Hemopoiesis is regulated by the complex interplay between the bone marrow microenvironment and hemopoietic stem cells and progenitors. The local production of cytokines plays a critical role in this process. Using long-term bone marrow cultures, we show here that monoclonal antibodies directed against the CD44 v4 and CD44 v6 epitopes stimulate myelopoiesis (CD44 v4 and CD44 v6) and lymphopoiesis (CD44 v6). In the bone marrow cell population, CD44 v4 and CD44 v6 epitopes are found virtually exclusively on double-positive bone marrow macrophages. The anti-CD44 v4 and v6 antibodies act on bone marrow macrophages to stimulate granulocyte-macrophage colony-stimulating factor (GM-CSF) production (v4 and v6) and interleukin-6 (IL-6) production (v6). This profile of cytokine production explains the differential stimulation of hemopoiesis by the 2 antibodies. We suggest that the antibodies mimic ligand(s) that stimulate GM-CSF or IL-6 production by bone marrow–derived macrophages by binding to CD44 family members that bear CD44 v4 and CD44 v6 epitopes on these cells.
Introduction
Differentiation of hemopoietic cells is regulated by the bone marrow microenvironment, which consists of extracellular matrix, cytokines, and accessory cells. A number of adhesion molecules that trigger cell–cell interactions and cell–matrix interactions are expressed on cells of the hemopoietic microenvironment, as well as on hemopoietic stem/progenitor cells (HSPCs; reviewed by Quesenberry1 and Chabannon and Torok-Storb2). Members of the CD44 adhesion protein family are expressed on both cells of the hemopoietic microenvironment and HSPCs.3,4 The transmembrane proteins of the CD44 family are composed of a common N-terminal extracellular portion, a transmembrane domain, and a cytoplasmic tail. They differ in the extracellular domain by the incorporation or exclusion of amino acid stretches encoded by at least 10 exons that are subjected to alternative splicing (for recent reviews, see Naor et al5 and Ponta et al6). Although still ill understood, it is likely that different members of the CD44 protein family have some functions in common but differ in others. They play decisive roles not only in a variety of physiologic conditions, such as lymphocyte trafficking, limb development, axon growth, and wound healing, but also in pathologic conditions, such as tumorigenesis, metastasis formation, and autoimmune diseases.5 6
An involvement of CD44 in hemopoiesis has been demonstrated by the use of CD44-specific antibodies. In long-term bone marrow cultures (LTBMCs) obtained from different species, some antibodies inhibited hemopoiesis,7,8 whereas others enhanced hemopoiesis.9,10 Activation or inhibition depended on the epitope recognized by the antibodies, but all antibodies used in these studies bound to extracellular sequences common to all CD44 proteins (pan-CD44 antibodies). Therefore, these antibodies did not allow the discrimination between contributions to hemopoiesis made by the CD44 standard protein (CD44s), which lacks variably spliced exon v1-v10 sequences, and CD44 variants (CD44v), containing variant exon sequences. Recently, we have used human variant exon–specific and pan-specific CD44 antibodies and have demonstrated that they block different stages of myelopoiesis in LTBMCs.11 We showed that an early stage of myelopoiesis prior to cobblestone area formation is inhibited by the pan-CD44 antibody, whereas the CD44 variant–specific antibody interfered with myelopoiesis after commitment of the cells to the myeloid lineage.
To gain insight into the mechanism of action of CD44 in both myelopoiesis and lymphopoiesis, we expanded our study to murine LTBMCs. We used antibodies directed against 3 CD44 variant exon–specific epitopes. An antibody recognizing an epitope on sequences of variant exon v7 had no influence on myelopoiesis and lymphopoiesis. Interestingly, the other antibodies had activating properties. The v4-specific antibody and the v6-specific antibody increased myelopoiesis. Furthermore, the v6-specific antibody in addition augmented lymphopoiesis. The antibodies stimulated an increase in production of the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-6 (IL-6) in bone marrow macrophages, suggesting a mechanism for their action.
Materials and methods
Mice
(C57Bl/6 × DBA)F1 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were kept under specific pathogen-free conditions. Mice were used for experiments at the age of 8 to 12 weeks. Where indicated, mice were irradiated with 8 Gy using a whole-body irradiation chamber with a 67Cs source.
LTBMCs
Mice were killed by cervical dislocation, femurs and tibias were removed, and bone marrow cells were flushed out of the bones with phosphate-buffered saline (PBS) containing 5% fetal calf serum (FCS) using a 21-gauge needle. For myeloid cultures, bone marrow cells at a concentration of 106/mL were cultured in 6-well plates in Dulbecco modified Eagle medium (DMEM) supplemented with 20% horse serum (Linaris GmbH, Wertheim-Bettinger, Germany) and 10−6 M hydrocortisone sodium salt.12 Cultures were incubated at 33°C in 5% CO2 in air and fed weekly by exchanging half of the culturing medium. Lymphoid cultures were prepared as described.13 Briefly, bone marrow cells were incubated in RPMI 1640 medium (Gibco Laboratories, NY) supplemented with 2 mM L-glutamine, 5 × 10−5 M 2-mercaptoethanol, and 5% FCS (StemCell Technologies, Vancouver, BC, Canada) at 37°C and 5% CO2. Antibodies were added where indicated.
Colony-forming unit assay
Bone marrow cells or nonadherent cells from LTBMCs were plated in 24-well plates in methylcellulose (StemCell Technologies) supplemented with 20% FCS and 2 mM glutamine and cultured at 37°C in a 5% CO2 atmosphere14 at a concentration of 104 cells/mL. For culturing of granulocyte-macrophage colony-forming units (CFUs), we added 10 ng/mL GM-CSF. For growth of macrophage CFUs, we cultured cells in the presence of 15% conditioned medium from L929 cells14 as a source of macrophage colony-stimulating factor (M-CSF). Conditioned medium from Wehi-3B cells was used as a source of IL-3 for culturing early myeloid progenitors. Pre–B-cell CFUs were cultured in the presence of 15% supernatant of an IL-7–transfected cell line (kindly provided by Dr Karasuyama, Basel Institute for Immunology, Switzerland) and 5 × 10−4 M 2-mercaptoethanol.
Long-term culture-initiating cell assay
The assay was performed according to the protocol provided by Stem Cell Technologies. Cells from the adherent layer of LTBMCs were plated in DMEM supplemented with 20% horse serum (StemCell Technologies) and hydrocortisone (10−6 M; Sigma, Munich, Germany) in limiting dilutions into wells containing S17 bone marrow stromal cells. Cultures were fed weekly, and the numbers of wells containing colonies were evaluated after 2 weeks.
Spleen CFU assay
Cells were injected into lethally irradiated mice (4 × 104 cells/mouse) to determine spleen CFUs. After 8 days, animals were killed and the spleens were collected and fixed in Tellesnicky solution. Colonies were calculated after several hours of fixation.
Bone marrow–derived macrophage cultures
Bone marrow–derived macrophages were cultured according to a protocol described previously.15 Bone marrow cells at a concentration of 1 × 106 cells/mL were cultivated in DMEM containing 2 mM glutamine, 0.37% (wt/vol) NaHCO3, 10% (vol/vol) heat-inactivated FCS, and 10% (vol/vol) L929 cell–conditioned medium as a source of M-CSF. Cultures were maintained at 37°C in a 5% CO2 atmosphere for 8 days. A total of 99% of the cells in the culture dish were phagocytic for latex particles.
Cell purification
To purify the CD44+ and CD44− cell populations by panning, we incubated the bone marrow cell suspension for 30 minutes on plastic dishes precoated with CD44-specific antibodies (10 μg/mL, 12 hours at 4°C). Thereafter, bound and unbound cells were collected and assayed immediately. For fluorescence-activated cell sorting (FACS), the bone marrow cells were incubated with CD44 v4- or v6-specific antibodies, followed by incubation with secondary anti-rat phycoerythrin (PE)–labeled antibodies. Sorting was performed on a FACStar Plus flow cytometer (Becton Dickinson, Heidelberg, Germany). For magnetic cell sorting (MACS), the bone marrow cell suspension was incubated with CD44 v4- or v6-specific antibodies, followed by incubation with secondary anti-rat antibodies labeled with magnetic beads. After 30 minutes of incubation at 4°C and washing, cells were applied to a magnetic column. Thereafter, the bound cells were released and assayed immediately.
HSPCs were enriched by panning. A bone marrow cell suspension (106 cells/mL) was incubated for 2 hours at 4°C on plastic dishes precoated with lineage-specific antibodies: CD4, CD8a, CD11b, CD11c, CD45R, and er-119 (Miltenyi Biotec GmbH, Germany). Unbound cells were harvested, washed twice with PBS plus 5% FCS, and assayed.
Antibodies
The pan-CD44 hybridoma cell line IM-7 (rat IgG2b) was obtained from American Type Culture Collection (cat. no. TIB237; Rockville, MD). The anti-murine CD44 v4 and v6 monoclonal antibodies 10D1 and 9A4 have been described previously.16 The murine CD44 v7-specific monoclonal antibody 3E10 was generated and characterized as before16 using a rat immunized with a glutathione-S–transferase (GST)–murine CD44 v6-v7 fusion protein. Antibodies were purified by standard procedures from hybridoma-conditioned media by passage over protein G–Sepharose 4B. All preparations were proven to be endotoxin free. Where indicated, F(ab′)2 fragments were obtained by pepsin digestion using an ImmunoPure F(ab′)2 preparation kit (Pierce, Rockford, IL). Epitope mapping was performed using custom PepSpots filters containing 12-mer peptides scanning the CD44 variant exon–encoded protein sequence concerned (Jerini Biotools GmbH, Berlin, Germany). The filters were incubated with the appropriate antibody followed by a horseradish peroxidase–labeled secondary antibody, according to the manufacturer's instructions. Antibody binding to the filters was detected using an enhanced chemiluminescence (ECL) kit (Amersham, Freiburg, Germany).
Flow cytometry
The monoclonal antibodies 10D1 and 9A4 were labeled withN-hydroxysuccinimide (NHS)–fluorescein (Pierce) according to the manufacturer's instructions. PE-labeled mouse anti-rat IgG and PE-labeled anti-mouse CD11b/CD18, as well as isotype control antibodies, were obtained from Becton Dickinson Pharmingen (Heidelberg, Germany). For flow cytometry, 5 × 105 cells were incubated with fluorescein isothiocyanate (FITC)– or PE-labeled antibodies at a concentration of 10 μg/mL at 4°C for 30 minutes and washed twice with FACS buffer (PBS, 3% FCS, 0.01% NaN3). Fluorescence was analyzed on a FACStar Plus flow cytometer (Becton Dickinson).
Enzyme-linked immunosorbent assay
Conditioned medium (CM) samples from LTBMCs or bone marrow–derived macrophage cultures (BMDMCs) treated with CD44-specific monoclonal antibodies or with F(ab′)2 fragments of monoclonal antibodies were kept at −80°C until use. CM samples were tested by enzyme-linked immunosorbent assay (ELISA; Endogen, Woburn, MA) for GM-CSF, M-CSF, IL-3, IL-4, IL-6, and IL-10 according to the instructions of the manufacturer.
Reverse transcriptase–polymerase chain reaction and exon-specific PCR analysis
Bone marrow–derived macrophages were isolated (see above), and RNA was prepared as described.17 Reverse transcriptase–polymerase chain reaction (RT-PCR) and exon-specific PCR analysis were performed as described.18 The primers used for amplification of CD44 variant exons were those described previously18 for the amplification of rat sequences.
Results
CD44 variant–specific antibodies stimulate hemopoiesis
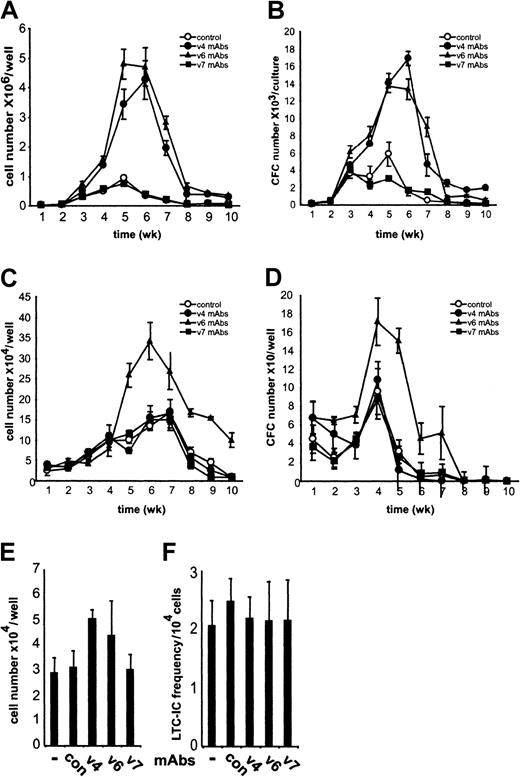
To investigate the contribution of CD44 variant proteins to murine hemopoiesis, we established myeloid and lymphoid LTBMCs in the presence of CD44 variant–specific antibodies. The generation of nonadherent cells and progenitor cells was compared with that in untreated LTBMCs (Figure 1). The production of nonadherent cells was strongly repressed in myeloid and lymphoid LTBMCs by treatment with the pan-CD44 antibody IM7 (not shown), in agreement with previous publications.7 8 The treatment of both myeloid and lymphoid LTBMCs with CD44 v6-specific antibodies dramatically increased the number of nonadherent cells determined during 10 weeks of culture (Figure 1A,C). CD44 exon v4–specific antibodies exhibited stimulatory activity only in myeloid LTBMCs (Figure 1A). The elevated number of nonadherent cells was not due to increased detachment of adherent cells because in LTBMCs at 5 weeks, when the stimulation by the antibodies was maximal, the number of adherent cells in CD44 v4- or v6-treated cultures was not reduced (Figure 1E). The number and morphology of cobblestone areas were also unchanged in CD44 v4- and v6-treated LTBMCs as compared with controls (not shown). Furthermore, the number of long-term culture-initiating cells (LTC-ICs) in the adherent layer of LTBMCs after 5 weeks was similar between CD44 v4- and v6-treated cultures and controls (Figure 1F). CD44 exon v7–specific antibodies did not affect hemopoiesis when applied to either myeloid or lymphoid LTBMCs (Figure 1A,C).
CD44 variant–specific antibodies stimulate myelopoiesis and lymphopoiesis.
Myeloid (A,B) or lymphoid (C,D) LTBMCs were grown in the absence or presence of CD44 exon v4-, v6-, or v7-specific antibodies (10 μg/mL). (A,C) Nonadherent cells were harvested weekly during feedings of 3 separate cultures; numbers were counted and expressed as mean ± SD. (B,D) Nonadherent cells harvested from LTBMCs were plated at a concentration of 104 cells/mL in 4 separate methylcellulose cultures supplemented with IL-3. After 7 days in culture, the number of colonies was counted, recalculated for the total number of cells obtained originally from each LTBMC culture well, and expressed as mean number of colonies per LTBMC culture well ± SD. The experiment was repeated 4 times with similar results. (E,F) Nonadherent cells were washed off from myeloid LTBMCs cultured for 5 weeks in the presence or absence of antibodies. The numbers of nonadherent cells were similar to those obtained in A. Adherent cells were harvested (by trypsinization), counted (E), and tested for LTC-ICs in a 2-week limiting dilution assay (F). The data shown are the means (± SD) of 2 experiments.
CD44 variant–specific antibodies stimulate myelopoiesis and lymphopoiesis.
Myeloid (A,B) or lymphoid (C,D) LTBMCs were grown in the absence or presence of CD44 exon v4-, v6-, or v7-specific antibodies (10 μg/mL). (A,C) Nonadherent cells were harvested weekly during feedings of 3 separate cultures; numbers were counted and expressed as mean ± SD. (B,D) Nonadherent cells harvested from LTBMCs were plated at a concentration of 104 cells/mL in 4 separate methylcellulose cultures supplemented with IL-3. After 7 days in culture, the number of colonies was counted, recalculated for the total number of cells obtained originally from each LTBMC culture well, and expressed as mean number of colonies per LTBMC culture well ± SD. The experiment was repeated 4 times with similar results. (E,F) Nonadherent cells were washed off from myeloid LTBMCs cultured for 5 weeks in the presence or absence of antibodies. The numbers of nonadherent cells were similar to those obtained in A. Adherent cells were harvested (by trypsinization), counted (E), and tested for LTC-ICs in a 2-week limiting dilution assay (F). The data shown are the means (± SD) of 2 experiments.
To analyze whether antibody treatment affects the number of hemopoietic progenitor cells in the nonadherent cell population, we plated cells harvested from LTBMCs at various times in methylcellulose cultures in the presence of IL-3, which promotes the growth of myeloid progenitors. Similar to the effect on the production of nonadherent cells, CD44 v6-specific antibodies enhanced the number of colony-forming cells (CFCs) in both myeloid and lymphoid LTBMCs, whereas CD44 v4-specific antibodies increased only the number of myeloid progenitors (Figure 1B,D). Colonies formed by progenitor cells obtained from LTBMCs cultured with or without CD44 v4 or v6 antibodies looked morphologically alike (data not shown). CD44 v7-specific antibodies had no effect on the generation of hemopoietic progenitors in LTBMCs. Consistent with previous publications, the pan-CD44 antibody IM7 abrogated CFC production in both myeloid and lymphoid LTBMCs (not shown). Thus, the CD44 variant–specific antibodies influenced the proliferation of progenitors.
CD44 variant–specific antibodies induce the release of factors stimulating hemopoiesis
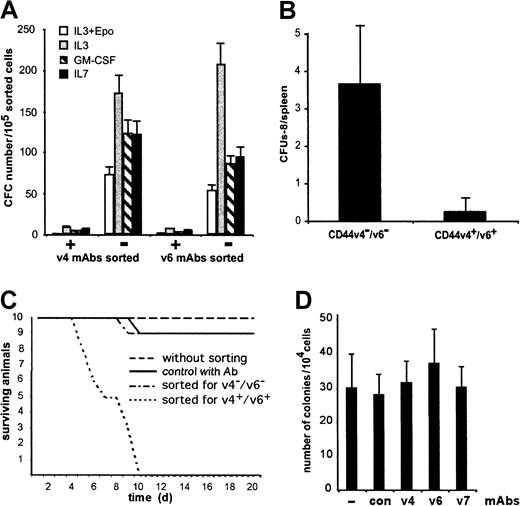
We considered 3 possibilities as to how the CD44 v4- and v6-specific antibodies could mediate their stimulatory actions on hemopoiesis: (1) The antibodies could bind to CD44 variant proteins on the cell surface of progenitor cells and thereby stimulate their proliferation. (2) The antibodies could bind to CD44 variants on both HSPCs and cells of the adherent layer and interfere with adhesive interactions between these cells, resulting in increased susceptibility of hemopoietic progenitors to growth factors. (3) The antibodies could bind to cells of the adherent layer and induce the synthesis of factors that stimulate proliferation of hemopoietic progenitors. To differentiate among these possibilities, we first addressed the question of whether the CD44 variant–specific antibodies could in principle directly stimulate hemopoietic progenitor cell proliferation. To do this, CD44 variant epitopes would need to be present on the surface of progenitor cells. We therefore examined whether hemopoietic progenitors express CD44 variants. We isolated CD44 v4- or v6-positive cells from bone marrow by FACS, by panning, or by MACS. The separated cells represented about 10% of the total bone marrow cell population, and expression of the respective epitopes was checked by immunohistochemistry. The enriched populations of CD44 variant–positive cells (80% to 90% pure) were then tested for the presence of committed progenitor cells in methylcellulose assays using different lineage-specific cytokines (Figure2A), or for the presence of polypotent hemopoietic progenitors, either by spleen colony assays (Figure 2B) or by their ability to reconstitute lethally irradiated mice (Figure 2C). In none of these assays could we detect progenitor cells in the CD44 variant–positive compartments. To further rule out the possibility that the sorting methods might have missed progenitor cells with weak expression of CD44 v4 or v6, we tested whether progenitor cells would respond to the CD44 variant antibodies in CFU assays. In the absence of any adherent LTBMCs, the number (Figure 2D) and size (not shown) of CFUs were similar independent of whether anti-CD44 variant antibodies were present. Together, these results suggest that the CD44 variant antibodies do not directly stimulate progenitor proliferation.
Hemopoietic progenitor cells are not directly stimulated by CD44 variant–specific antibodies.
CD44 v4- and/or v6-positive or negative bone marrow cells were examined for the presence of hematopoietic progenitor cells. (A) Bone marrow cells were sorted with CD44 variant–specific antibodies by MACS, and the sorted cells were cultivated in semisolid methylcellulose cultures with lineage-specific cytokines. GM-CSF was used to assay for granulocyte-macrophage progenitors (CFU-GM); IL-7 was used to assay for B-cell progenitors (CFU-B); and IL-3 + Epo was added to determine early erythroid progenitors (erythroid burst-forming units; BFU-e). Data shown are means (± SD) of the colony numbers obtained with 4 different wells, representing 1 of 5 independent experiments, when cells were separated by panning or FACS. (B) Bone marrow cells were sorted by MACS. The sorted cells were injected into 10 lethally irradiated mice at a concentration of 104 cells per mouse. After 8 days, the numbers of colonies formed on spleens (CFUs-8) were counted and expressed as means ± SD. Similar results were obtained with cells separated by panning or FACS. (C) Bone marrow cells were used unsorted or sorted with CD44 variant–specific antibodies by MACS. The CD44 variant–positive and –negative cell populations were injected into 10 lethally irradiated mice. The control group with antibody represents mice reconstituted with unseparated bone marrow cells together with anti-CD44 v4 and v6 monoclonal antibodies. The data are the result of one experiment. A second independent experiment gave similar results. (D) Nonadherent cells from intact myeloid LTBMCs were harvested and cultured in methylcellulose as described in Figure 1B and D, except that CD44 variant antibodies (10 μg/mL) were added as indicated.
Hemopoietic progenitor cells are not directly stimulated by CD44 variant–specific antibodies.
CD44 v4- and/or v6-positive or negative bone marrow cells were examined for the presence of hematopoietic progenitor cells. (A) Bone marrow cells were sorted with CD44 variant–specific antibodies by MACS, and the sorted cells were cultivated in semisolid methylcellulose cultures with lineage-specific cytokines. GM-CSF was used to assay for granulocyte-macrophage progenitors (CFU-GM); IL-7 was used to assay for B-cell progenitors (CFU-B); and IL-3 + Epo was added to determine early erythroid progenitors (erythroid burst-forming units; BFU-e). Data shown are means (± SD) of the colony numbers obtained with 4 different wells, representing 1 of 5 independent experiments, when cells were separated by panning or FACS. (B) Bone marrow cells were sorted by MACS. The sorted cells were injected into 10 lethally irradiated mice at a concentration of 104 cells per mouse. After 8 days, the numbers of colonies formed on spleens (CFUs-8) were counted and expressed as means ± SD. Similar results were obtained with cells separated by panning or FACS. (C) Bone marrow cells were used unsorted or sorted with CD44 variant–specific antibodies by MACS. The CD44 variant–positive and –negative cell populations were injected into 10 lethally irradiated mice. The control group with antibody represents mice reconstituted with unseparated bone marrow cells together with anti-CD44 v4 and v6 monoclonal antibodies. The data are the result of one experiment. A second independent experiment gave similar results. (D) Nonadherent cells from intact myeloid LTBMCs were harvested and cultured in methylcellulose as described in Figure 1B and D, except that CD44 variant antibodies (10 μg/mL) were added as indicated.
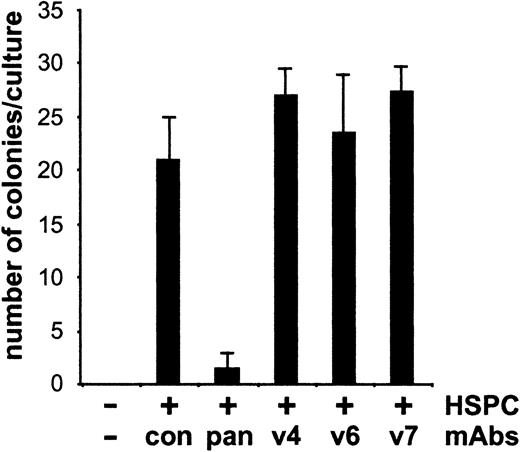
To analyze whether the antibodies might interfere with binding of progenitor cells to adherent cells of the LTBMCs, we enriched HSPCs by negative selection with lineage-specific antibodies. These cells were then incubated for 4 hours with irradiated adherent layers of LTBMCs, either in the absence or presence of CD44 variant–specific antibodies. The bound cells were cultivated for an additional 14 days, and cobblestone area formation was examined. The successful formation of cobblestone areas in both the presence or absence of the antibodies indicated that binding of HSPCs to the hemopoietic stromal microenvironment did not depend on CD44 v4, v6, and v7 (Figure3).
Adhesion of HSPCs to adherent layer of LTBMCs is CD44 variant independent.
Enriched HSPCs were incubated in 4 wells at a concentration of 103 cells/well with irradiated adherent cells of LTBMCs for 4 hours with CD44-specific antibodies (10 μg/mL) as indicated. After washing to remove unbound cells and monoclonal antibodies, bound cells were further cultured for 14 days, and the numbers of cobblestone areas were counted and presented as means ± SD. Con indicates control antibody (10 μg/mL); pan, IM7 antibody (10 μg/mL). The experiment was repeated 3 times with similar results.
Adhesion of HSPCs to adherent layer of LTBMCs is CD44 variant independent.
Enriched HSPCs were incubated in 4 wells at a concentration of 103 cells/well with irradiated adherent cells of LTBMCs for 4 hours with CD44-specific antibodies (10 μg/mL) as indicated. After washing to remove unbound cells and monoclonal antibodies, bound cells were further cultured for 14 days, and the numbers of cobblestone areas were counted and presented as means ± SD. Con indicates control antibody (10 μg/mL); pan, IM7 antibody (10 μg/mL). The experiment was repeated 3 times with similar results.
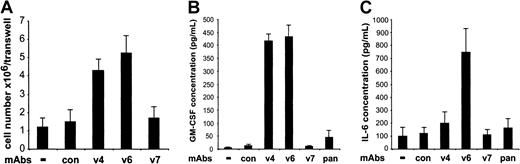
To test whether CD44 variant antibodies might interact with adherent cells of LTBMCs and thereby induce the synthesis or release of an activity that stimulates proliferation of hemopoietic progenitor cells, we separated HSPCs and adherent cells of LTBMCs by a membrane and applied CD44-specific antibodies to the cultures, as indicated (Figure4A). Whereas the number of HSPCs in the upper chamber was increased when the adherent cells of LTBMCs were incubated with CD44 v4 or v6 antibodies, the treatment with CD44 v7 antibody had no effect. Thus, we conclude from these experiments and those described in Figure 2 that the targets for the CD44 variant–specific antibodies are adherent cells of the hemopoietic microenvironment and that the binding of the antibodies results in the release of a membrane-permeable cytokine that stimulates progenitor cells.
CD44 variant–specific antibodies stimulate adherent cells in LTBMCs to produce colony-forming activity.
(A) Where indicated, CD44 v4-, v6-, and v7-specific antibodies (10 μg/mL) were applied to adherent layers of LTBMCs (3 wells/group) in a transwell chamber. HSPCs, enriched by panning, were cultivated in the upper well at 37°C at a concentration of 106 cells/well. After 5 days of culture, the cells in the upper well of the transwell chambers were harvested and counted, and the numbers were expressed as means ± SD. (B) Conditioned media from 3 separate cultures of LTBMCs treated for 48 hours with CD44-specific antibodies, as indicated, were tested for GM-CSF by ELISA. (C) Conditioned media from LTBMCs (n = 3) treated for 48 hours with CD44-specific antibodies were tested for IL-6 by ELISA. Con indicates control antibody (10 μg/mL); pan, IM7 antibody (10 μg/mL). The experiment was repeated 3 times with similar results.
CD44 variant–specific antibodies stimulate adherent cells in LTBMCs to produce colony-forming activity.
(A) Where indicated, CD44 v4-, v6-, and v7-specific antibodies (10 μg/mL) were applied to adherent layers of LTBMCs (3 wells/group) in a transwell chamber. HSPCs, enriched by panning, were cultivated in the upper well at 37°C at a concentration of 106 cells/well. After 5 days of culture, the cells in the upper well of the transwell chambers were harvested and counted, and the numbers were expressed as means ± SD. (B) Conditioned media from 3 separate cultures of LTBMCs treated for 48 hours with CD44-specific antibodies, as indicated, were tested for GM-CSF by ELISA. (C) Conditioned media from LTBMCs (n = 3) treated for 48 hours with CD44-specific antibodies were tested for IL-6 by ELISA. Con indicates control antibody (10 μg/mL); pan, IM7 antibody (10 μg/mL). The experiment was repeated 3 times with similar results.
IL-6 and GM-CSF production in LTBMCs is stimulated by CD44 variant–specific antibody treatment
To identify the colony-promoting activity produced by the hemopoietic microenvironment, we tested conditioned media from LTBMCs treated with CD44-specific antibodies for the presence of IL-3, IL-4, IL-6, G-CSF, M-CSF, and GM-CSF. Of these cytokines, GM-CSF production was dramatically increased in LTBMCs upon treatment with CD44 v4- and v6-specific antibodies (Figure 4B), and IL-6 was increased upon treatment with CD44 v6-specific antibodies (Figure 4C). The levels of the cytokines IL-4, IL-3, G-CSF, and M-CSF did not change upon treatment with CD44 v4 or v6 antibodies (not shown). CD44 v7-specific antibodies and the pan-CD44 antibody IM7 had no effect on cytokine production.
Bone marrow macrophages express CD44 v4 and v6 epitopes
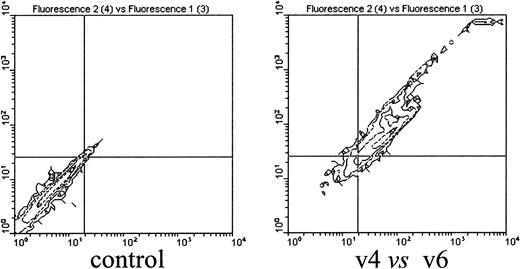
To identify the cellular targets of the CD44 v4- and v6-specific antibodies, we costained CD44 variant–positive cells in the bone marrow cell population with various cell lineage markers and analyzed them by FACS. This double staining revealed that CD44 v4 and v6 epitopes are found predominantly in the CD11b+ population, identifying bone marrow macrophages as the CD44 v4/v6-positive cells. Of the CD11b+ cells, 80% to 90% were positive for CD44 v4 and v6 staining. Importantly, virtually all cells were double positive for v4 and v6 expression (Figure 5). Staining was performed using one antibody directly labeled with FITC and the other antibody visualized by treatment with secondary PE-labeled antibodies.
Bone marrow–derived macrophages are CD44 v4 and v6 positive.
A 99% pure population of bone marrow–derived macrophages cultured as described in “Materials and methods” was double stained with anti-CD44 v4 and CD44 v6 antibodies. Cells (5 × 105) were first incubated with the unlabeled primary antibody followed by incubation with PE-labeled secondary antibodies, and then the cells were treated with the FITC-labeled antibodies. Fluorescence was measured in a FACScan cytometer (Becton Dickinson). In the experiment shown, v6 antibodies were FITC labeled. In similar experiments, FITC-labeled v4 antibodies gave the same result (not shown).
Bone marrow–derived macrophages are CD44 v4 and v6 positive.
A 99% pure population of bone marrow–derived macrophages cultured as described in “Materials and methods” was double stained with anti-CD44 v4 and CD44 v6 antibodies. Cells (5 × 105) were first incubated with the unlabeled primary antibody followed by incubation with PE-labeled secondary antibodies, and then the cells were treated with the FITC-labeled antibodies. Fluorescence was measured in a FACScan cytometer (Becton Dickinson). In the experiment shown, v6 antibodies were FITC labeled. In similar experiments, FITC-labeled v4 antibodies gave the same result (not shown).
The staining of CD11b+ cells with CD44 variant–specific antibodies suggests that macrophages in the adherent layer of LTBMCs are the target for the CD44 variant–specific antibodies.
Cytokine production by purified macrophages
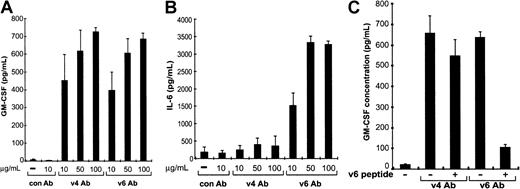
Our data suggested that the CD44 v4- and v6-specific antibodies bind to bone marrow macrophages and induce the release of GM-CSF and IL-6, resulting in an increased proliferation of myeloid and lymphoid progenitor cells. To prove this, we treated BMDMCs with F(ab′)2 fragments of the CD44 v4- and v6-specific antibodies (to avoid nonspecific activation via the Fc receptor) and 48 hours later analyzed the conditioned medium for IL-6 and GM-CSF content. Treatment with the F(ab′)2 fragments of the CD44 v6-specific antibody increased the concentrations of GM-CSF and IL-6 in the conditioned medium, whereas the F(ab′)2 fragments of the CD44 v4-specific antibody increased only the concentration of GM-CSF (Figure 6A,B). F(ab′)2fragments of IM7 antibodies had no influence on GM-CSF and IL-6 production (not shown). The specificity of the antibody action was further underlined by the fact that the CD44 v6 antibody effect was completely abrogated by the addition of a peptide covering the sequence of the epitope recognized by the antibody (Figure 6C; the sequence of the epitope is depicted in Figure 7). These results are therefore in perfect agreement with our proposed mechanism of action of the antibodies in LTBMCs.
CD44 variant–specific antibodies induce bone marrow–derived macrophages to secrete GM-CSF and IL-6.
Macrophages were stimulated with different amounts of CD44 v4 and CD44 v6 antibodies. Conditioned media from macrophage cultures (4 cultures) treated for 48 hours with CD44 variant–specific antibodies at concentrations indicated were tested by ELISA for the production of GM-CSF (A) or IL-6 (B). (C) Bone marrow–derived macrophage cultures (4 cultures) were treated with CD44 variant antibodies (10 μg/mL) together with a peptide covering the epitope recognized by the CD44 v6-specific antibody (AATQQETWFQNGWQ; kindly provided by Benda and Co, Vienna; concentration 10 μg/mL), and the concentration of GM-CSF was determined in the conditioned medium. The experiments described in A and B were repeated 4 times, and the experiment in C was done twice. In each case, similar results were obtained in the replicate experiments.
CD44 variant–specific antibodies induce bone marrow–derived macrophages to secrete GM-CSF and IL-6.
Macrophages were stimulated with different amounts of CD44 v4 and CD44 v6 antibodies. Conditioned media from macrophage cultures (4 cultures) treated for 48 hours with CD44 variant–specific antibodies at concentrations indicated were tested by ELISA for the production of GM-CSF (A) or IL-6 (B). (C) Bone marrow–derived macrophage cultures (4 cultures) were treated with CD44 variant antibodies (10 μg/mL) together with a peptide covering the epitope recognized by the CD44 v6-specific antibody (AATQQETWFQNGWQ; kindly provided by Benda and Co, Vienna; concentration 10 μg/mL), and the concentration of GM-CSF was determined in the conditioned medium. The experiments described in A and B were repeated 4 times, and the experiment in C was done twice. In each case, similar results were obtained in the replicate experiments.
Identification of epitopes recognized by CD44 v6-specific antibodies.
The CD44 exon v6 sequences of human (A), rat (B), and mouse (C) are shown.42 Underlined are the epitopes recognized by the human-specific antibody VFF18,11 the rat-specific antibody 1.1ASML,43 and the murine-specific antibody 9A4, as determined by peptide mapping. The gray areas identify conserved amino acids.
Identification of epitopes recognized by CD44 v6-specific antibodies.
The CD44 exon v6 sequences of human (A), rat (B), and mouse (C) are shown.42 Underlined are the epitopes recognized by the human-specific antibody VFF18,11 the rat-specific antibody 1.1ASML,43 and the murine-specific antibody 9A4, as determined by peptide mapping. The gray areas identify conserved amino acids.
Discussion
In an attempt to understand the role of CD44 variants in murine hemopoiesis, we have treated murine lymphopoietic or myelopoietic LTBMCs with several CD44-specific antibodies and measured their influence on the production of nonadherent cells and progenitors. In agreement with earlier findings,7 8 a pan-CD44 antibody strongly repressed myelopoiesis and lymphopoiesis. In contrast, 2 variant-specific antibodies recognizing epitopes encoded by CD44 exon v6 and v4, respectively, enhanced hemopoiesis.
Our data demonstrate that in the bone marrow cell population, CD44 v4 and v6 epitopes are expressed almost exclusively on macrophages. The epitopes for the CD44 exon v6 and v4 are found on the same cells, as revealed by double staining with both antibodies and FACS analysis. They are, however, not expressed on the same protein because RT-PCR analysis of RNA derived from purified macrophage cultures with variant exon-specific primers19 demonstrated inclusion of each exon in separate RNA species (not shown). Activation of LTBMCs with the 2 specific antibodies led to different effects: the CD44 v4-specific antibodies activated myelopoiesis, whereas the v6-specific antibodies activated both lymphopoiesis and myelopoiesis. Consistent with this observation, we found that stimulation with the different antibodies led to secretion of different cytokines: GM-CSF with v4-specific antibodies, and GM-CSF and IL-6 with v6-specific ones. IL-6 production would explain why CD44 v6 antibodies enhance both myelopoiesis and lymphopoiesis because IL-6 is the cytokine that induces proliferation of progenitor cells prior to commitment.20-26 GM-CSF secretion could explain the stimulation of myelopoiesis because this cytokine is specific for the myeloid differentiation pathway.
Recently, the repression of myeloid maturation by a CD44 v6-specific antibody (VFF18) in human LTBMCs has been described.11 In contrast to the observation here, the human v6-specific antibody had no effect on CFU production. A similar observation that 2 antibodies recognize similar regions of a protein but have different effects has been made for the anti-CD44 antibodies J-173 and KM81. They recognize similar but not identical regions of the CD44 HA-binding domain. Although both interfere with HA binding, J-173 stimulates lymphocytes and NK cells,27 whereas KM81 is inhibitory for a T-cell hybridoma.28 We have mapped the epitope recognized by the murine CD44 v6 antibody 9A4 and have compared it with the epitope recognized by the human exon v6-specific antibody VFF18 (Figure 7). The 2 epitopes are overlapping but do not cover exactly the same homologous sequence of the CD44 v6 region. We thus conclude that like the J-173 and KM81 antibodies, the 9A4 and VFF18 antibodies have different biologic effects because they bind to spatially disparate epitopes.
Here we have shown that the targets of the activating CD44 variant–specific antibodies are bone marrow macrophages. Because the binding of the antibodies to macrophages results in cytokine secretion, we assume that a signal cascade is activated by the antibodies. The notion that CD44 proteins are involved in signaling is supported by studies linking CD44 with the coactivation of lymphocytes,27,29-32 induction of genes,33and activation of signaling molecules.34-36 Furthermore, the production of cytokines in lymphocytes seems to be mediated by CD44.37 Interestingly, the induction of the synthesis of the cytokine M-CSF has also been observed in peripheral macrophages upon treatment with CD44-specific antibodies,38 although it should be noted that the increase in cytokine protein was only marginal.
Although it is possible that the CD44 antibodies prevent binding to macrophages of a molecule that inhibits hemopoiesis, the most likely interpretation of the stimulatory effect of the CD44 variant–specific antibodies is that the antibodies simulate the binding of a stimulatory ligand, which might be located on a cell or be a component of the extracellular matrix. This hypothesis would speak for the existence of a ligand that binds specifically to CD44 variant proteins. We have previously shown that CD44 variant proteins have increased affinity to hyaluronan (HA) and other glycosaminoglycans (GAGs).39,40Thus, binding of HA or other GAGs as natural ligands might be simulated by the antibodies. In fact, stimulation of hemopoiesis by HA has been shown.15,41 Furthermore, the target of HA action is bone marrow macrophages, similar to the action of the CD44 variant–specific antibodies described here. However, binding of macrophages to HA results predominantly in the production of IL-6 and small amounts of IL-1β. Furthermore, the increase in IL-6, which appeared to be the critical cytokine for hemopoiesis in these studies, was not mediated through CD44 but by another, as yet unidentified, HA receptor on the macrophages.15 Thus, we suggest that the CD44 variant antibodies simulate binding of a stimulatory ligand other than HA.
In summary, we have shown here that different variants of CD44 on macrophages of the hemopoietic microenvironment play a decisive role in myelopoiesis and lymphopoiesis by triggering the secretion of lineage-specific cytokines.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Helmut Ponta, Forschungszentrum Karlsruhe, Institute of Toxicology and Genetics, PO Box 3640, D-76021 Karlsruhe, Germany; e-mail: helmut.ponta@itg.fzk.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal