Abstract
CD44 is the major cell surface receptor for the extracellular matrix glycosaminoglycan hyaluronan and is implicated in a variety of biological events that include embryonic morphogenesis, lymphocyte recirculation, inflammation, and tumor metastasis. CD44 delivers activation signals to T lymphocytes, B lymphocytes, natural killer cells, polymorphonuclear leukocytes, and macrophages by stimulating protein tyrosine phosphorylation and calcium influx. The mechanism of signal transduction via CD44 remains undefined, although CD44 was shown to physically associate with intracellular protein tyrosine kinase Lck in T lymphocytes. In the present report, we show that a significant proportion of CD44 in human peripheral blood T lymphocytes and endothelial cells is associated with low-density plasma membrane fractions that represent specialized plasma membrane domains enriched in glycosphingolipids and glycosylphosphatidylinositol (GPI)-anchored proteins. CD44 and the GPI-anchored CD59 do not appear to directly interact in the low-density membrane fractions. In human peripheral blood T lymphocytes, 20% to 30% of the Src family protein tyrosine kinases, Lck and Fyn, are recovered from these fractions. CD44-associated protein kinase activity was selectively recovered from the low-density membrane fractions, corresponding to glycosphingolipid-rich plasma membrane microdomains. Reprecipitation of the in vitro phosphorylated proteins showed that CD44 associates not only with Lck but also with Fyn kinase in these membrane domains. Our results suggest that cellular stimulation via CD44 may proceed through the signaling machinery of glycosphingolipid-enriched plasma membrane microdomains and, hence, depend on the functional integrity of such domains.
CD44 IS A TYPE I transmembrane glycoprotein expressed in a variety of cell types, including lymphocytes, macrophages, erythrocytes, fibroblasts, epithelial cells, and endothelial cells.1 Although CD44 is encoded by a single gene, alternative RNA splicing gives rise to a large number of isoforms with distinct cellular distribution patterns. Posttranslational addition of N- and O-glycans and chondroitin/heparan sulfate moieties make CD44 a highly heterogeneous molecule. Furthermore, expression of certain defined isoforms is associated with specific cellular behavior.2 CD44 is a major receptor for the extracellular glycosaminoglycan hyaluronic acid (HA).3Interaction with HA underlies a wide spectrum of CD44 functions in embryonic morphogenesis and organogenesis, lymphocyte homing, hematopoiesis, cellular activation, and tumor progression.1 4-6
Signaling via CD44 has been well documented using anti-CD44 monoclonal antibody (MoAb) as well as its natural ligand. Specific anti-CD44 MoAb induces T lymphocytes to secrete interleukin-2 and proliferate, both directly and in response to suboptimal doses of mitogenic anti-CD3 or anti-CD2 pairs7-12; stimulates cytotoxic effector functions in cytotoxic T lymphocytes (CTLs) and polymorphonulear leukocytes (PMNs)12-14; and augments natural killer (NK) cell cytotoxicity15-17 and macrophage proinflammatory cytokine secretion.18 Similarly, HA binding to CD44 stimulates T lymphocytes19,20 and macrophages.21-24 In B cells, both anti-CD44 MoAb and HA stimulate proliferation and differentiation into antibody-producing cells.25 Marked differences were shown in the ability of cell surface CD44 to bind HA and in the capacity of anti-CD44 MoAbs to stimulate target cells.1 Recent studies have shown that HA fragments, but not polymeric HA, activate macrophages via CD44.23,24 HA fragments are generated by activated leukocytes in chronic inflammatory lesions26,27 that might lead to further recruitment of leukocytes. CD44-HA interaction contributes directly to tumor development,28 and elevated hyaluronidase production by tumors generates HA fragments that promote neoangiogenesis.29
Despite the well-documented signaling capacity of CD44, the mechanism of signal transduction via CD44 remains poorly understood. The recent demonstration that CD44 coprecipitates Lck in human T lymphocytes30 provides one important link in the CD44 signaling pathway. A significant proportion of CD44 resists solubilization in nonionic detergents,31 and recent investigations have shown that CD44 molecules lacking the cytoplasmic tail are still detergent-insoluble, possibly because of their association with Triton X-100 (TX-100)-insoluble lipids.31,32 This behavior is similar to that of most glycosylphosphatidylinositol (GPI)-anchored glycoproteins,33 which are confined to the external leaflet of the plasma membrane. Because many GPI-anchored molecules transduce signals34 and associate with Src family PTKs in specialized plasma membrane domains,35 36 we investigated whether CD44 also shares this property. Our results show that only the CD44 molecules present in glycosphingolipid (GSL)-rich low-density membrane domains associate with active Lck as well as Fyn kinases.
MATERIALS AND METHODS
Reagents and antibodies.
TX-100 was from Merck (Darmstadt, Germany). Brij-58 (polyoxyethylene 20 cetyl ether) and horseradish peroxidase (HRP)-conjugated cholera toxin were from Sigma Chemie (Buchs, Switzerland). Protease inhibitors aprotinin, leupeptin, Pefabloc SC, biotin-X-NHS ester, and octylglucoside (OTG) were from Boehringer Mannheim (Mannheim, Germany). HRP-conjugated streptavidin and the enhanced chemiluminescence (ECL) reagent were from Amersham (Buckinghamshire, UK). Mouse MoAb against human CD2 (MEM-65), CD44 (MEM-85), CD45 (MEM-28), CD59 (MEM-43), and major histocompatibility antigen I (MHC-I; MEM-147) were kind gifts from Dr Vaclav Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). Rabbit polyclonal antibodies against Lck and Fyn kinases, HRP-conjugated goat antimouse and goat antirabbit IgG and protein A/G immunoprecipitation beads were from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Rabbit polyclonal antibody against caveolin and antiphosphotyrosine MoAb PY20 were from Transduction Labs (Lexington, KY). Alkaline phosphatase (AP)-conjugated goat antimouse IgG was from PharMingen (San Diego, CA). BCA protein quantitation kit and AP-ECL detection kit were from Bio-Rad (Richmond, CA).
Distribution of cellular proteins and GM1 ganglioside in equilibrium density gradients.
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats. One hundred million PBMC or PHA blasts were washed in TKM buffer (50 mmol/L Tris-HCl, pH 7.4, 25 mmol/L KCl, 5 mmol/L MgCl2, and 1 mmol/L EGTA) and lysed in 0.750 mL of lysis buffer (TKM buffer containing 0.5% TX-100 or 0.5% Brij-58 and the protease inhibitors leupeptin [1 μmol/L], aprotinin [2 μg/mL], and Pefabloc SC [2 mmol/L] and 100 μmol/L sodium orthovanadate) for 30 minutes on ice. After adding sucrose to 40%, the lysate was placed at the bottom of a SW41 tube, overlaid with 6.0 mL of 36% sucrose followed by 3.5 mL of 5% sucrose in TKM buffer, and centrifuged at 250,000g for 16 to 20 hours at 4°C. Eleven 1-mL fractions (excluding the pellet) were collected from the top and stored at −20°C. Human umbilical vein-derived endothelial cell line ECV304 obtained from ATCC (Rockville, MD) was maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS). Confluent cultures in 10-cm diameter Petri dishes were overlaid with 750 μL of lysis buffer containing 0.5% TX-100 or 60 mmol/L OTG, scraped, and incubated for 30 minutes on ice. Further steps were the same as described above for PBMC. To analyze the detergent extractability of cell surface proteins, postnuclear supernatants were removed by centrifuging the detergent lysate at full speed in a table-top centrifuge for 5 minutes, and the nuclear pellet was solubilized in 750 μL of TKM buffer by sonication.
The presence of various cell surface and intracellular proteins in the density gradient fractions, total detergent extract, or solubilized nuclear pellet was evaluated by Western blotting as described earlier.37 Briefly, 20 μL of the samples solubilized in 6× sample buffer were electrophoresed and transferred to nitrocellulose filters. After blocking, proteins were detected using appropriate first and HRP-conjugated second antibodies by ECL. Distribution of the GM1 ganglioside was analyzed by dot-immunoassay using HRP-conjugated cholera toxin as detailed elsewhere.37Western blot and dot-blot luminograms were quantitated by laser scanning densitometry (Molecular Dynamics, Sunnyvale, CA).
Cell surface biotinylation and immunoprecipitation.
Confluent cultures of ECV304 cells were biotinylated with 50 μg/mL Biotin-X-NHS ester in 5 mL of biotinylation buffer (10 mmol/L sodium borate buffer, pH 8.9) at room temperature for 15 minutes, washed in TKM buffer, and lysed in 0.750 mL of lysis buffer containing 0.5% TX-100. After equilibrium gradient centrifugation, low-density fractions (3 through 6, that correspond to the 5% to 36% sucrose interface; Fig 1) were pooled. One milliliter of the pool, precleared with Pansorbin (Calbiochem, San Diego, CA), was added to protein A/G beads coated with MoAb against CD44 or CD59. After incubation at 4°C for 2 hours, the beads were washed in lysis buffer and proteins eluted by boiling in reducing sample buffer were detected by Western blot using streptavidin-HRP and ECL reagent.
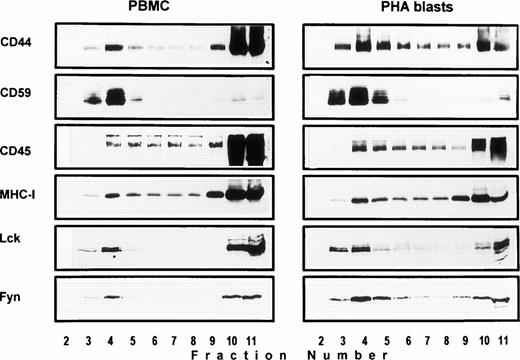
Association of CD44 with low-density plasma membrane fractions in human T lymphocytes. One hundred million human PBMC (left panel) or PHA blasts (right panel) were extracted in TKM buffer containing 0.5% TX-100, and the lysates were subjected to equilibrium gradient centrifugation as described in the Materials and Methods. Eleven 1-mL fractions were collected from the top, and 20 μL of each fraction (2 through 11) was electrophoresed under nonreducing (CD44, CD45, MHC-I, and CD59) or reducing (Lck and Fyn) conditions. Separated proteins were detected by ECL-based Western blot. Fractions 3 through 6 correspond to the 5% to 36% sucrose interface. Data shown are representative of at least three experiments. Identical distribution profiles were obtained when 0.5% Brij-58 was used for extraction. Fraction 1 did not contain any of the cell surface or intracellular proteins tested.
Association of CD44 with low-density plasma membrane fractions in human T lymphocytes. One hundred million human PBMC (left panel) or PHA blasts (right panel) were extracted in TKM buffer containing 0.5% TX-100, and the lysates were subjected to equilibrium gradient centrifugation as described in the Materials and Methods. Eleven 1-mL fractions were collected from the top, and 20 μL of each fraction (2 through 11) was electrophoresed under nonreducing (CD44, CD45, MHC-I, and CD59) or reducing (Lck and Fyn) conditions. Separated proteins were detected by ECL-based Western blot. Fractions 3 through 6 correspond to the 5% to 36% sucrose interface. Data shown are representative of at least three experiments. Identical distribution profiles were obtained when 0.5% Brij-58 was used for extraction. Fraction 1 did not contain any of the cell surface or intracellular proteins tested.
Cell stimulation via CD44.
Twenty-four–well Falcon microtiter plates were coated with 50 μg/mL anti-CD44 MoAb (MEM-85) in phosphate-buffered saline (PBS) for 2 hours at 37°C. Freshly isolated PBMC from healthy volunteers were suspended in RPMI at 2 × 106/mL and equilibrated to 37°C, and 1 mL was added to the wells. At the indicated time points, cells were pelleted down in a microfuge. The cell pellet and cells adhering to the plate were lysed in 100 μL of boiling sodium dodecyl sulfate (SDS) lysis buffer (10 mmol/L Tris pH 7.4, 1% SDS, 100 μmol/L sodium orthovanadate), pooled, and sonicated briefly. Aliquots of the lysates were analyzed for phosphotyrosylated proteins or the kinases by Western blot.
Immune complex kinase assays.
Twenty-five microliters of protein A/G plus beads was incubated with 5 μg of the indicated antibodies in 500 μL PBS for 30 minutes at 4°C and FCS was added to 0.5% (to minimize nonspecific background). This mixture was incubated for a further 30 minutes and pelleted down. For immunoprecipitation from the total cell lysates, 10 ×106 PBMC were lysed in 1 mL of lysis buffer containing 0.5% Brij-58 and the nuclei were removed by centrifugation. One milliliter of total lysate or pooled floating membrane fractions (3 through 6) or the bottom fractions (9 through 11) from the equilibrium gradients of Brij-58 lysate were precleared with Pansorbin and added to Ab-coated protein A/G beads. After incubation at 4°C for 2 to 4 hours on a rotating wheel, the beads were washed twice in TKM-0.5% Brij-58, washed once in kinase buffer (20 mmol/L MOPS, pH 7.4, 5 mmol/L MgCl2, 5 mmol/L MnCl2, 100 μmol/L sodium orthovanadate), and finally suspended in 25 μL of kinase buffer. Kinase reaction was initiated by the addition of 2 μCi of [γ-32P]-ATP (5,000 Ci/mmol; Amersham) in 5 μL kinase buffer. After incubation for 15 minutes at 30°C, the reaction was stopped by adding 6× sample buffer and boiling. Alternatively, SDS was added to 0.4% to disrupt the membrane complexes and diluted with TKM-0.5% Brij-58 to 500 μL volume, and the in vitro phosphorylated proteins were reprecipitated with antibodies against phosphotyrosine, Lck, or Fyn. Immunoprecipitated proteins were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
RESULTS
Solubility of CD44 in non-ionic detergents has been reported to be cell type specific.31 Whereas a significant fraction of CD44 in fibroblasts was detergent-insoluble, lymphocyte and epithelial CD44 were completely solubilized in TX-100. In fibroblasts, only the detergent-insoluble fraction of CD44 floated up to the low-density fractions in equilibrium density gradients.32 However, when we analyzed the flotation properties of different cell surface molecules with GPI or polypeptide membrane anchor in human peripheral blood T lymphocytes lysed in 0.5% TX-100, we observed that 20% to 30% of the extracted CD44 floated up to the low-density fractions at the 5% to 36% sucrose interface (Fig 1). All CD44 was extracted under these conditions (data not shown). Almost all of the GPI-anchored CD59 (Fig 1) and more than 60% of the cholera toxin binding GM1 ganglioside (Fig 2A) were recovered from the floating fractions 3 through 6, which also contained small amounts of MHC-I and CD45 (Fig 1). Similar results were obtained with PHA blasts (Fig 1, right panel; and Fig 2A). Protein estimation by BCA method showed that the floating low-density fractions (3 through 6) contained less than 20% of the total cellular proteins extracted, and the bulk remained at the bottom of the gradient; the total protein profile across the gradient is shown in Fig 2B.
Distribution of the glycosphingolipid GM1 in equilibrium sedimentation gradients. In (A), 10 μL of the gradient fractions shown in Fig 1 was dot-blotted onto nitrocellulose filters, blocked, and probed with HRP-conjugated cholera toxin followed by ECL detection. The total protein profile of the gradient fractions of TX-100 lysate of PBMC is shown in (B). A similar profile obtained for PHA blasts is not shown.
Distribution of the glycosphingolipid GM1 in equilibrium sedimentation gradients. In (A), 10 μL of the gradient fractions shown in Fig 1 was dot-blotted onto nitrocellulose filters, blocked, and probed with HRP-conjugated cholera toxin followed by ECL detection. The total protein profile of the gradient fractions of TX-100 lysate of PBMC is shown in (B). A similar profile obtained for PHA blasts is not shown.
We next examined the behavior of CD44 in the human endothelial cell line ECV304 expressing caveolin, a protein also recovered preferentially in low-density membrane fractions.38 Here again, CD44 was almost completely extracted by TX-100 (data not shown), yet 30% to 40% of CD44 was detected in GPI and caveolin-rich floating membrane fractions (Fig 3A, upper panel). An identical distribution profile was obtained even after a stronger extraction in 60 mmol/L OTG (Fig 3A, lower panel) or 2% TX-100 (data not shown), suggesting that the floating CD59, caveolin, and CD44 were still associated with buoyant membrane lipid complexes. We have observed a similar pattern for murine GPI-linked Thy-1 molecules, which were completely extracted by OTG while remaining floatable in equilibrium gradients.37
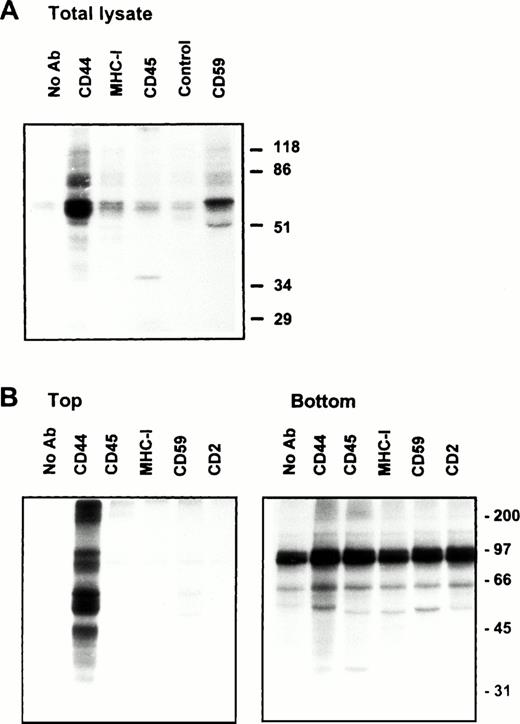
(A) Endothelial cell CD44 partitions into caveolin containing low-density membrane fractions. ECV304 cells grown in 10-cm Petri dishes were extracted in TKM-0.5% TX-100 (upper panel) or 60 mmol/L OTG (lower panel) at 4°C for 30 minutes. The lysates were subjected to equilibrium sedimentation, and the fractions were tested for the distribution of CD44, CD59, or caveolin as described in Fig 1. Caveolin was analyzed under reducing conditions. (B) CD44 and CD59 do not interact directly in the low-density membrane fractions. Surface biotinylated ECV304 cells were extracted with TKM-0.5% TX-100 and ultracentrifuged in sucrose gradients, and the GPI-rich top fractions 3 through 6 were pooled. One milliliter of the pool was precleared with Pansorbin and immunoprecipitated with protein A/G beads coated with antibodies against CD59 or CD44. SDS-PAGE separated proteins were detected by Western blot using streptavidin-HRP.
(A) Endothelial cell CD44 partitions into caveolin containing low-density membrane fractions. ECV304 cells grown in 10-cm Petri dishes were extracted in TKM-0.5% TX-100 (upper panel) or 60 mmol/L OTG (lower panel) at 4°C for 30 minutes. The lysates were subjected to equilibrium sedimentation, and the fractions were tested for the distribution of CD44, CD59, or caveolin as described in Fig 1. Caveolin was analyzed under reducing conditions. (B) CD44 and CD59 do not interact directly in the low-density membrane fractions. Surface biotinylated ECV304 cells were extracted with TKM-0.5% TX-100 and ultracentrifuged in sucrose gradients, and the GPI-rich top fractions 3 through 6 were pooled. One milliliter of the pool was precleared with Pansorbin and immunoprecipitated with protein A/G beads coated with antibodies against CD59 or CD44. SDS-PAGE separated proteins were detected by Western blot using streptavidin-HRP.
On the plasma membrane, GSLs and cholesterol form specialized plasma membrane microdomains39 that could be isolated as detergent-resistant, low-density plasma membrane vesicles.40 41 Our results show that a significant fraction of lymphocyte and endothelial cell CD44 is recovered in membrane fractions enriched in GPI-anchored receptors and GSLs, whether the cells expressed caveolin or not. To investigate if CD44 and CD59 are present in the same membrane complex in the floating membrane fractions, we surface-biotinylated ECV304 cells, extracted in 0.5% TX-100, and immunoprecipitated CD44 or CD59 from low-density membrane fractions using the same lysis buffer for all the washing steps. CD44 antibodies immunoprecipitated a 90-kD molecule and did not coprecipitate the 18- to 22-kD CD59 (Fig 3B); similarly, anti-CD59 did not coprecipitate CD44, suggesting that recovery of CD44 in GPI-rich membrane complexes does not arise from a direct interaction with CD59.
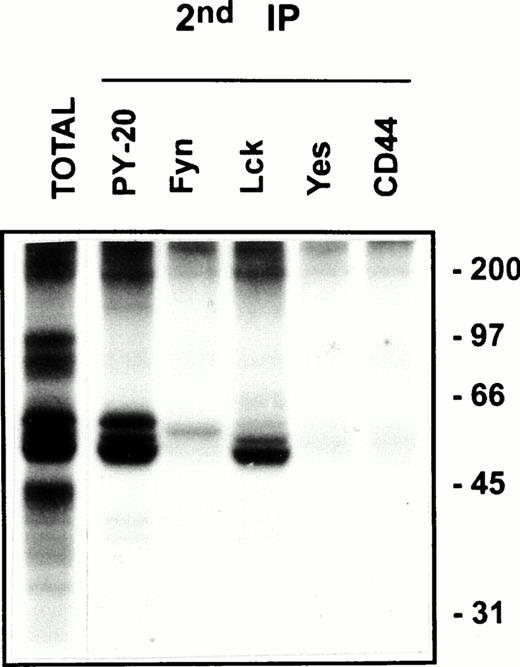
Protein tyrosine phosphorylation is an important step in transmembrane signaling,42 and stimulation via CD44 leads to increased tyrosine phosphorylation.12,43,44 Stimulation of freshly isolated human PBMC by immobilized anti-CD44 MoAb rapidly induced phosphorylation of 59-, 56-, 42-, and 26-kD proteins on tyrosine residues, which persisted up to 45 minutes after stimulation (Fig 4A). The 59- and 56-kD phosphotyrosylated proteins correspond to the positions of Lck and Fyn tyrosine kinases (Fig 4B). This rapid induction of tyrosine phosphorylation could result from activation of the Lck tyrosine kinase reported to be physically associated with CD44,30 but the mode of interaction between CD44 and Lck remains undefined. We observed that, in freshly isolated PBMC, 20% to 30% of Lck and Fyn kinases was recovered in the same floating fractions as CD44 and CD59, which was increased to 50% to 60% in PHA blasts (Fig 1). This finding prompted us to investigate if CD44 interacted with Lck as a result of its association with distinct membrane domains. To this end, kinase assays were performed on CD44 immunoprecipitated from total detergent lysates or pooled density gradient fractions (Fig5). Proteins at 55 to 60 and 80 to 90 kD were strongly phosphorylated in the CD44 immunoprecipitate from total detergent extracts (Fig 5A), a pattern closely resembling but stronger than that of CD59. These interactions seem to be specific, because the CD45 immunoprecipitate from total detergent extract showed phosphorylation of proteins in the Src kinase region (albeit much less strongly than the CD44 immunoprecipitate) and of an additional protein of 35 kD (Fig 5A) that has been shown to specifically associate with CD45.45 When the experiment was performed on density gradient fractions, we observed that the kinase activities associated with CD44 and CD59 were recovered exclusively from the low-density membrane complexes (Fig 5B, left panel, top). The CD59-associated kinase activities in the top fractions were more apparent after a longer exposure (data not shown). The CD44-associated phosphoproteins, particularly those in the 95-, 40- to 45-kD region were more prominent in the low-density membrane fractions than in total cell lysates. The phosphoprotein profile after kinase assay on CD44 immunoprecipitated from the pooled bottom fractions 9 through 11, which contained more than half of cellular CD44 and the kinases (Fig 1) and 80% of the cellular proteins (Fig 2), was not significantly different from that of control immunoprecipitations (Fig 5B, right panel, bottom). However, the CD45-associated 35-kD phosphoprotein was recovered only from the bottom fractions. Reprecipitation of the in vitro phosphorylated proteins associated with CD44 from the floating membrane fractions showed that they are tyrosine phosphorylated and correspond to the multiply phosphorylated forms of Lck and Fyn (Fig6). Fyn appears to be less efficiently phosphorylated than Lck, suggesting that Lck is primarily responsible for CD44-associated tyrosine kinase activities in T lymphocytes. The 80- to 90-kD phosphoprotein does not appear to be CD44, because anti-CD44 did not precipitate it (Fig 6). Tyrosine-phosphorylated proteins of similar molecular weight have been observed in association with several GPI-anchored proteins,35 46 raising the possibility that they could be the putative, signal-transducing transmembrane linkers, but their identities remain to be established.
Stimulation of protein tyrosine phosphorylation via CD44. Freshly isolated human PBMC were stimulated by immobilized anti-CD44 MoAb as described in the Materials and Methods. At the indicated time points, cells were lysed and equivalent amounts of lysates were electrophoresed under reducing conditions, blotted, and probed for tyrosine phosphorylated proteins by PY20 followed by GAM-AP and ECL detection (upper panel). Lane 1, unstimulated cells; lanes 2 through 6, cells plated in CD44 MoAb-coated wells, lane 7, cells plated in control mouse IgG-coated wells (C). Equivalent protein loading in the wells was verified by Ponceau-S staining of the blots, as well as by probing for Lck and Fyn kinases (lower panels).
Stimulation of protein tyrosine phosphorylation via CD44. Freshly isolated human PBMC were stimulated by immobilized anti-CD44 MoAb as described in the Materials and Methods. At the indicated time points, cells were lysed and equivalent amounts of lysates were electrophoresed under reducing conditions, blotted, and probed for tyrosine phosphorylated proteins by PY20 followed by GAM-AP and ECL detection (upper panel). Lane 1, unstimulated cells; lanes 2 through 6, cells plated in CD44 MoAb-coated wells, lane 7, cells plated in control mouse IgG-coated wells (C). Equivalent protein loading in the wells was verified by Ponceau-S staining of the blots, as well as by probing for Lck and Fyn kinases (lower panels).
Kinase activities associated with CD44 are localized in low-density plasma membrane domains. (A) One milliliter of total Brij-58 lysate from 10 ×106 PBMC was precleared with Pansorbin and immunoprecipitated on protein A/G beads coated with indicated antibodies or a control MoAb (against hapten TNP) and blocked subsequently with FCS. After washing, the beads were assayed for the associated kinase activity at 30°C for 15 minutes. The reaction was stopped by boiling in sample buffer, and the phosphorylated proteins separated by SDS-PAGE were detected by autoradiography. (B) After gradient centrifugation of the Brij-58 extract as in Fig 1, the top (3 through 5) and bottom (9 through 11) fractions were pooled separately. The bottom pool containing 80% of the cellular proteins was diluted four times to equalize the protein concentration. Immunoprecipitation with indicated antibodies from the pooled top or bottom fractions and kinase reaction were performed as in (A). The autoradiograms were obtained after overnight exposure at room temperature. Similar results were obtained with PHA blasts (not shown). Kinase activities associated with CD44 are better preserved in whole lysates or gradient top fractions when Brij-58 was used; experiments with TX-100–lysed samples required a much longer exposure time, but showed an identical phosphoprotein profile (not shown).
Kinase activities associated with CD44 are localized in low-density plasma membrane domains. (A) One milliliter of total Brij-58 lysate from 10 ×106 PBMC was precleared with Pansorbin and immunoprecipitated on protein A/G beads coated with indicated antibodies or a control MoAb (against hapten TNP) and blocked subsequently with FCS. After washing, the beads were assayed for the associated kinase activity at 30°C for 15 minutes. The reaction was stopped by boiling in sample buffer, and the phosphorylated proteins separated by SDS-PAGE were detected by autoradiography. (B) After gradient centrifugation of the Brij-58 extract as in Fig 1, the top (3 through 5) and bottom (9 through 11) fractions were pooled separately. The bottom pool containing 80% of the cellular proteins was diluted four times to equalize the protein concentration. Immunoprecipitation with indicated antibodies from the pooled top or bottom fractions and kinase reaction were performed as in (A). The autoradiograms were obtained after overnight exposure at room temperature. Similar results were obtained with PHA blasts (not shown). Kinase activities associated with CD44 are better preserved in whole lysates or gradient top fractions when Brij-58 was used; experiments with TX-100–lysed samples required a much longer exposure time, but showed an identical phosphoprotein profile (not shown).
CD44 is associated with Lck and Fyn kinases in the low-density membrane domains. After kinase assay on CD44 immunoprecipitates from pooled low-density fractions as in Fig 5B, left panel top, the immune complexes were washed in 0.5% Brij-58 lysis buffer, dissociated by SDS, and reimmunoprecipitated using the indicated antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and visualized by autoradiography after 4 days of exposure. The total phosphoprotein profile associated with the primary CD44 immunoprecipitate is shown in the first lane.
CD44 is associated with Lck and Fyn kinases in the low-density membrane domains. After kinase assay on CD44 immunoprecipitates from pooled low-density fractions as in Fig 5B, left panel top, the immune complexes were washed in 0.5% Brij-58 lysis buffer, dissociated by SDS, and reimmunoprecipitated using the indicated antibodies. The immunoprecipitated proteins were separated by SDS-PAGE and visualized by autoradiography after 4 days of exposure. The total phosphoprotein profile associated with the primary CD44 immunoprecipitate is shown in the first lane.
DISCUSSION
CD44 transduces activation signals in T lymphocytes, CTLs, PMNs, NK cells, B lymphocytes, and macrophages,7-25 but how exactly these signals are transduced across the plasma membrane remains poorly understood. MoAb-induced CD44 cross-linking increases protein tyrosine phosphorylation, calcium influx, and gene activation in target cells.10,12,22,43 Interaction of CD44 with cytoskeletal networks and GTP,47 intracellular PTK Lck,30and p185 HER2 transmembrane oncoprotein with PTK activity48have been implicated as transmembrane signaling mechanisms. In the present report, we show that (1) only a small proportion of all CD44 receptors associates with Lck, (2) this interaction occurs in specialized plasma membrane microdomains, and (3) in addition to Lck, Fyn is also recovered in association with CD44 from these membrane domains. These membrane domains, enriched in GSLs and GPI-anchored glycoproteins, also harbor a number of other signaling molecules and thus represent privileged signaling sites in the plasma membrane.39
GSL-rich plasma membrane rafts are conveniently isolated as floating membrane vesicles in isopyknic density gradients.40,41Whereas the association with buoyant membrane domains of GPI-anchored proteins arises from specific interactions of their lipid anchors with GSLs,49 the acyl modification of CD44 at the C-terminus47 is unlikely to associate the molecule with low-density membrane complexes, because tailless mutants of CD44 were still detergent-insoluble and buoyant.32 Although CD44 does not seem to interact directly with GPI-anchored proteins (see Fig 3B), interactions with GSLs through the carbohydrate head groups that could engage a fraction of CD44 in such low-density membrane complexes are not ruled out. It is interesting to note that GSLs are also potent signal transducers50,51 as GPI-anchored glycoproteins, and that their expression, like that of CD44, is modulated in metastatic tumor cells.52
Ligand-mediated interactions with transmembrane signal transducing receptors having PTK activity in their cytoplasmic domains underlie the signaling capacity of some GPI-anchored proteins such as receptors for ciliary neurotrophic factor (CNTFR-α) and glial-cell derived neurotrophic factor (GDNFR-α).53 Interactions with integrins constitutes another mode of signaling via GPI-anchored proteins, eg, CD87 (uPAR) and CD16b (FcγRIIIB).54,55 Most other GPI-anchored proteins appear to signal through their association with intracellular Src family PTKs in GSL-rich plasma membrane domains.35,36,56 FcεRI, a transmembrane protein complex, also appears to signal through its association with Lyn kinase in similar membrane domains57 and critically depends on the integrity of such domains for signaling.58 In addition to PTKs, the GPI, GSLs, and caveolin-rich domains contain other signal transduction molecules that include G proteins,38 calcium channels, and enzymes involved in phosphoinositide metabolism.59,60 Cross-linking is believed to induce coalescence of these domains, initiate protein tyrosine phosphorylation, and generate activation signals.39
Unlike the GPI-anchored glycoproteins, which are confined to the external leaflet of the plasma membrane, CD44 traverses the membrane bilayer. However, the cytoplasmic domain of CD44 does not seem to contain any sequence motif that could mediate binding of Src kinases.30 Whether the interactions of CD44 with Lck and Fyn depend entirely on the lipid milieu of the buoyant membrane domains or involve additional linker protein(s) is not known at present. Nevertheless, this association raises the possibility that signaling via CD44 can result from the aggregation of functional membrane rafts as in the case of several GPI-anchored proteins. The observation that HA polymers are poor agonists compared to fragmented HA23supports this hypothesis and suggests that HA polymers might immobilize the membrane domains and prevent their coalescence. Also, it should be noted that F(ab)2 fragments of anti-CD44 MoAb significantly inhibit the HA-induced proliferation of B lymphocytes,25probably preventing domain coalescence. Considerable variations in the stimulatory effects of HA on human T cells versus mouse T cells and B cells versus T cells25 could arise from the differences in the polymer composition of commercially obtained HA23 as much as from the cell-type specificity of the responses. Moreover, some anti-CD44 MoAbs stimulate cells, whereas others are ineffective or even inhibitory1 and suppress inflammation.61 62Clearly, further investigations are required to fully understand the subtleties of signaling via CD44 in different cell types.
Recently, it has been demonstrated that induction of HA binding capacity on T cells could result from activation-induced clustering of CD44 followed by disulfide bond-mediated dimerization.63Whether homodimerization is required for signaling via CD44 is not known. Covalent dimerization involves the Cys 286 residue in the transmembrane domain of CD4463 that is essential for binding high levels of HA.64 It is noteworthy that (1) the transmembrane domain alone without the cytoplasmic tail is sufficient to confer detergent insolubility and buoyancy,32 and (2) only a small fraction of total cell surface CD44 is detergent insoluble, buoyant,32 can form homodimers to confer increased HA binding,63 and associates with Src family PTKs in the low-density membrane domains (this report). We are currently investigating whether the fraction of CD44 associated with glycosphingolipid-rich membrane rafts is both signal competent CD44 and contributes to high-affinity HA binding induced upon cellular activation.
ACKNOWLEDGMENT
The authors thank Dr Vaclav Horejsi for his generous gift of antibodies.
Supported by the Swiss National Science Foundation Grant No. 31-39 709.93 and by Swiss Cancer League Grant No. 462-2-1997. S.I. is supported by a grant from Sir Jules Thorn Charitable Trusts.
Address reprint requests to Daniel C. Hoessli, MD, Department of Pathology, Centre Médical Universitaire, 1 rue Michel Servet, 1211 Geneva 4, Switzerland; e-mail:Daniel.Hoessli@medecine.unige.ch.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal