 | 77 | 10 | 77 |
| ≥1.16 | 6 | 29 | 7 | 37 | 22 | 23 | 3 | 21 |
| Unknown | 19 | 11 | 32 | 4 | ||||
| Cellploidy4-150 | ||||||||
| Diploid | 5 | 25 | 4 | 22 | 21 | 23 | 0 | 0 |
| Hypodiploid <46 | 0 | 0 | 1 | 6 | 4 | 4 | 0 | 0 |
| Hyperdiploid 47-50 | 4 | 20 | 1 | 6 | 11 | 12 | 1 | 9 |
| Hyperdiploid >50 | 10 | 50 | 8 | 44 | 21 | 23 | 5 | 45.5 |
| Pseudodiploid | 1 | 5 | 4 | 22 | 31 | 34 | 5 | 45.5 |
| Polyploid | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 |
| Not evaluable4-151 | 16 | 10 | 24 | 5 | ||||
| Not determined | 8 | 2 | 13 | 1 | ||||
| Chromosome structural anomalies4-150 | ||||||||
| Absent | 8 | 57 | 7 | 41 | 28 | 34 | 2 | 18 |
| Present | 6 | 43 | 10 | 59 | 54 | 66 | 9 | 82 |
| Not evaluable | 22 | 11 | 32 | 5 | ||||
| Not determined | 8 | 2 | 13 | 1 | ||||
Percentage of patients with available information.
Including Down’s syndrome.
Treatment results.
Treatment results are updated as of January 1, 1998.
Induction treatment according to protocol I could be evaluated in 217 of 218 patients: 1 RG patient was lost to follow-up shortly after diagnosis. Two hundred twelve (98%) patients achieved a CR (Table 4). Two patients died early during induction treatment: 1 RG patient with hyperleukocytosis died of cerebrovascular hemorrhage at day 6 and 1 SRG patient with Down’s syndrome died of pseudomonas septicemia and cardiac failure; 3 patients died of progressive disease. Three EG patients underwent allogeneic BMT (2 from a related and 1 from an unrelated donor).
Treatment Results of DCLSG Protocol ALL-7
| . | SRG (without protocol II) . | SRT (SRG with protocol II) . | RG . | EG . | All Patients . |
|---|---|---|---|---|---|
| No. of patients | 44 | 30 | 127 | 17 | 218 |
| No CR | 1 | 0 | 1 | 3 | 5 |
| Early death | 1 | 0 | 1 | 0 | 2 |
| No response | 0 | 0 | 0 | 3 | 3 |
| Lost to follow-up | 0 | 0 | 1 | 0 | 1 |
| CR achieved | 43 (98%) | 30 (100%) | 125 (99%) | 14 (82%) | 212 (98%) |
| Death in CR | 0 | 1 | 3 | 2 | 6 |
| Relapses | 19 | 6 | 37 | 1 | 63 |
| During treatment | 6 | 1 | 16 | 1 | 24 |
| After treatment | 13 | 5 | 21 | 0 | 39 |
| Site of relapse | |||||
| BM/blood | 12 | 4 | 26 | 1 | 43 |
| CNS | 5 | 0 | 7 | 0 | 12 |
| BM/CNS | 1 | 0 | 1 | 0 | 2 |
| BM/CNS/testis | 0 | 0 | 1 | 0 | 1 |
| BM/testis | 0 | 1 | 1 | 0 | 2 |
| Testis | 0 | 1 | 1 | 0 | 2 |
| Abdominal mass | 1 | 0 | 0 | 0 | 1 |
| Second malignancy | 0 | 0 | 1 | 1 | 2 |
| In continuous CR | 24 | 23 | 84 | 10 | 141 |
| Patients alive at last follow-up | 33 | 26 | 100 | 11 | 170 |
| Median follow-up | 87 | 71 | 80 | 89 | 79 |
| Range (mo) | 60-105 | 48-82 | 49-110 | 59-103 | 48-110 |
| EFS (% ±SE) | |||||
| At 2 yrs | 72.7 ± 6.7 | 90.0 ± 5.5 | 81.0 ± 3.5 | 63.3 ± 12.0 | 79.2 ± 2.8 |
| At 5 yrs | 54.5 ± 7.5 | 76.7 ± 7.7 | 66.6 ± 4.2 | 63.3 ± 12.0 | 65.3 ± 3.2 |
| No. event-free alive at 5 yrs | 24 | 21 | 78 | 9 | 132 |
| Survival | |||||
| At 2 yrs | 95.5 ± 3.1 | 93.3 ± 4.6 | 89.8 ± 2.7 | 62.5 ± 12.1 | 89.4 ± 2.1 |
| At 5 yrs | 75.0 ± 6.5 | 86.7 ± 6.2 | 80.2 ± 3.6 | 62.5 ± 12.1 | 78.7 ± 2.8 |
| No. alive at 5 yrs | 32 | 24 | 92 | 9 | 157 |
| . | SRG (without protocol II) . | SRT (SRG with protocol II) . | RG . | EG . | All Patients . |
|---|---|---|---|---|---|
| No. of patients | 44 | 30 | 127 | 17 | 218 |
| No CR | 1 | 0 | 1 | 3 | 5 |
| Early death | 1 | 0 | 1 | 0 | 2 |
| No response | 0 | 0 | 0 | 3 | 3 |
| Lost to follow-up | 0 | 0 | 1 | 0 | 1 |
| CR achieved | 43 (98%) | 30 (100%) | 125 (99%) | 14 (82%) | 212 (98%) |
| Death in CR | 0 | 1 | 3 | 2 | 6 |
| Relapses | 19 | 6 | 37 | 1 | 63 |
| During treatment | 6 | 1 | 16 | 1 | 24 |
| After treatment | 13 | 5 | 21 | 0 | 39 |
| Site of relapse | |||||
| BM/blood | 12 | 4 | 26 | 1 | 43 |
| CNS | 5 | 0 | 7 | 0 | 12 |
| BM/CNS | 1 | 0 | 1 | 0 | 2 |
| BM/CNS/testis | 0 | 0 | 1 | 0 | 1 |
| BM/testis | 0 | 1 | 1 | 0 | 2 |
| Testis | 0 | 1 | 1 | 0 | 2 |
| Abdominal mass | 1 | 0 | 0 | 0 | 1 |
| Second malignancy | 0 | 0 | 1 | 1 | 2 |
| In continuous CR | 24 | 23 | 84 | 10 | 141 |
| Patients alive at last follow-up | 33 | 26 | 100 | 11 | 170 |
| Median follow-up | 87 | 71 | 80 | 89 | 79 |
| Range (mo) | 60-105 | 48-82 | 49-110 | 59-103 | 48-110 |
| EFS (% ±SE) | |||||
| At 2 yrs | 72.7 ± 6.7 | 90.0 ± 5.5 | 81.0 ± 3.5 | 63.3 ± 12.0 | 79.2 ± 2.8 |
| At 5 yrs | 54.5 ± 7.5 | 76.7 ± 7.7 | 66.6 ± 4.2 | 63.3 ± 12.0 | 65.3 ± 3.2 |
| No. event-free alive at 5 yrs | 24 | 21 | 78 | 9 | 132 |
| Survival | |||||
| At 2 yrs | 95.5 ± 3.1 | 93.3 ± 4.6 | 89.8 ± 2.7 | 62.5 ± 12.1 | 89.4 ± 2.1 |
| At 5 yrs | 75.0 ± 6.5 | 86.7 ± 6.2 | 80.2 ± 3.6 | 62.5 ± 12.1 | 78.7 ± 2.8 |
| No. alive at 5 yrs | 32 | 24 | 92 | 9 | 157 |
In 14 patients, major protocol violations occurred after remission: RG treatment applied to 1 SRG patient, too early cessation of therapy (1 SRG patient), allogeneic bone marrow transplantation in 1 RG patient, treatment for relapse ALL not confirmed by DCLSG laboratory (2 RG patients), discrepancy in steroid response between DCLSG and institution (1 RG patient), protocol S refused by already randomized RG patients (4), treatment reduction because of toxicity (1 RG and 1 EG patient), and protocol E not applied (2 EG patients). One EG patient was lost for follow-up after CR.
Six patients died in first CR, 2 of them after protocol violation. The causes of death were septicemia (1 SRG and 1 RG patient), interstitial pneumonia (1 SRG patient), and complications of bone marrow transplantation (1 RG and 2 EG patients).
Sixty-three patients relapsed: 20 patients during therapy, 37 patients after therapy, and 6 patients after major treatment modifications. The site of relapse is shown in Table 4. Remarkably, no CNS relapses occurred in the SRT group (SRG patients who had received protocol II). Two patients developed a second malignancy: 1 RG patient with initial CNS disease who had received cranial radiotherapy developed thyroid carcinoma 40 months after diagnosis of ALL; 1 SRG patient developed acute myeloid leukemia (FAB-type M1) 2 years after treatment of an isolated CNS relapse with chemotherapy and craniospinal irradiation.
One hundred forty-one patients are in first CCR at last follow-up.
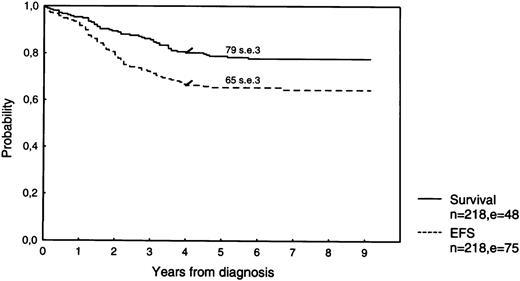
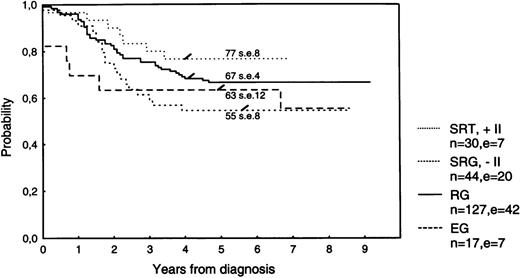
The estimated 5-year survival is 79.7% (SE 2.5%) for the total group of 259 patients, 78.7% (SE 2.8%) for the 218 protocol patients (Fig 1), and 84.9% (SE 5.7%) for the nonprotocol patients. Estimates for a 5-year EFS are 66.4% (SE 3.0%), 65.3% (SE 3.2%; Fig 1), and 72.3% (SE 7.1%), respectively. The 5-year EFS according to treatment group is 63.5% (SE 5.6%) for all 74 SRG patients, 66.6% (SE 4.2%) for RG patients, and 63.3% (SE 12.0%) for EG patients (Fig 2).
Survival and EFS of 218 newly diagnosed ALL-patients treated according to DCSLG protocol ALL-7.
Survival and EFS of 218 newly diagnosed ALL-patients treated according to DCSLG protocol ALL-7.
EFS according to risk group. Stratification after treatment with DCLSG protocol ALL-7. SRT, standard-risk group receiving protocol II; SRG, standard-risk group not receiving protocol II; RG, risk group; EG, experimental group.
EFS according to risk group. Stratification after treatment with DCLSG protocol ALL-7. SRT, standard-risk group receiving protocol II; SRG, standard-risk group not receiving protocol II; RG, risk group; EG, experimental group.
The 5-year EFS for SRG patients not receiving protocol II is 54.5% (SE 7.5%) and for SRG patients receiving protocol II is 76.7% (SE 7.7%; logrank P = .049). The 5-year survival rates are 75.0% (SE 6.5%) and 86.7% (SE 6.2%), respectively (logrank, P = .25; Table 4).
CNS Relapses in Childhood ALL: Treatment Regimens With BFM Backbone
| Study . | Patients . | CRT . | MTX(g) . | eTIT . | Reported Isolated CNS Relapse (%) . | Median Follow-Up (yrs) . | Reference . | |
|---|---|---|---|---|---|---|---|---|
| N . | %7-150 . | |||||||
| DCLSG-ALL-7 | 218 | − | 5 | − | 5.5 | 5 | 35 | |
| SRG − protocol II | 44 | 20 | − | 5 | − | 11.4 | ||
| SRG + protocol II | 30 | 14 | − | 5 | − | 0 | ||
| RG | 127 | 58 | − | 5 | − | 5.5 | ||
| EG | 17 | 8 | − | 5 | − | 0 | ||
| BFM-ALL-86 | 998 | 1.8 | 5 | 1 | ||||
| SRG − protocol II | 110 | 11 | − | 5 | − | 1.8 | ||
| SRG + protocol II | 175 | 18 | − | 5 | − | 1.1 | ||
| RG | 610 | 61 | + | 5 | − | 1.1 | ||
| EG | 103 | 10 | + | 5 | − | 6.8 | ||
| GATLA | 3 | 22 | ||||||
| MRG | 490 | − | − | 2 | − | 3.1 | ||
| INS-89 | ||||||||
| SRG | 39 | 17 | − | 5 | + | 0.0 | 3.5 | 24 |
| RG | 155 | 69 | − | 5 | + | 2.3 | ||
| AIEOP ALL-88 | 6 | 23 | ||||||
| SR | 79 | 19 | − | 5 | − | 6.3 | ||
| IR | 119 | 30 | − | 5 | + | 0.8 | ||
| HR | 198 | 50 | + | 5 | − | 7.1 | ||
| CCG 1882 | 317 | − | + | − | − | 2.3 | 5 | 26 |
| HR | 319 | − | − | − | + | 3.6 | 5 | |
| Study . | Patients . | CRT . | MTX(g) . | eTIT . | Reported Isolated CNS Relapse (%) . | Median Follow-Up (yrs) . | Reference . | |
|---|---|---|---|---|---|---|---|---|
| N . | %7-150 . | |||||||
| DCLSG-ALL-7 | 218 | − | 5 | − | 5.5 | 5 | 35 | |
| SRG − protocol II | 44 | 20 | − | 5 | − | 11.4 | ||
| SRG + protocol II | 30 | 14 | − | 5 | − | 0 | ||
| RG | 127 | 58 | − | 5 | − | 5.5 | ||
| EG | 17 | 8 | − | 5 | − | 0 | ||
| BFM-ALL-86 | 998 | 1.8 | 5 | 1 | ||||
| SRG − protocol II | 110 | 11 | − | 5 | − | 1.8 | ||
| SRG + protocol II | 175 | 18 | − | 5 | − | 1.1 | ||
| RG | 610 | 61 | + | 5 | − | 1.1 | ||
| EG | 103 | 10 | + | 5 | − | 6.8 | ||
| GATLA | 3 | 22 | ||||||
| MRG | 490 | − | − | 2 | − | 3.1 | ||
| INS-89 | ||||||||
| SRG | 39 | 17 | − | 5 | + | 0.0 | 3.5 | 24 |
| RG | 155 | 69 | − | 5 | + | 2.3 | ||
| AIEOP ALL-88 | 6 | 23 | ||||||
| SR | 79 | 19 | − | 5 | − | 6.3 | ||
| IR | 119 | 30 | − | 5 | + | 0.8 | ||
| HR | 198 | 50 | + | 5 | − | 7.1 | ||
| CCG 1882 | 317 | − | + | − | − | 2.3 | 5 | 26 |
| HR | 319 | − | − | − | + | 3.6 | 5 | |
Abbreviations: MTX (g), high-dose intravenous methotrexate (grams per square meter of body surface per dose); CRT, cranial radiotherapy; eTIT, extended triple intrathecal therapy (diadreson-F, methotrexate, cytosine-arabinoside).
Percentage of total ALL population.
The overall isolated CNS relapse rate at 5 years was 5.5%. The incidence was 11.4% in SRG patients not receiving early reinduction with protocol II, whereas no CNS relapses occured in SRG patients receiving protocol II (Table 5).
Prognostic Factors in Study DCLSG-ALL-7
| Risk Factor . | No. of Patients (218 total) . | EFS . | P Value . | |
|---|---|---|---|---|
| 5 yr (%) . | SE (%) . | |||
| Sex | ||||
| M | 123 | 63.1 | 4.4 | .40 |
| F | 95 | 68.1 | 4.8 | |
| Age (yrs) | ||||
| 1-9 | 177 | 67.0 | 3.5 | .25 |
| ≥10 | 38 | 59.5 | 8.1 | |
| WBC | ||||
| <50,000 | 172 | 66.7 | 3.6 | .16 |
| ≥50,000 | 46 | 60.1 | 7.3 | |
| Hb (mmol/L) | ||||
| <5 | 118 | 68.4 | 4.3 | .21 |
| ≥5 | 99 | 61.2 | 5.0 | |
| FAB (n = 216) | ||||
| L-1 | 166 | 64.8 | 3.7 | .40 |
| L-2 | 47 | 71.7 | 6.8 | |
| Immunophenotype (n = 197) | ||||
| C-ALL | 115 | 69.9 | 4.3 | .11 |
| Pre-B-ALL | 48 | 60.3 | 7.1 | |
| T-ALL | 34 | 57.8 | 8.6 | |
| DNA index (n = 124)6-150 | ||||
| <1.16 | 88 | 70.4 | 4.9 | .25 |
| ≥1.16 | 36 | 80.6 | 6.6 | |
| Ploidy (n = 109)6-150 | ||||
| Diploid | 25 | 80.0 | 8.0 | .16 |
| Hyperdiploid 47-50 | 12 | 41.7 | 14.2 | |
| Hyperdiploid >50 chr | 44 | 72.1 | 6.8 | |
| Pseudodiploid | 28 | 64.1 | 9.1 | |
| Chromosome structural anomalies (n = 124) | ||||
| Absent | 45 | 79.6 | 6.1 | .019 |
| Present | 79 | 59.0 | 5.6 | |
| Prednisone response (n = 210) | ||||
| <1,000 blasts/μL | 198 | 64.4 | 3.4 | .86 |
| ≥1,000 blasts/μL | 12 | 74.4 | 12.7 | |
| Risk Factor . | No. of Patients (218 total) . | EFS . | P Value . | |
|---|---|---|---|---|
| 5 yr (%) . | SE (%) . | |||
| Sex | ||||
| M | 123 | 63.1 | 4.4 | .40 |
| F | 95 | 68.1 | 4.8 | |
| Age (yrs) | ||||
| 1-9 | 177 | 67.0 | 3.5 | .25 |
| ≥10 | 38 | 59.5 | 8.1 | |
| WBC | ||||
| <50,000 | 172 | 66.7 | 3.6 | .16 |
| ≥50,000 | 46 | 60.1 | 7.3 | |
| Hb (mmol/L) | ||||
| <5 | 118 | 68.4 | 4.3 | .21 |
| ≥5 | 99 | 61.2 | 5.0 | |
| FAB (n = 216) | ||||
| L-1 | 166 | 64.8 | 3.7 | .40 |
| L-2 | 47 | 71.7 | 6.8 | |
| Immunophenotype (n = 197) | ||||
| C-ALL | 115 | 69.9 | 4.3 | .11 |
| Pre-B-ALL | 48 | 60.3 | 7.1 | |
| T-ALL | 34 | 57.8 | 8.6 | |
| DNA index (n = 124)6-150 | ||||
| <1.16 | 88 | 70.4 | 4.9 | .25 |
| ≥1.16 | 36 | 80.6 | 6.6 | |
| Ploidy (n = 109)6-150 | ||||
| Diploid | 25 | 80.0 | 8.0 | .16 |
| Hyperdiploid 47-50 | 12 | 41.7 | 14.2 | |
| Hyperdiploid >50 chr | 44 | 72.1 | 6.8 | |
| Pseudodiploid | 28 | 64.1 | 9.1 | |
| Chromosome structural anomalies (n = 124) | ||||
| Absent | 45 | 79.6 | 6.1 | .019 |
| Present | 79 | 59.0 | 5.6 | |
| Prednisone response (n = 210) | ||||
| <1,000 blasts/μL | 198 | 64.4 | 3.4 | .86 |
| ≥1,000 blasts/μL | 12 | 74.4 | 12.7 | |
Only B-progenitor ALL categories.
Randomized study on late intensification with protocol S for RG patients.
Of 127 RG patients, 117 were eligible for randomization; 10 patients could not be randomized for the following reasons: death during induction (1), lost to follow-up (1), relapse (4), death in CR (2), and major protocol violations (2). Informed consent was obtained from 51 (44%) of 117 eligible patients: 25 were randomized into group 1 (without protocol S) and 26 into group 2 (with protocol S). Patient and disease characteristics did not significantly differ between randomized and nonrandomized patients or between RG-1 and RG-2 patients. The estimated 5-year EFS, based on randomization group, is 76.0% (SE 8.5%) for RG-1 patients and 65.4% (SE 9.3%) for RG-2 patients (P = .34) and 69.6% (SE 4.8%) for nonrandomized RG patients (P = .20). Analysis of the results according to whether protocol S was actually applied or not showed no significant difference in 5-year EFS of 64% (SE 10%) for 25 patients receiving protocol S and 72% (SE 5%) for 92 patients not receiving protocol S.
Prognostic factor analysis.
None of the factors tested proved to be of significant influence on the outcome of treatment except for chromosomal anomalies, but these characteristics were determined in only 62% of the patients (Table 6). Because of the few significant or marginally significant factors in the univariate analysis, a multivariate Cox’s regression analysis is not reported.
Comparison of DCLSG ALL-7 and ALL-BFM-86.
No significant differences between DCLSG ALL-7 and ALL-BFM-86 patients were found in the distribution of sex, leukemic cell mass (RF), WBC, DNA index, immunophenotype, prednisone response at day 8, patients with Down’s syndrome, or initial CNS involvement (data not shown). However, the following differences were observed between the two study populations.
(1) In the BFM study, patients ≤18 years of age at diagnosis were eligible, but in DCLSG ALL-7, only patients ≤15 years of age were eligible. The proportion of children ≥14 years of age is higher (68/998 [7%]) in the BFM study than in the DCLSG study (3/218 [1%]). (2) For SRG patients, the proportion of patients not receiving protocol II is significantly higher in the DCLSG ALL-7 study (44/74 [59%]) than in the ALL-BFM-86 study (110/285 [39%]). (3) During the study ALL-BFM-86, the total treatment duration was changed from 18 to 24 months, but not so in DCLSG ALL-7. Of 998 patients entered into ALL-BFM-86, 143 were treated for 18 months and 855 were treated for 24 months.1 In the DCLSG ALL-7 study, the total treatment duration was 18 months for all 218 patients. (4) Cranial irradiation was applied as CNS prophylaxis to all the BFM RG and EG patients; in the DCLSG study, it was applied only to patients with initial CNS involvement (n = 5).
Keeping in mind these differences, several comparisons have been performed. EFS at 5 years was 73% (SE 1%) for the 998 ALL-BFM-86 patients and 65.3% (SE 3.2%) for the 218 DCLSG patients (P = .021, Z-test). After exclusion of SRG patients not receiving protocol II (110 patients in ALL-BFM-86 and 44 patients in DCLSG), the 5-year EFS is 74% (SE 1.5%) and 68.0% (SE 3.6%), respectively (P = .12, Z-test). The 5-year EFS of 143 consecutive ALL-BFM-86 patients treated for 18 months was 67% (SE 4%), compared with 64.6% (SE 4.0%) for the first 143 patients treated according to the DCLSG ALL-7 protocol (P = .67, Z-test).
To evaluate the role of cranial irradiation, the treatment results of the 127 RG and 17 EG patients treated according to the DCLSG protocol ALL-7 were compared with those of 90 RG and 24 EG patients treated for 18 months according to the ALL-BFM-86 protocol. The 5-year EFS was 66.6% (SE 4.2%) for DCLSG RG patients and 73% (SE 5%) for BFM RG patients (P = .33), and 63.3% (SE 12.0%) and 50% (SE 10%), respectively, for EG patients (P = .40, Z-test).
To rule out any influence of the patients 16 years of age and older in the BFM study, these analyses were also performed excluding this older age group. No difference was found between the 5-year EFS of 89 DCLSG RG patients (68.2%, SE 5.0%) and 89 BFM RG patients (72.5%, SE 4.8%; logrank, P = .77) or between the 5-year EFS of 13 DCLSG (59.8%, SE 14.0%) and 23 BFM EG patients (52.2%, SE 10.4%; logrank,P = .99; Fig 3).
EFS of patients stratified into RG or EG, ≤15 years of age at diagnosis, and treated for 18 months according to protocol ALL-BFM-86 or DCLSG ALL-7.
EFS of patients stratified into RG or EG, ≤15 years of age at diagnosis, and treated for 18 months according to protocol ALL-BFM-86 or DCLSG ALL-7.
Within this same subgroup the 5-year EFS of T-ALL patients in the ALL-BFM-86 (n = 17, irradiated) and in the DCLSG ALL-7 (n = 26, nonirradiated) was 65% (±12) and 60% (±10%), respectively (logrank, P = .71). In the DCLSG ALL-7 study, 3 of 6 T-ALL prednisone good responders (PGR) with WBC counts greater than 100 × 109/L relapsed and 7 of 20 T-ALL-PGR patients with WBC counts ≤100 × 109/L relapsed.
DISCUSSION
The DCLSG ALL-7 study has confirmed the results of the ALL BFM-86 study and has shown that, in the context of BFM-oriented treatment, (1) delayed reinduction treatment (protocol II) is required even for children with low leukemic cell mass at diagnosis and (2) late intensification (protocol S) does not improve the outcome for children with high(er) tumor burden (RG patients).
The 5-year EFS of 218 protocol ALL-7-patients significantly differed from that of 998 ALL BFM-86 protocol patients (65.3% [SE 3.2%] and 73% [SE 1%], respectively; Z-test, P = .02).1However, restricting the analysis to similar patient groups, no significant differences in 5-year EFS rates could be found: 64.6% (SE 4.0%) and 67% (SE 4%), respectively (P = .67, Z-test).
Cranial irradiation (18-24 Gy) is highly effective in preventing CNS relapse, but also adds late adverse side effects on intellectual, psychomotor, and neuroendocrine functioning, especially in young children. In addition, there is an enhanced risk for developing a second tumor.
The results of the ALL BFM-81 study had already demonstrated that cranial irradiation can safely be substituted by systemic medium-dose MTX and intrathecal chemotherapy in ALL patients with low leukemic cell mass at diagnosis.18
The results of the population-based, multicenter DCLSG ALL-7 study show that cranial radiotherapy is not needed in patients with ALL treated with BFM-oriented treatment.
Possible exceptions are children with initial CNS involvement and T-ALL patients with a high leukemic cell mass at diagnosis (WBC ≥100 × 109/L)19; unfortunately, the number of T-ALL patients in the ALL-7 study is too small to draw any conclusions on the latter subject.
Remarkably, no isolated CNS relapse occurred in 30 ALL-7 SRG patients receiving protocol II, compared with 5 in 44 patients not receiving protocol II. This difference might be attributed to the administration of dexamethasone during protocol II, which penetrates well in the CSF and has a higher antileukemic cell activity.20 21
Cranial irradiation (12 Gy) was still applied in MRG and HRG patients in protocol ALL-BFM-90 and is still applied in HRG patients in the current protocol ALL-BFM-95.22
However, other studies using BFM-oriented treatment have spared children with ALL the exposure to cranial irradiation by applying intermediate- or high-dose MTX infusions and intrathecal chemotherapy (Table 5). The results of the GATLA protocol for medium-risk patients are consistent with those of the DCLSG ALL-7 study.23 The results of the Italian AIEOP protocol-8824 and those of the Israeli ISN-89 study25 suggest that triple intrathecal chemotherapy extended throughout maintenance treatment, as originally applied in the POG ALinC 13 study,26 might further reduce the incidence of CNS relapse in a large proportion of children with ALL. In the CCG 1882-modified BFM study, no significant difference was observed in the incidence of isolated CNS relapse in children with high-risk ALL (1 to 9 years of age and with a WBC count ≥50 × 109/L; or ≥10 years of age, excluding those with lymphomatous features) and rapid early response (≤25% bone marrow blasts on day 7), randomized to receive either intrathecal MTX and cranial irradiation or intensive ith MTX alone.27
Systemic medium- or high-dose MTX and extended intrathecal chemotherapy are apparently as effective in preventing CNS relapse as cranial irradiation, but long-term side effects are still to be evaluated. Statistically significant decreases in overall and verbal intelligence quotients and in arithmetic achievement were found in 16 (62%) of 26 patients treated with parenteral MTX for CNS prophylaxis only.28 Abnormal MRI and CCT scans were seen in 15 (38%) of 39 patients who had received intrathecal MTX and systemic medium- to high-dose MTX.
In 20% of these 39 patients, widening of sulci or ventricles, indicating brain atrophy, were observed; no possible signs of leukoencephalopathy or calcifications were observed.29
Currently, late side effects of CNS prophylaxis with chemotherapy only seem to be less severe than those of cranial irradiation, but long-term follow-up is needed to establish late sequelae of this treatment modality.
In contrast to the BFM-86 study, sex, WBC, Hb value, FAB type, immunophenotype, DNA index, and chromosome ploidy (only relevant for B-progenitor ALL) were not of significant prognostic value in the DCLSG ALL-7 study, but this may partly be due to the very intensive therapy for all patients and to small sample size.
Many investigators have found that DNA ploidy and structural chromosomal abnormalities are independent prognostic factors, but this significance can be lost if therapy changes.30,31 Also, when patients with translocation t(9;22) (N = 2) and t(4;11) (N = 4) were excluded from such analysis, differences in EFS disappeared between patients with and without leukemic cell chromosomal structural abnormalities.32 However, if patients with leukemic karyotype t(9;22) and t(4;11) are excluded in the DCLSG ALL 7 study, the significant difference in 5-year EFS rate for patients with (N = 73) and without (N = 45) chromosomal structural abnormalities persists: 61.1% (SE 5.7%) and 79.5% (SE 6.1%), respectively (logrank,P = .037).
The persistence of peripheral blood blasts after 1 week of (multiagent) chemotherapy was shown to confer a poor prognosis in St Jude Total Therapy Study XI33 and in study ALL-BFM-86.1 In DCLSG ALL-7, the persistence of peripheral blood blasts after 7 days of monotherapy with prednisone and 1 intrathecal dose of MTX and prednisolone had no prognostic significance (P = .86). However, the proportions of patients with circulating leukemic blasts in the peripheral blood at diagnosis and after 1 week of treatment were substantially different in the St Jude study and the DCLSG study: 84% versus 97% at diagnosis and 14% versus 73% after 1 week, respectively. The proportion of prednisone poor responders was not significantly different in the ALL-BFM-86 and the DCLSG ALL-7 study (9.5% and 6%, respectively; P = .08).
Drug sensitivity testing using the MTT assay has been performed in a minority (27%) of the patients; the results have been published previously.34
The duration of treatment in the DCLSG ALL-7 study (18 months) was shorter than in most treatment protocols for children with ALL (2 to 5 years).
The optimal length of treatment is not well established. A meta-analysis of 42 clinical trials, involving 12,000 randomized children, could not show a difference between 5 years and 3 years of maintenance treatment; also, the risk of relapse by the addition of a third year of maintenance was counterbalanced by an increase in the risk of death during remission.35 On the other hand, the results of the randomized clinical trials ALL-BFM-81 and 83, comparing 18 versus 24 months of total therapy duration, showed an advantage for a therapy duration of 24 months.18
Therefore, the outcome of the DCLSG-ALL-7 study might have improved if the total duration of treatment had been 24 months instead of 18 months.
Recently developed, more sensitive PCR-based techniques for the detection of minimal residual disease (MRD) at several time points during treatment may contribute to determine the optimal length of treatment for individual future patients.36
Before the introduction of the BFM-oriented treatment, children with ALL in The Netherlands were treated according to protocols based on Pinkel’s Total Therapy.37,38 In the DCLSG ALL-6 protocol (accrual period 1984 to 1988) for children with ALL-NHR, defined as age of 0 to 15 years, an initial WBC count of less than 50 × 109/L, the absence of a mediastinal mass and/or cerebromeningeal leukemia at diagnosis (comprising 71% of all ALL patients), the combination of intravenous medium-dose MTX (2 g/m2, 3×), extended triple intrathecal chemotherapy during the first year of maintenance treatment, the substitution of prednison by dexamethasone during induction and biweekly pulses, and a total duration of treatment of 24 months has proved to be highly effective, especially in preventing CNS relapse (1.1%), with an 8-year EFS rate of 81% ± 3%.2
The 5-year EFS rate of children with ALL-NHR characteristics in the DCLSG ALL-7 study using DCLSG-ALL-6 criteria (n = 116; excluding SRG patients not receiving protocol II) was 70.7% ± 4.2%, compared with 82.6% ± 2.7% after treatment with protocol ALL-6 (P= .023; logrank test).
We conclude that children with newly diagnosed ALL and treated with BFM-oriented treatment do not need cranial radiotherapy for CNS prophylaxis, with the possible exception of T-ALL patients with a high WBC count. Also, children with low leukemic cell mass at diagnosis do require delayed reinduction with protocol II, including dexamethasone, and this therapeutic strategy significantly reduces the CNS relapse rate. The omission of cranial radiotherapy will most likely reduce the long-term adverse effects of treatment. It is not clear yet if extended intrathecal treatment during mainenance therapy is necessary when the described treatment components are being used. Prospective studies of neuropsychologic and the treatment-related morbidity are needed.
Although different treatment strategies may give excellent outcome, the results of the protocols DCLSG ALL-6 and ALL-7 have prompted the DCLSG to reintroduce ALL-6 treatment for children with ALL-NHR in The Netherlands. For ALL-HR patients (all other patients with ALL), the ALL-6 backbone has been intensified using the results of a promising institutional pilot study (1984 to 1988) of the Pediatric Oncology Center Groningen, but without cranial irradiation (DCLSG protocol ALL-9).
APPENDIX I
DCLSG Board Members 1988.
W.A. Kamps, J.A. Rammeloo, K. Hählen, E.F. van Leeuwen, F.A.E. Nabben, A. Postma, E.J.M. Sjamsoedin-Visser, E.Th. van ‘t Veer-Korthof, G.A.M. de Vaan, F.C. de Waal, and R.S. Weening.
NWKGC Members 1988.
R. Slater (Amsterdam), A. Hagemeijer (Rotterdam), E. van den Berg-de Ruiter (Groningen), A. Hamers (Maastricht), C.G. Beverstock (Leiden), A. Geurts van Kessel (Nijmegen), S.L. Bhola (Utrecht), W. Kroes (Amsterdam), and M. van den Blij-Philipsen (Veldhoven).
APPENDIX II Treatment According to DCLSG Protocol ALL-7
| Drug . | Dose . | Applied on Days* . |
|---|---|---|
| Protocol I | ||
| Prednisone (prephase orally) | 60 mg/m2 | −7-0 |
| Prednisone (orally) | 60 mg/m2 | 1-28 |
| Vincristine (IV) | 1.5 mg/m2 (max. 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 40 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (IV) | 10,000 IU/m2 | 19, 22, 25, 28, 31, 34, 37, 40 |
| Cyclophosphamide (IV) | 1,000 mg/m2 | 43, 71 |
| Cytosine arabinoside | 75 mg/m2 | 45-48, 52-55, 59-62, 66-69 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 43-70 |
| Methotrexate (ith) | According to age* | −7, 1, 45, 59 |
| Protocol M | ||
| 6-Mercaptopurine (orally) | 25 mg/m2 | 1-56 |
| Methotrexate (24-h infusion)† | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (ith) | According to age* | 8, 22, 36, 50 |
| Protocol E | ||
| Prednisone (orally) | 100 mg/m2 | 1-7, 15-21, 29-35, 43-49 |
| Cytosine arabinoside | 2,000 mg/m2 every 12 h | 1, 2, 29, 30 |
| Ifosfamide (1-h infusion) | 1,000 mg/m2 every 12 h | 15, 16, 43, 44 |
| Mitoxantrone (IV) | 10 mg/m2 | 1, 15, 29, 43 |
| Methotrexate (24-h infusion) | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (ith) | According to age* | 8, 22, 36, 50 |
| Protocol II | ||
| Dexamethasone (orally) | 10 mg/m2 | 1-21 |
| Vincristine (IV) | 1.5 mg/m2 (max. 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (IV) | 10,000 IU/m2 | 8, 11, 15, 18 |
| Cyclophosphamide (IV) | 1,000 mg/m2 | 36 |
| Cytosine arabinoside | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanin (orally) | 60 mg/m2 | 36-49 |
| Methotrexate (ith) | According to age* | 38, 45 |
| Protocol S (late intensification) | ||
| Prednisone (orally) | 100 mg/m2 | 1-7, 15-21 |
| Vindesine (IV) | 3 mg/m2 (max. 5 mg) | 1, 8, 15, 22 |
| Teniposide (IV) | 150 mg/m2 | 1, 8, 15, 22 |
| Ifosfamide (IV) | 1,000 mg/m2 | 1, 2 |
| Cytosine arabinoside (3-h infusion) | 2,000 mg/m2 every 12 h | 15, 16 |
| Maintenance treatment up to 18 mo after diagnosis: | 6-Mercaptopurine 50 mg/m2 daily (orally) and methotrexate 20 mg/m2 weekly (orally) |
| Drug . | Dose . | Applied on Days* . |
|---|---|---|
| Protocol I | ||
| Prednisone (prephase orally) | 60 mg/m2 | −7-0 |
| Prednisone (orally) | 60 mg/m2 | 1-28 |
| Vincristine (IV) | 1.5 mg/m2 (max. 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 40 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (IV) | 10,000 IU/m2 | 19, 22, 25, 28, 31, 34, 37, 40 |
| Cyclophosphamide (IV) | 1,000 mg/m2 | 43, 71 |
| Cytosine arabinoside | 75 mg/m2 | 45-48, 52-55, 59-62, 66-69 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 43-70 |
| Methotrexate (ith) | According to age* | −7, 1, 45, 59 |
| Protocol M | ||
| 6-Mercaptopurine (orally) | 25 mg/m2 | 1-56 |
| Methotrexate (24-h infusion)† | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (ith) | According to age* | 8, 22, 36, 50 |
| Protocol E | ||
| Prednisone (orally) | 100 mg/m2 | 1-7, 15-21, 29-35, 43-49 |
| Cytosine arabinoside | 2,000 mg/m2 every 12 h | 1, 2, 29, 30 |
| Ifosfamide (1-h infusion) | 1,000 mg/m2 every 12 h | 15, 16, 43, 44 |
| Mitoxantrone (IV) | 10 mg/m2 | 1, 15, 29, 43 |
| Methotrexate (24-h infusion) | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (ith) | According to age* | 8, 22, 36, 50 |
| Protocol II | ||
| Dexamethasone (orally) | 10 mg/m2 | 1-21 |
| Vincristine (IV) | 1.5 mg/m2 (max. 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| L-Asparaginase (IV) | 10,000 IU/m2 | 8, 11, 15, 18 |
| Cyclophosphamide (IV) | 1,000 mg/m2 | 36 |
| Cytosine arabinoside | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanin (orally) | 60 mg/m2 | 36-49 |
| Methotrexate (ith) | According to age* | 38, 45 |
| Protocol S (late intensification) | ||
| Prednisone (orally) | 100 mg/m2 | 1-7, 15-21 |
| Vindesine (IV) | 3 mg/m2 (max. 5 mg) | 1, 8, 15, 22 |
| Teniposide (IV) | 150 mg/m2 | 1, 8, 15, 22 |
| Ifosfamide (IV) | 1,000 mg/m2 | 1, 2 |
| Cytosine arabinoside (3-h infusion) | 2,000 mg/m2 every 12 h | 15, 16 |
| Maintenance treatment up to 18 mo after diagnosis: | 6-Mercaptopurine 50 mg/m2 daily (orally) and methotrexate 20 mg/m2 weekly (orally) |
Abbreviations: ith, intrathecally; IV, intravenously; IU, international units.
Less than 1 year: 6 mg; 1 year: 8 mg; 2 years: 10 mg; ≥3 years: 12 mg.
Leukovorin rescue: 36 to 72 hours after the start of MTX infusion: 15 mg/m2 leukovorin, every 3 hours; IV (6×) and orally or IV (4×), respectively.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to W.A. Kamps, MD, PhD, Dutch Childhood Leukemia Study Group, PO Box 43515, 2504 AM The Hague, The Netherlands; e-mail: snwlk@wxs.nl.