It has been shown that the novel synthetic triterpenoid CDDO inhibits proliferation and induces differentiation and apoptosis in myeloid leukemia cells. In the current study the effects of the C-28 methyl ester of CDDO, CDDO-Me, were analyzed on cell growth and apoptosis of leukemic cell lines and primary acute myelogenous leukemia (AML). CDDO-Me decreased the viability of leukemic cell lines, including multidrug resistant (MDR)-1–overexpressing, p53null HL-60-Dox and of primary AML cells, and it was 3- to 5-fold more active than CDDO. CDDO-Me induced a loss of mitochondrial membrane potential, induction of caspase-3 cleavage, increase in annexin V binding and DNA fragmentation, suggesting the induction of apoptosis. CDDO-Me induced pro-apoptotic Bax protein that preceded caspase activation. Furthermore, CDDO-Me inhibited the activation of ERK1/2, as determined by the inhibition of mitochondrial ERK1/2 phosphorylation, and it blocked Bcl-2 phosphorylation, rendering Bcl-2 less anti-apoptotic. CDDO-Me induced granulo-monocytic differentiation in HL-60 cells and monocytic differentiation in primary cells. Of significance, colony formation of AML progenitors was significantly inhibited in a dose-dependent fashion, whereas normal CD34+ progenitor cells were less affected. Combinations with ATRA or the RXR-specific ligand LG100268 enhanced the effects of CDDO-Me on cell viability and terminal differentiation of myeloid leukemic cell lines. In conclusion, CDDO-Me is an MDR-1– and a p53-independent compound that exerts strong antiproliferative, apoptotic, and differentiating effects in myeloid leukemic cell lines and in primary AML samples when given in submicromolar concentrations. Differential effects of CDDO-Me on leukemic and normal progenitor cells suggest that CDDO-Me has potential as a novel compound in the treatment of hematologic malignancies.
Introduction
Acute myelogenous leukemia (AML) remains incurable in most patients, largely because of its resistance to chemotherapy. The therapeutic regimens used have not changed in the past 3 decades and usually include cytosine arabinoside (ara-C) and anthracycline analogs.1,2 Recently, topoisomerase inhibitors, cytokines, and multidrug resistant (MDR)-1 blockers have been evaluated, but they failed to have major impact on patient survival.3-7
Most chemotherapy agents used in the treatment of hematologic malignancies eliminate cells by inducing apoptosis, and many factors that inhibit chemotherapy-induced apoptosis have been identified. The initiation of a cascade of cysteine proteases of the ICE/ced3 family (caspases) plays a pivotal role in apoptosis.8 Theextrinsic death receptor pathway, triggered by members of the tumor necrosis factor family, is activated when the proximal regulator caspase-8 is recruited into the death receptor complex. The Bcl-2 family of proteins, on the other hand, seems to play a central role in the regulation of the mitochondrial (intrinsic) apoptotic pathway. In particular, Bcl-2 and Bcl-XLoverexpression prevent the mitochondrial release of cytochromec, caspase activation, and apoptosis (for review, see9-11). Bax is a pro-apoptotic member of the Bcl-2 family that dimerizes with itself or with Bcl-2/Bcl-XL, and an increase in the levels of free Bax, or Bax homodimers, promotes apoptosis.12,13 Bcl-2 has been the subject of intense study as a mechanism of chemoresistance because of its ability to suppress chemotherapy-induced apoptosis.12,14,15 Recent studies have demonstrated that phosphorylation of Bcl-2 at the serine 70 site is required to inhibit apoptosis.16,17 Protein kinase (PKC)-α and the mitogen-activated protein (MAP) kinases ERK1 (p44) and ERK2 (p42) were identified as physiologic Bcl-2 kinases,18,19 and PKC-α expression was observed to modulate the prognostic impact of Bcl-2 in AML.20 These collective results strongly suggest that Bcl-2 phosphorylation plays a role in the resistance of cells to chemotherapy-induced apoptosis.
Triterpenoids, together with their close chemical relatives, steroids, are members of a larger family of structurally related compounds called cyclosqualenoids.21 Oleanolic and ursolic acids are both derived from squalene and have definite, though weak, anti-inflammatory and anticarcinogenic properties.22,232-Cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) is a novel synthetic triterpenoid that is more than 10 000-fold more potent than its parent compound, oleanolic acid, in suppressing the γ-interferon–induced synthesis of nitric oxide by mouse macrophages.24 Further, CDDO was shown to have potent differentiating, antiproliferative, and anti-inflammatory properties.24,25 It was reported to activate caspase-8 and -3 and to induce mitochondrial cytochrome c release in leukemic cell lines and in osteosarcoma cells.26,27 In the current study, we analyzed the effects of a novel C-28 methyl ester of CDDO, CDDO-Me,28 on the growth and apoptosis of leukemic cell lines and primary blast cells from patients with AML. We investigated the molecular mechanism of CDDO-Me–induced apoptosis and the effects of combinations of CDDO-Me with cytosine–ara-C and retinoids because of their established activity in the clinical therapy for AML.
Patients, materials, and methods
Reagents
Stock solutions of CDDO-Me29 at 10 mM in dimethyl sulfoxide (DMSO) were stored at −20°C. Working solutions were prepared in DMSO and added directly to culture medium. ATRA was purchased from Sigma Chemical (St Louis, MO) and kept in 100% ethanol solution at −20°C. RXR-specific ligand LG100268 was kindly provided by Dr Richard Heyman. Caspase-3 inhibitor Z-DEVD-fmk and bongkrekic acid (BA) were obtained from Calbiochem (La Jolla, CA). Cyclosporin A (CyA) was purchased from Sandoz. Fas-signaling antibody CH11 and Fas-blocking antibody ZB4 were obtained from Immunotech (Miami, FL).
Cell lines
HL-60, KG-1, U937, and Jurkat cell lines were obtained from the American Type Culture Collection (Rockville, MD). NB4 cells were kindly provided by Dr M. Lanotte.30 HL-60–doxorubicin-resistant cells31 (HL-60-DOX) were also used. U937/Bcl-2 and its appropriate vector controls (U937/pCEP) were provided by Dr S. Grant.32 U937 cells were transfected with WT, S70A, or S70E cDNA containing cytomegalovirus plasmids17 by electroporation (200 V, 975 μF capacitance) and were selected and maintained in the medium plus 500 μg/mL G418 (Gibco BRL, Gaithersburg, MD).
Patients
Samples of bone marrow or peripheral blood were obtained for in vitro studies from patients with newly diagnosed or recurrent AML with high (more than 70%) blast count and from patients with myeloid transformation of chronic myeloid leukemia (CML). Informed consent was obtained following institutional guidelines. Mononuclear cells were separated by Ficoll-Hypaque (Sigma Chemical) density-gradient centrifugation.
Suspension culture of leukemic cells
Leukemic cell lines were cultured at a density of 3.0 × 105 cells/mL, and AML mononuclear cells were cultured at 5 × 105 cells/mL in the presence or absence of indicated concentrations of CDDO-Me. Appropriate amounts of DMSO (final concentration less than 0.05%) were included as control. For cytotoxicity studies, 1 μM ara-C was added to the cultures. After 24 to 72 hours, viable cells were counted with the trypan blue dye exclusion method using a hematocytometer.
Cell kinetic and DNA fragmentation studies
Cell cycle kinetics was determined by staining cells with acridine orange for cellular DNA and RNA content and was followed by flow cytometric analysis as described.33 Samples were measured in a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using the 488-nm line of a 15-nm argon laser and filter settings for green (530 nm) (DNA) and red (585 nm) (RNA) fluorescence. Ten thousand events were stored in list mode for analysis. The percentage of cells in the sub-G1 peak defined the proportion of apoptotic cells in the tested populations. Cell debris was defined as events in the lowest 10% range of fluorescence, and these results were eliminated from analysis. Cell-cycle kinetics was analyzed using ModFit software (Verity Software House, Topsham, ME).
Acute myelogenous leukemia blast colony assay
A previously described method was used to measure AML blast colony formation.34 35 Briefly, 1 × 105T-cell–depleted, nonadherent, low-density bone marrow cells were plated in 0.8% methylcellulose in Iscoves modified Dulbecco medium (IMDM; Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum and 15 ng/mL recombinant human granulocyte–macrophage colony-stimulating factor (hGM-CSF). CDDO-Me was added at the initiation of cultures at concentrations ranging from 0.05 to 0.5 μM. AML blast colonies were evaluated under a microscope on day 7 of culture in duplicate dishes.
Granulocyte-erythroid-macrophage-megakaryocyte colony-forming unit assay
In 3 experiments, 2 × 105 CD34+ cells isolated from normal bone marrow (n = 1) or G-CSF–stimulated peripheral blood (n = 2) were plated in 0.8% methylcellulose with IMDM, 1 U/mL human erythropoietin (Terry Fox Laboratories, Vancouver, BC, Canada), and 50 ng/mL recombinant hGM-CSF. CDDO-Me was added at the initiation of cultures at concentrations ranging from 0.05 to 0.5 μM. All cultures were evaluated after 14 days for the number of erythroid burst-forming unit (BFU-E) colonies, defined as aggregates of more than 500 hemoglobinized cells or 3 or more erythroid subcolonies, and granulocyte–macrophage colony-forming unit (CFU-GM) colonies, defined as a cluster of 40 or more granulocytes, monocyte–macrophages, or both.
Western blot analysis
An equal amount of protein lysate was placed on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for 2 hours at 100 V, followed by transfer of the protein to a Nytran membrane (Schleicher & Schuell, Keene, NH) and immunoblotting. Polyclonal rabbit antibodies to Bcl-2, Bcl-XL, and Bax36 37 were kindly provided by Dr J. C. Reed. Antibodies against poly (ADP-ribose) polymerase (PARP) was obtained from PharMingen (San Diego, CA), DFF-45 was from Oncogene (Cambridge, MA), XIAP was from Transduction Laboratories (Lexington, KY), caspase-3 was from PharMingen, and phospho-specific anti-pERK1/2 antibodies were from Calbiochem. A specific antibody recognizing only the p20-processed caspase-3 band was provided by Idun Pharmaceutical (La Jolla, CA).
Cell fractionation and Bax immunolocalization studies
Subcellular fractionation of cells was performed by a previously described method.38 Briefly, cells were swollen in ice-cold hypotonic HEPES buffer (10 mM HEPES, pH 7.4, 5 mM MgCl2, 40 mM KCl, 1 mM PMSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin) for 30 minutes, aspirated repeatedly through a 25-gauge needle (25 strokes), and centrifuged at 200g to pellet the nuclei. The resultant supernatant was then centrifuged at 10 000g to pellet the heavy-membrane fraction containing the mitochondria. The heavy-membrane supernatant was centrifuged at 150 000g to pellet the plasma membranes, and the supernatant represented the cytosol (Cyt). Subcellular fractions were subjected to denaturing electrophoresis in a 12% acrylamide–0.1% SDS gel and transferred to nitrocellulose for Bax Western blot analysis.
Northern blot analysis
The Bax probe was obtained by cloning the polymerase chain reaction products of amplified cDNA. The sequence was compared with GenBank data to ensure that the correct cDNA was cloned. Twenty micrograms total RNA was denatured and run overnight on a 1% formamide agarose gel at 30 V. After staining in ethidium bromide, RNA was transferred to a Nitran filter and left overnight in 10× sodium chloride–sodium citrate, followed by drying at 80°C. Hybridization was carried out at 42°C for 20 hours, and the filters were washed under highly stringent conditions. Signals were analyzed with a Betascope 603 (Betagen, Waltham, MA).
Metabolic labeling, immunoprecipitation, and immunoblot analysis
Cells were labeled with [32P]orthophosphoric acid and then treated with 0.1 μM CDDO-Me, after which Bcl-2 was analyzed by immunoprecipitation, as previously described.17 39Samples were electrophoresed in a 12% acrylamide–0.1% SDS gel, transferred to nitrocellulose, and exposed to Hyperfilm (Amersham Pharmacia Biotech, United Kingdom) at −80°C. The same blot was used for Western blot analysis with anti–Bcl-2 antisera.
In vitro ERK assay
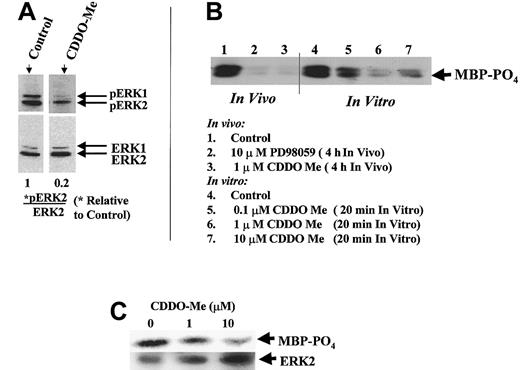
The effect of CDDO-Me was determined using an in vitro MAP kinase assay kit from Upstate Biotechnology (Lake Placid, NY) and ERK1/2 antibody from Santa Cruz Biotechnology (Santa Cruz, CA). For each sample, ERK 1/2 was immunoprecipitated from 2 × 107K562 or 1 × 107 HL60 cells using a specific anti-ERK 1/2 antibody and Protein A agarose (Life Technologies, Rockville, MD). The ERK-containing agarose pellet was resuspended in assay buffer containing an inhibitor cocktail (PKC inhibitor peptide, PKA inhibitor peptide, and compound R24571) to block possible contaminating non-ERK kinases. Where appropriate, varying concentrations (0.1, 1, and 10 μM) of CDDO-Me were added. Dephosphorylated myelin basic protein (MBP; 25 μg) was used as substrate. Phosphorylation of MBP was observed by using an anti–phospho-MBP antibody. As a negative control, a lysate containing inactive ERK (obtained from K562 cells treated for 4 hours in vivo with 10 μM MEK inhibitor PD98059) was used in the assay. The amount of ERK2 immunoprecipitated from each sample was determined by using anti-ERK2 antibody.
For K562 cells, a control experiment was performed to determine that CDDO-Me could at least inhibit ERK upstream, if not directly. K562 cells were treated in vivo for 4 hours with 1 μM CDDO-Me, and lysate from these cells was used in the in vitro kinase assay.
Immunophenotyping
Phycoerythrin-conjugated anti-CD11b, fluorescein isothiocyanate (FITC)–conjugated anti-CD14 monoclonal antibody (mAb) (Becton Dickinson) and phycoerythrin-conjugated anti-CD95 mAb (PharMingen) were used at a 1:10 dilution. Percentage positive cells was calculated by subtracting the percentage of cells with a fluorescence intensity greater than the set marker using the isotype control (background) from the percentage of cells with a fluorescence intensity greater than the same marker using the specific antibody.40
Annexin V staining
Cells were washed in phosphate-buffered saline and resuspended in 100 μL binding buffer containing annexin V (Roche Diagnostic, Indianapolis, IN). Cells were analyzed by flow cytometry after the addition of propidium iodide (PI).41 Annexin V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and PI stains the cellular DNA of those cells with a compromised cell membrane. This allows for live cells (unstained with either fluorochrome) to be discriminated from apoptotic cells (stained only with annexin V) and necrotic cells (stained with both annexin and PI).42
Cytofluorometric analysis of the Δψm
To evaluate the Δψm, cells were incubated with the cationic lipophilic dye chlorophenyl-X–rosamine (CMXRos; 150 nM; Molecular Probes, Eugene, OR).43 CMXRos is incorporated into mitochondria driven by the Δψm and reacts with thiol residues to form covalent thiol ester bonds. CMXRos fluorescence was recorded by flow cytometry in fluorescence channel 3 (FL-3). Background values of the apoptosis of control cells cultured without the CDDO-Me or in DMSO-solvent control (less than 10% CMXRos-low) were subtracted from the values obtained under experimental conditions.
Detection of active caspases
Cell-permeable fluorigenic substrate Phi-Phi-Lux-G1D2 was administered to monitor caspase activity according to the manufacturer's recommendations (OncoImmunin, Kensington, MD).46 Briefly, 106 cells were resuspended in 5 μL substrate solution and incubated for 1 hour at 37°C in the dark. After incubation, cells were washed, and the fluorescence emission was determined using the FL-1 channel of a Becton Dickinson FACScan flow cytometer.
Statistics
Results are expressed as means ± SEM. Levels of significance were evaluated by a 2-tailed paired Student ttest, and P < .05 was considered significant.
Results
CDDO-Me decreases viability and induces apoptosis in leukemic cell lines
CDDO-Me decreased the viability in leukemic HL-60, KG-1, and NB4 cells, with respective IC50 values of 0.4, 0.4, and 0.27 μM, as determined by cell counts at 48 hours. To study the mechanism of growth inhibition, we analyzed the effect of CDDO-Me on cell cycle and apoptosis in HL-60 cells. CDDO-Me at 0.05 and 0.1 μM inhibited cell growth in a dose- and time-dependent fashion, and no viable cells were recovered at 0.5 μM (Figure1A). Although cell-cycle measurements revealed no significant differences in cell-cycle distribution, a dose-dependent increase in annexin V binding in CDDO-Me–treated cells was seen (Figure 1B), suggesting that apoptosis contributed to CDDO-Me–induced growth arrest. HL-60 cells are p53null, suggesting that the cytotoxic effect of CDDO-Me is p53 independent. MDR-1 overexpressing HL-60–Dox cells were also sensitive to CDDO-Me, and blocking MDR-1 using the specific inhibitor PSC-833 did not enhance CDDO-Me cytotoxicity (data not shown). Hence, CDDO-Me appears to be p53 and MDR-1 independent.
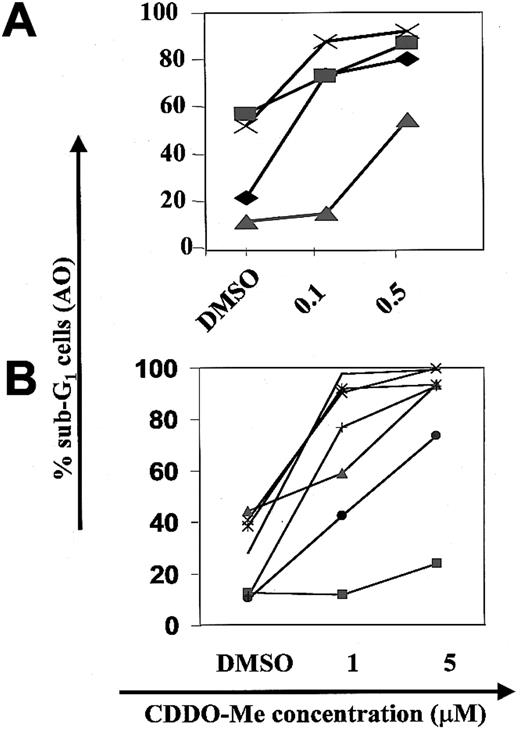
CDDO-Me inhibition of cell growth and induction of apoptosis in HL-60 cells.
HL-60 cells were incubated with different concentrations (0.05, 0.1, 0.5 μM) of CDDO-Me or DMSO (control) for 96 hours. The effect on cell growth was examined by cell count (A). Apoptosis was measured by staining with fluorescein isothiocyanate-labeled annexin V, which binds phosphatidylserine with high affinity (B). Cells were simultaneously stained with PI and analyzed by flow cytometry. At 0.5 μM CDDO-Me, more than 95% of cells were annexin V–PI-positive at 48 hours (not shown).
CDDO-Me inhibition of cell growth and induction of apoptosis in HL-60 cells.
HL-60 cells were incubated with different concentrations (0.05, 0.1, 0.5 μM) of CDDO-Me or DMSO (control) for 96 hours. The effect on cell growth was examined by cell count (A). Apoptosis was measured by staining with fluorescein isothiocyanate-labeled annexin V, which binds phosphatidylserine with high affinity (B). Cells were simultaneously stained with PI and analyzed by flow cytometry. At 0.5 μM CDDO-Me, more than 95% of cells were annexin V–PI-positive at 48 hours (not shown).
CDDO-Me decreases viability, induces apoptosis, and inhibits colony formation in primary acute myelogenous leukemia cells
In primary AML cells, CDDO-Me induced a dose-dependent increase in the percentage of apoptotic cells, as determined by DNA flow cytometry (n = 4, Figure 2A). The paired mean difference between 0.1 μM CDDO-Me and DMSO-treated cells was 26.9% ± 10.8% (CDDO-Me–DMSO) and 43.2% ± 6.0% at 0.5 μM CDDO-Me (P < .01). In an additional series of 7 patient samples, apoptosis was induced in 6 of 7 AML samples at 1 μM CDDO-Me, and 5 μM induced apoptosis in all samples with more than 70% apoptotic cells in 6 of 7 samples (Figure 2B). CDDO-Me also induced apoptosis in CML blast crisis samples in vitro (in 3 of 4 samples at 1 μM, in all 4 samples at 5 μM).
CDDO-Me induction of apoptosis in primary AML samples.
AML blasts from AML samples 1 to 4 were grown in the presence of 0.1 and 0.5 μM CDDO-Me (A), and AML samples 5 to 11 at 1 or 5 μM for 48 hours (B). Apoptosis was measured by subG1-DNA flow cytometry (AO, acridine orange).
CDDO-Me induction of apoptosis in primary AML samples.
AML blasts from AML samples 1 to 4 were grown in the presence of 0.1 and 0.5 μM CDDO-Me (A), and AML samples 5 to 11 at 1 or 5 μM for 48 hours (B). Apoptosis was measured by subG1-DNA flow cytometry (AO, acridine orange).
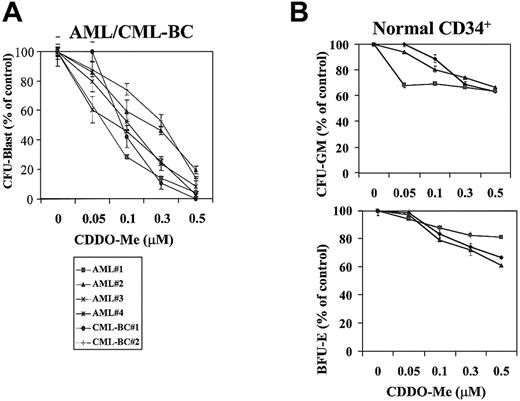
We then examined the effect of CDDO-Me on clonogenic AML cells. Colony formation of AML progenitors was significantly inhibited in a dose-dependent fashion, with 46.7% ± 6.6% surviving colonies at 0.1 μM CDDO-Me and only 8.8% ± 3.8% at 0.5 μM (Figure3). CDDO-Me at 0.5 μM also inhibited more than 50% of colonies in 2 CML myeloid blast crisis samples. In contrast, 64.5% ± 1.1% and 60.8% ± 2.5% CFU-GM and CFU-E from normal CD34+ cells survived treatment with 0.5 μM CDDO-Me. The difference between AML and normal progenitors was highly significant (P < .02).
Effect of CDDO-Me on leukemic and normal clonogenic progenitors was studied.
(A) CDDO-Me inhibition of AML clonogenic progenitor growth. Data represent average results from 4 different AML and 2 CML-BC samples. Results are expressed as the mean ± SEM of the number of colonies in the presence of increasing concentrations of CDDO-Me (0.05, 0.1, 0.3, 0.5 μM) compared with the number in control cells. The mean number of CFU-blast colonies in the control cultures of experiments 1, 2 3, 4, 5, and 6 were 1268.5 ± 29.0, 350.0 ± 18.4, 473.5 ± 12.0, 360.5 ± 12.0, 65.5 ± 6.4, and 526 ± 14.1, respectively. (B) Effect of CDDO-Me on the growth of normal myeloid and erythroid progenitors using magnetically separated CD34+cells. The mean numbers of CFU-GM colonies in the control cultures of experiments 1, 2, and 3 were 687.5 ± 7.5, 995.5 ± 14.5, and 331 ± 7, respectively; the mean numbers of BFU-E colonies were 384.5.5 ± 14.5, 585.5 ± 7.5, and 259.5 ± 7.5.
Effect of CDDO-Me on leukemic and normal clonogenic progenitors was studied.
(A) CDDO-Me inhibition of AML clonogenic progenitor growth. Data represent average results from 4 different AML and 2 CML-BC samples. Results are expressed as the mean ± SEM of the number of colonies in the presence of increasing concentrations of CDDO-Me (0.05, 0.1, 0.3, 0.5 μM) compared with the number in control cells. The mean number of CFU-blast colonies in the control cultures of experiments 1, 2 3, 4, 5, and 6 were 1268.5 ± 29.0, 350.0 ± 18.4, 473.5 ± 12.0, 360.5 ± 12.0, 65.5 ± 6.4, and 526 ± 14.1, respectively. (B) Effect of CDDO-Me on the growth of normal myeloid and erythroid progenitors using magnetically separated CD34+cells. The mean numbers of CFU-GM colonies in the control cultures of experiments 1, 2, and 3 were 687.5 ± 7.5, 995.5 ± 14.5, and 331 ± 7, respectively; the mean numbers of BFU-E colonies were 384.5.5 ± 14.5, 585.5 ± 7.5, and 259.5 ± 7.5.
CDDO-Me induces changes in the apoptotic machinery
To determine the sequence of molecular changes during CDDO-Me–induced cell death, we performed time-course studies of apoptosis in U937 and HL-60 cells. Loss of mitochondrial membrane potential (Δψm) was described in a number of different models of apoptosis. CDDO-Me–treated U937 cells exhibited a time-dependent decrease in Δψm (Figure4A), with complete loss of mitochondrial membrane potential at 6 hours. Similarly, exposure of HL-60 to 1 μM CDDO-Me for 2 and 4 hours decreased the percentage of cells with intact Δψm by 46% and 57%. We next used pharmacologic inhibitors of permeability transition (CyA44 and BA45) to validate the role of mitochondrial disruption. CyA partially inhibited CDDO-Me–triggered Δψm loss, providing further evidence that CDDO-Me affects Δψm(Figure 4C). CyA also prevented CDDO-Me–induced cell killing at 4 hours (HL-60 control, 4.8 × 105 cells/mL; 1 μM CDDO-Me, 2.8 × 105 cells/mL; CDDO-Me + CyA, 4.1 × 105 cells/mL). Similarly, BA reduced mitochondrial depolarization by CDDO-Me (77% vs 48% CMXRos-high cells) and decreased the percentage of cells with activated caspases by 60%.
CDDO-Me–induced decrease in the mitochondrial membrane potential and caspase activation in U937 cells.
Cells were assayed for apoptosis after 2, 4, and 6 hours of treatment with 1 μM CDDO-Me. (A) CMXRos assay (y-axis) to evaluate the reduction in the mitochondrial membrane potential. Cells expressing CMXRos below the threshold level were considered apoptotic. (C) Partial inhibition of CDDO-Me–induced loss of mitochondrial membrane potential by CyA. HL-60 cells were pretreated with the pharmacologic inhibitor of permeability transition CyA (10 μM) followed by CDDO-Me (1 μM) exposure for 2 or 4 hours. Loss of the mitochondrial membrane potential (ψm) was measured by CMXRos staining. No effect was observed from CyA alone (not shown). (D) HL-60 cells were exposed to 1 μM CDDO-Me for 2, 4, 6, and 8 hours. Caspase-3 cleavage was studied by Western blot analysis using antibody to caspase-3 (pro–caspase-3 at 32 kd and cleaved caspase-3 at 17 kd). (B) Activated caspase-3 (x-axis), as measured by conversion of Phi-Phi-Lux, and PI (y-axis). Cells showing a high conversion of Phi-Phi-Lux are considered apoptotic.
CDDO-Me–induced decrease in the mitochondrial membrane potential and caspase activation in U937 cells.
Cells were assayed for apoptosis after 2, 4, and 6 hours of treatment with 1 μM CDDO-Me. (A) CMXRos assay (y-axis) to evaluate the reduction in the mitochondrial membrane potential. Cells expressing CMXRos below the threshold level were considered apoptotic. (C) Partial inhibition of CDDO-Me–induced loss of mitochondrial membrane potential by CyA. HL-60 cells were pretreated with the pharmacologic inhibitor of permeability transition CyA (10 μM) followed by CDDO-Me (1 μM) exposure for 2 or 4 hours. Loss of the mitochondrial membrane potential (ψm) was measured by CMXRos staining. No effect was observed from CyA alone (not shown). (D) HL-60 cells were exposed to 1 μM CDDO-Me for 2, 4, 6, and 8 hours. Caspase-3 cleavage was studied by Western blot analysis using antibody to caspase-3 (pro–caspase-3 at 32 kd and cleaved caspase-3 at 17 kd). (B) Activated caspase-3 (x-axis), as measured by conversion of Phi-Phi-Lux, and PI (y-axis). Cells showing a high conversion of Phi-Phi-Lux are considered apoptotic.
Because caspase-3 has a pivotal role in the intrinsic apoptosis pathway,8,47 48 we examined the effect of CDDO-Me on cleavage of caspase-3 by Western blot analysis. CDDO-Me–induced activation of caspase-3 resulted in the appearance of the 17-kd proteolytic product of caspase-3 at 2 hours and complete disappearance of uncleaved 32-kd caspase-3 after 6 hours (Figure 4D). Using the fluorigenic caspase-3 substrate Phi-Phi-Lux, 24% and 67% of U937 cells were Phi-Phi-Lux-positive at 4 and 6 hours after CDDO-Me (1 μM; Figure 4C). We also observed cleavage of the caspase-3 substrates PARP and DFF-45 starting at 4 hours (data not shown). Finally, translocation of phosphatidylserine to the cell surface was detected at 4 and 6 hours. Pretreatment of U937 and HL-60 cells with the caspase-3 inhibitor Z-DEVD-fmk (25 μM) significantly reduced annexin V positivity (Figure 5A), diminished the cleavage of Phi-Phi-Lux, and prevented CDDO-Me–induced cytotoxicity, as determined by cell counts. Western blot analysis using an antibody specific for cleaved caspase-3 demonstrated disappearance of the cleavage product (Figure 5B). Notably, pretreatment with Z-DEVD-fmk prevented mitochondrial depolarization (4% vs 40%) in U937 cells, suggesting the existence of a caspase–mitochondrial amplification loop. These data establish a key role of caspase-3 in CDDO-Me–induced apoptosis. Collectively, results demonstrate that CDDO-Me induces apoptosis by decreasing Δψm and activation of caspase-3 (at 2 hours or less), followed by cleavage of caspase-3 substrates and plasma membrane changes between 4 and 6 hours.
Caspase-3 inhibitor Z-DEVD-fmk blocks CDDO-Me–induced annexin V positivity and caspase-3 cleavage in leukemic cells.
(A) Evidence of apoptosis was identified by staining with annexin V (x-axis) and PI (y-axis) after 6 hours of 1 μM CDDO-Me treatment. DMSO, which was used as a solvent, was used as the control treatment. Cells binding annexin V and retaining PI were apoptotic (lower right quadrant); double-positive cells underwent secondary necrosis (upper right quadrant). In panel 3, U937 and HL-60 cells were pretreated with 25 μM Z-DEVD-fmk (a caspase-3 inhibitor) for 1 hour followed by 6 hours of exposure to CDDO-Me (1 μM). (B) Western blot analysis shows the appearance of the cleaved caspase-3 band at 2, 4, and 6 hours of CDDO-Me exposure (detected with antibody that recognizes only cleaved caspase-3, IDUN) and absence of the cleaved product in DEVD-pretreated cells.
Caspase-3 inhibitor Z-DEVD-fmk blocks CDDO-Me–induced annexin V positivity and caspase-3 cleavage in leukemic cells.
(A) Evidence of apoptosis was identified by staining with annexin V (x-axis) and PI (y-axis) after 6 hours of 1 μM CDDO-Me treatment. DMSO, which was used as a solvent, was used as the control treatment. Cells binding annexin V and retaining PI were apoptotic (lower right quadrant); double-positive cells underwent secondary necrosis (upper right quadrant). In panel 3, U937 and HL-60 cells were pretreated with 25 μM Z-DEVD-fmk (a caspase-3 inhibitor) for 1 hour followed by 6 hours of exposure to CDDO-Me (1 μM). (B) Western blot analysis shows the appearance of the cleaved caspase-3 band at 2, 4, and 6 hours of CDDO-Me exposure (detected with antibody that recognizes only cleaved caspase-3, IDUN) and absence of the cleaved product in DEVD-pretreated cells.
We also investigated the relationship between the sensitivity of leukemic cells to CDDO-Me and Fas signaling. No induction of Fas was observed by flow cytometry in several cell types tested. Anti–Fas blocking antibody ZB4 did not prevent CDDO-Me–induced killing in NB4 or Jurkat cells, and Fas-activating antibody CH11 did not sensitize cells to CDDO-Me cytotoxicity (not shown). In addition, no changes in caspase-8 were detected by Western blot analysis in HL-60 and U937 cells despite massive cell death. These data suggest that the induction of the FasL–Fas–caspase-8 pathway is not essential in the execution of CDDO-Me–induced cell death. Because Bcl-2/Bax is known to regulate mitochondrial membrane integrity, we examined the effects of CDDO-Me on Bcl-2 and Bax levels. By Northern blot analysis, CDDO-Me induced Bax mRNA levels in both HL-60 and U937 cells (Figure6A). These changes paralleled the increase in Bax protein levels, whereas Bcl-2 protein was only minimally affected in HL-60 and NB4 cells, despite substantial cell killing (Figure 6B). At high concentrations of CDDO-Me, Bcl-2 cleavage was observed when cell viability decreased by more than 50% (data not shown), presumably as a consequence of caspase-3 activation.49 Induction of apoptosis was shown to shift Bax from the cytosol to mitochondrial membranes, where it directly induces cytochrome c release.50 We therefore performed subcellular fractionation studies to isolate mitochondrial and cytosolic fractions of control and CDDO-Me–treated cells. As shown in Figure 6C, CDDO-Me induced Bax protein levels, coincident with a Bax decrease in the cytosolic and with an increase in the mitochondrial (heavy membrane) fraction. The new band at 18 kd detected in the total lysate and in the mitochondrial but not in the cytosolic fraction likely represents a cleavage product of Bax.51
CDDO-Me–induced Bax expression and translocation to the mitochondria.
(A) Northern blot using Bax probe for HL-60 and U937 cells treated with 1 μM CDDO-Me for 6 hours. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase (B) NB4 cells were treated with the indicated concentrations of CDDO-Me. Protein lysates were analyzed by Western blot with anti-Bcl-2 and anti-Bax antibodies. (C) Western blot of subcellular fractions to detect Bax in mitochondria heavy membrane (HM) or cytosol (cyt). Tot, total protein. A band detected at 18 kd likely represents a cleavage product of Bax.51
CDDO-Me–induced Bax expression and translocation to the mitochondria.
(A) Northern blot using Bax probe for HL-60 and U937 cells treated with 1 μM CDDO-Me for 6 hours. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase (B) NB4 cells were treated with the indicated concentrations of CDDO-Me. Protein lysates were analyzed by Western blot with anti-Bcl-2 and anti-Bax antibodies. (C) Western blot of subcellular fractions to detect Bax in mitochondria heavy membrane (HM) or cytosol (cyt). Tot, total protein. A band detected at 18 kd likely represents a cleavage product of Bax.51
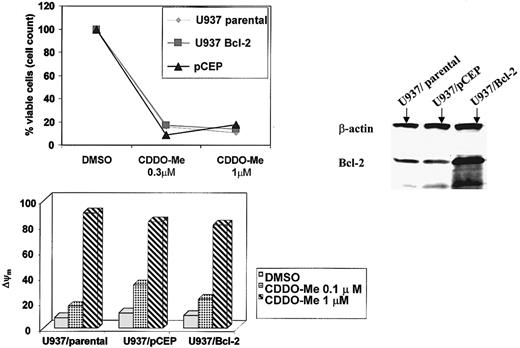
We next examined the effect of Bcl-2 overexpression on CDDO-Me–induced cytotoxicity. Surprisingly, U937/Bcl-2 cells were not protected from CDDO-Me–induced cytotoxicity as detected by cell growth and Δψm (Figure 7). These results suggest that CDDO-Me induces apoptosis independent of Bcl-2 levels and that posttranslational modifications of the protein may affect its anti-apoptotic function.
Lack of protection from CDDO-Me–induced apoptosis conferred by Bcl-2 in U937 cells.
U937 cells transduced with Bcl-2 (U937/Bcl-2) and their empty-vector counterparts (pCEP) were treated with the indicated concentrations of CDDO-Me for 24 hours. Growth inhibition was determined by cell count; changes in the mitochondrial membrane potential were measured by CMXRos staining (Δψm). Western blot demonstrates elevated Bcl-2 levels in the U937/Bcl-2 cells.
Lack of protection from CDDO-Me–induced apoptosis conferred by Bcl-2 in U937 cells.
U937 cells transduced with Bcl-2 (U937/Bcl-2) and their empty-vector counterparts (pCEP) were treated with the indicated concentrations of CDDO-Me for 24 hours. Growth inhibition was determined by cell count; changes in the mitochondrial membrane potential were measured by CMXRos staining (Δψm). Western blot demonstrates elevated Bcl-2 levels in the U937/Bcl-2 cells.
CDDO-Me abrogates Bcl-2 phosphorylation
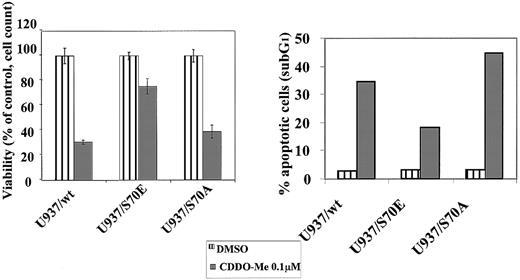
We then determined the effect of CDDO-Me on Bcl-2 phosphorylation by performing metabolic labeling studies with32P-orthophosphoric acid. After treatment with 0.1 μM CDDO-Me (a concentration that induces apoptosis), Bcl-2 phosphorylation was virtually abrogated (Figure8).52 To further investigate mechanisms of inhibition of Bcl-2 phosphorylation that could play a role in CDDO-Me–induced cell death, we selected U937 cells that were stably transfected with a serine 70→alanine, Bcl-2 mutant (S70A). Previous studies demonstrated that the S70A mutant was unable to be phosphorylated and that this mutation was also incapable of protecting cells from chemotherapy-induced apoptosis.17 The U937/S70A Bcl-2 cells were found to be sensitive to CDDO-Me (60% decrease in viability after 0.1 μM CDDO-Me). In contrast, a Ser→Glu mutant of Bcl-2, S70E, which may mimic a phosphate charge and was shown to potently suppress apoptosis, was found to be much more resistant to CDDO-Me–induced apoptosis compared with wild-type Bcl-2 (75% viable cells after 0.1 μM CDDO-Me compared with 30% for U937/wt; Figure9). These data indicate that the phosphorylation status of Bcl-2 contributes to cell sensitivity to CDDO-Me–induced killing. Of note, U937/wt and the S70E and S70A Bcl-2 transfectants express roughly equivalent levels of Bcl-2 protein as determined by densitometry (data not shown), suggesting that the observed differential effects are not related to differences in Bcl-2 expression.
CDDO-Me blockade of Bcl-2 phosphorylation.
U937 cells were radiolabeled with [32P]orthophosphate, treated with 0.1 μM CDDO-Me, and lysed, after which Bcl-2 was immunoprecipitated. Protein was subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to x-ray film to identify the phosphorylated Bcl-2. The identity of the protein was confirmed by Western blot analysis using the same filter. Pre, pre-immune serum.
CDDO-Me blockade of Bcl-2 phosphorylation.
U937 cells were radiolabeled with [32P]orthophosphate, treated with 0.1 μM CDDO-Me, and lysed, after which Bcl-2 was immunoprecipitated. Protein was subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to x-ray film to identify the phosphorylated Bcl-2. The identity of the protein was confirmed by Western blot analysis using the same filter. Pre, pre-immune serum.
Partial protection from CDDO-Me–induced apoptosis conferred by the U937/S70E Bcl-2 mutant.
Stable transfectants of U937 cells using a serine→alanine Bcl-2 mutant, S70A, and a Ser→Glu mutant, S70E, as well as U937/wt Bcl-2, were exposed to 0.1 μM CDDO-Me for 24 hours. Viability was determined by cell count with trypan blue exclusion, and apoptosis was measured by sub-G1 flow cytometry (acridine orange). S70E, which may mimic a potential phosphate charge, suppressed the CDDO-Me–induced apoptosis.
Partial protection from CDDO-Me–induced apoptosis conferred by the U937/S70E Bcl-2 mutant.
Stable transfectants of U937 cells using a serine→alanine Bcl-2 mutant, S70A, and a Ser→Glu mutant, S70E, as well as U937/wt Bcl-2, were exposed to 0.1 μM CDDO-Me for 24 hours. Viability was determined by cell count with trypan blue exclusion, and apoptosis was measured by sub-G1 flow cytometry (acridine orange). S70E, which may mimic a potential phosphate charge, suppressed the CDDO-Me–induced apoptosis.
Because PKC-α and the MAP kinases ERK1 (p44) and ERK2 (p42) have been identified as physiologic Bcl-2 kinases,18 19 studies were performed to assess their activation status. Western blot analysis of subcellular fractions of U937 cells revealed that little, if any, PKC-α was colocalized with Bcl-2 in the mitochondrial membranes of U937 cells (not shown), suggesting that mitochondrial PKC is not a likely target of CDDO-Me. However, both ERK1 and ERK2 were prominently detected in the mitochondrial membranes. Furthermore, although treatment of cells with 1 μM CDDO-Me for 3 hours had no effect on the total ERK1/2 protein levels (Figure10A), CDDO-Me blocked the activation of ERK1/2, as shown by the inhibition of ERK1/2 phosphorylation. Treatment of K562 cells with 1 μM CDDO-Me in vivo abrogated ERK kinase activity similar to specific MEK inhibitor PD98059 (Figure 10B, lanes 1-3). Of note, no effect on Akt activity was seen (data not shown). These findings suggest that CDDO-Me induces apoptosis by the inhibition of ERK1/2 and Bcl-2 phosphorylation.
CDDO-Me inhibits ERK1/2 activation–phosphorylation.
(A) Western blot analysis of U937 cells revealed inhibition of ERK1/2 phosphorylation (pERK1/2) after 1 μM CDDO-Me treatment for 3 hours but no effect on ERK1/2 protein levels (ERK1/2). The intensity of the bands was quantitated by densitometry and expressed as a ratio of pERK2/ERK2 relative to the control value. (B) K562 cells were treated in vivo for 4 hours with 1 μM CDDO-Me, and lysate of these cells was used in the in vitro assay (lanes 1-3). As a negative control, a lysate containing inactive ERK1/2 was used in the assay (after 4-hour treatment of intact K562 cells with 10 μM MEK inhibitor PD98059). Phosphorylation of ERK substrate MBP was observed using an anti–phospho-MBP antibody after SDS-PAGE. In a parallel experiment, active ERK was immunoprecipitated from K562 cells treated with 0.1, 1, and 10 μM of CDDO-Me in vitro, and MBP was added as substrate with a cocktail of kinase inhibitors (lanes 4-7). (C) Active ERK was immunoprecipitated from HL-60 cells, and kinase activity was determined by MBP phosphorylation. The amount of ERK2 immunoprecipitated from each sample was determined by using anti-ERK2 antibody.
CDDO-Me inhibits ERK1/2 activation–phosphorylation.
(A) Western blot analysis of U937 cells revealed inhibition of ERK1/2 phosphorylation (pERK1/2) after 1 μM CDDO-Me treatment for 3 hours but no effect on ERK1/2 protein levels (ERK1/2). The intensity of the bands was quantitated by densitometry and expressed as a ratio of pERK2/ERK2 relative to the control value. (B) K562 cells were treated in vivo for 4 hours with 1 μM CDDO-Me, and lysate of these cells was used in the in vitro assay (lanes 1-3). As a negative control, a lysate containing inactive ERK1/2 was used in the assay (after 4-hour treatment of intact K562 cells with 10 μM MEK inhibitor PD98059). Phosphorylation of ERK substrate MBP was observed using an anti–phospho-MBP antibody after SDS-PAGE. In a parallel experiment, active ERK was immunoprecipitated from K562 cells treated with 0.1, 1, and 10 μM of CDDO-Me in vitro, and MBP was added as substrate with a cocktail of kinase inhibitors (lanes 4-7). (C) Active ERK was immunoprecipitated from HL-60 cells, and kinase activity was determined by MBP phosphorylation. The amount of ERK2 immunoprecipitated from each sample was determined by using anti-ERK2 antibody.
To determine specific effects of CDDO-Me on ERK activity, we used an in vitro MAP kinase assay kit. ERK was immunoprecipitated from K562 cells because these cells contain the Bcr-Abl kinase and activated ERK present in these cells under basal conditions.53 CDDO-Me inhibited MBP phosphorylation in a dose-dependent manner (Figure 10B, lanes 4-7). Similar effects were observed in HL-60 cells (Figure 10C). These data suggest that there may be a direct effect of the compound on ERK kinase activity.
CDDO-Me induces differentiation and enhances the effects of ara-C and retinoids in leukemic cell lines
Although CDDO induces differentiation,24 our data demonstrated that its methyl ester is a more potent inducer of granulomonocytic differentiation. At 0.1 μM CDDO-Me, 86.6% of HL-60 cells expressed CD11b, whereas 1 μM CDDO was needed to exert a similar effect. Monocytic differentiation was induced in 2 of the 5 AML samples, as shown by induction of the monocytic differentiation marker CD14. CDDO-Me also enhanced ara-C–induced cell killing in primary AML (DMSO control, 24.9% ± 7.4%; 1 μM CDDO-Me, 50.5% ± 15%; 1 μM ara-C, 39.8% ± 8.2%; CDDO-Me + ara-C, 65.4% ± 10.2%; n = 6) (P < .01). In CML blast crisis samples, CDDO-Me also enhanced ara-C–induced cell death but induced differentiation in only 1 of 4 samples.
We then tested the combined effect of CDDO-Me and ATRA in leukemic cell lines. In HL-60, 59.4% and 21.2% of cells remained viable at 0.1 and 1 μM CDDO-Me; combining this with 0.5 μM ATRA further diminished the number of viable cells (31.6% and 9.6%, respectively). This decrease in viability was associated with increased DNA fragmentation. The combination decreased the numbers of viable cells in all cell lines tested (NB4, KG-1, HL-60) more than either CDDO-Me or ATRA alone, again suggesting that the combination of CDDO-Me and ATRA activates apoptotic pathways. In primary AML cells, ATRA enhanced CDDO-Me–induced apoptosis in 3 of 8 samples tested.
Others and we have previously demonstrated that ATRA down-regulates Bcl-2 mRNA transcription and protein expression.54 55 ATRA alone decreased Bcl-2 protein levels; however, there was no additive effect on Bcl-2 levels by combinations of ATRA and CDDO-Me. At the highest concentrations (0.5 μM CDDO-Me and 1 μM ATRA), Bcl-2 was cleaved (data not shown). Of interest, in the same experiment we also observed cleavage of XIAP, an IAP family member, a finding that correlates with a marked decrease of viability. XIAP cleavage product (30 kd) was detected in HL-60 cells at 0.5 μM CDDO-Me alone or at 0.3 μM CDDO-Me combined with 1 μM ATRA. However, cleavage of both Bcl-2 and XIAP occurred only after a pronounced decrease in cell viability and was likely to be a consequence of caspase activation, not the initiating event.
We then examined whether CDDO-Me in combination with ATRA enhances differentiation. HL-60 cells were cultured with 0.1 μM CDDO-Me for 72 hours, alone or in combination with 1 μM ATRA. CDDO-Me and ATRA were additive in inducing granulomonocytic differentiation: 25.8% ± 1.7% CDDO–Me- and 21% ± 2.5% ATRA-treated cells were positive for CD11b, whereas 52.6% ± 7.6% of cells treated with both agents expressed for CD11b (after subtraction of CD11b positivity in DMSO-treated controls).
We next tested whether combinations of CDDO-Me with the RXR-specific ligand LG100268 significantly potentiated the antileukemic effect of CDDO-Me. LG100268 at 10 nM, 100 nM, and 1 μM enhanced the killing of HL-60 cells in a dose-dependent manner (Table1). At 1 μM LG100268, cell growth was markedly inhibited, and the percentage of cells in S+G2M decreased by 50%. Collectively, our data show that combinations of CDDO-Me with either retinoids or rexinoids markedly decreased cell viability and induced terminal differentiation in myeloid leukemic cell lines.
RXR-specific ligand LG100268 enhances CDDO-Me-induced apoptosis in HL-60 cells (24 hours)
| LG100268 . | CDDO-Me . | |
|---|---|---|
| 0 . | 0.1 μM . | |
| 0 | 4.4 | 22.4 |
| 10 nM | 6.8 | 34.4 |
| 100 nM | 6.2 | 40.3 |
| 1 μM | 6.3 | 61.6 |
| LG100268 . | CDDO-Me . | |
|---|---|---|
| 0 . | 0.1 μM . | |
| 0 | 4.4 | 22.4 |
| 10 nM | 6.8 | 34.4 |
| 100 nM | 6.2 | 40.3 |
| 1 μM | 6.3 | 61.6 |
Results are expressed as a percentage of sub-G1 cells (flow cytometry).
Discussion
In this study, we observed that myeloid leukemia cell lines and primary AML blasts underwent rapid apoptosis when incubated with submicromolar concentrations of the novel triterpenoid, CDDO-Me. CDDO-Me was consistently more active than the parental compound, CDDO.25 Because HL-60 and U937 cells lack functional p53 protein, it appears that CDDO-Me triggers apoptosis independent of p53. HL-60–Dox cells overexpressing MDR-1 are sensitive to CDDO-Me–induced cell death, suggesting that CDDO-Me can induce apoptosis independent of the MDR-1 protein. This was further confirmed by the finding that the MDR-1 inhibitor, PSC 833, did not enhance CDDO-Me–induced killing.
CDDO-Me profoundly inhibited clonogenic cell growth in AML and in blast crisis CML, known to be resistant to most chemotherapeutic agents. In contrast, more than 50% of normal CFU-GM progenitor cells survived treatment with 0.5 μM CDDO-Me, a concentration that killed more than 90% of leukemic colonies in most AML. CDDO-Me also sensitized leukemic cells to apoptosis induced by ara-C.
In vitro studies have shown that ATRA alone or in combination with chemotherapeutic agents is effective in AML cells.56,57ATRA binds to the 3 RAR nuclear receptors, whereas LG100268 specifically binds to RXR receptors.58 RXRs play a central role in the cellular physiology of the nuclear receptor superfamily by virtue of their ability to modulate the activity of many other receptors. Therefore, we investigated the combined effects of CDDO-Me and retinoids on the viability, apoptosis, and differentiation of leukemic cells. Intriguingly, the combination of CDDO-Me with either ATRA or LG100268 exerted a potent cytotoxic effect in leukemic cells at concentrations that were ineffective when the agents were given singly. The primary mechanism of decreased leukemic cell survival appears to be the induction of apoptosis, as shown by DNA fragmentation and annexin V staining. ATRA also enhanced CDDO-Me–induced apoptosis in primary AML samples. Whether these effects can be attributed to the recent finding that CDDO-Me is an antagonist for the PPARγ nuclear receptor28 remains to be established.
Our findings further demonstrated that CDDO-Me induced the translocation of phosphatidylserine to the cell surface and caspase activation. Caspase-3 is the likely caspase that mediates CDDO-Me–induced apoptosis, as shown by Western blot analysis, flow cytometry using a florigenic substrate, and cleavage of the known caspase substrates PARP and DFF-45. Significantly, caspase-3 inhibition prevented the apoptosis of leukemic cells, establishing an essential role for caspase activation in CDDO-Me–induced apoptosis. In contrast, caspase-8 activation in Fas–Fas-ligand pathway does not play a critical role in the execution of CDDO-Me–induced cell death.
Early changes in mitochondrial membrane integrity point to involvement of the intrinsic cell death pathway. We studied whether apoptosis induced by CDDO-Me is the result of mitochondrial permeability transition changes by testing the effects of specific inhibitors of permeability transition pores, BA, and CyA on CDDO-Me–induced cell death. In addition to abolishing the apoptotic Δψmdisruption, the inhibitors of permeability transition also prevented caspase activation linked to apoptosis. These data suggest that a number of apoptotic events are secondary to mitochondrial damage.
The response of cells to apoptotic stimuli critically depends on the balance between pro-apoptotic and anti-apoptotic members of the Bcl-2 family. Bax promotes cell death, and its up-regulation has been associated with enhanced apoptosis.59,60 Although CDDO-Me did not affect the levels of Bcl-2 and Bcl-XL proteins, it induced Bax mRNA with a resulting increase in Bax protein levels. Results of subcellular fractionation studies demonstrated that CDDO-Me decreased the cytosolic and increased the mitochondrial Bax fraction. This translocation of Bax from the cytosol to mitochondrial membranes is known to directly induce cytochrome crelease.50,61 The critical role of Bax in apoptosis was recently demonstrated by the complete abrogation of the apoptotic response in cells that lacked functional Bax (Bax knockouts).62
The resistance of leukemic cells to chemotherapy-induced apoptosis remains the most significant problem in the treatment of AML. Specifically, high intracellular levels of Bcl-2 inhibit the apoptosis typically induced by chemotherapeutic agents.63,64Furthermore, posttranslational modifications of Bcl-2 appear to be involved in the regulation of apoptosis. In particular, Bcl-2 phosphorylation was associated with enhanced Bcl-2 function in some studies17,18,39,65 and with the inactivation of Bcl-2 in others.66-69 Phosphorylation at serine 70 was also found to be required for the anti-apoptotic function of Bcl-2 to be mediated by mitochondrial PKC-α.17,18 MAP kinases ERK1/2 have recently been identified as physiological Bcl-2 kinases.19Expression and constitutive activation of ERK was found in most samples from patients with AML.70 71 Because Bcl-2 overexpression failed to protect U937 cells from CDDO-Me–induced apoptosis, we hypothesize that CDDO-Me may inactivate Bcl-2 function with consequent mitochondrial damage, Bax release, and caspase activation. This was borne out in our in vivo labeling experiments, which demonstrated that CDDO-Me dramatically blocked Bcl-2 phosphorylation in U937 cells. In addition, a Ser→Glu mutant, S70E, which may mimic a potential phosphate charge, more potently suppressed CDDO-Me–induced apoptosis than wild-type Bcl-2. These data provide additional evidence that the phosphorylation status of Bcl-2 determines the sensitivity of cells to CDDO-Me–induced killing.
Bcl-2 must be targeted to mitochondrial membranes to efficiently suppress apoptosis.72 73 Subcellular fractionation studies revealed that ERK1/2, but not PKC-α, is present in the heavy-membrane fraction containing mitochondria in U937 cells. Our preliminary data demonstrated that the treatment of U937 cells with CDDO-Me inhibited phosphorylation (ie, activation) and ERK kinase activity, suggesting a direct inhibition of ERK by the compound. However, we cannot rule out the possibility that the ERK activator, MEK, was not co-immunoprecipitated and that the inhibition of ERK is the indirect result of MEK inhibition. Studies analyzing the inhibitory effect of CDDO-Me on the MAP kinase pathway and Bcl-2 dephosphorylation are now in progress.
In conclusion, our data provide the first evidence that exposure to the novel triterpenoid, CDDO-Me, induces apoptosis and inhibits colony formation of myeloid leukemic cells. CDDO-Me also directly affects Δψm, activates caspases, and induces expression of the pro-apoptotic Bax protein. Of potential clinical importance is the ability of CDDO-Me to enhance the cytotoxic effects of ara-C and retinoids, agents used in the therapy for AML. The observed effect of CDDO-Me on posttranslational modification of Bcl-2 and ERK1/2 is perhaps one of the factors responsible for the inactivation of their anti-apoptotic function, at least in some cellular systems. Hence, our data indicate the potential usefulness of CDDO-Me in the treatment of human myeloid leukemias, including blast crisis of CML. In addition, the independence of CDDO-Me from p53 and MDR-1, its cytotoxic effect at submicromolar concentrations, and its ability to induce differentiation make CDDO-Me a likely agent for the treatment of other types of cancer as well.
We thank Dr Steven Grant for Bcl-2–transfected leukemic cell lines, Tena Horton and Rosemarie Lauzon for their help in the preparation of the manuscript, and Dr Edward Sausville and Kenneth Snader and the RAID program (National Cancer Institute) for assistance in providing CDDO-Me.
Supported by grants from the National Institutes of Health (CA55164, CA49639, CA16672, CA44649, RO1 CA 78814), US grant 6097-02, Department of Defense (DAMD17-99-1-9168, DAMD17-98-1-8604), the National Foundation for Cancer Research, and the Oliver and Jennie Donaldson Trust. M.B.S. is Oscar M. Cohn Professor and M.A. is Stringer Professor for Cancer Treatment and Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Andreeff, Dept of Blood and Marrow Transplantation, Section of Molecular Hematology and Therapy, Box 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mandreef@mdanderson.org.





![Fig. 8. CDDO-Me blockade of Bcl-2 phosphorylation. / U937 cells were radiolabeled with [32P]orthophosphate, treated with 0.1 μM CDDO-Me, and lysed, after which Bcl-2 was immunoprecipitated. Protein was subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to x-ray film to identify the phosphorylated Bcl-2. The identity of the protein was confirmed by Western blot analysis using the same filter. Pre, pre-immune serum.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.326/6/m_h80121923008.jpeg?Expires=1765881952&Signature=AYnF6AiOeBaTAuJ1BkfWTFXJ-3xgVXekHkhQ5y1lFzQOz9Lp9bvuM8JR1Gg7fi6TOIqUVE0tzIDdBB-CMao4SPZCmfiAVjF5Z6w3niW4Nw~QDDA~3knuhhIvVBWcdutC666Dn9NzNaaxlHnj32Ob69ishcrkElbAe0xhjSDYWtIfwA-BocMHkjo3uTJFMVsmZ3L-c7812TsnXKyQT9mcuEhm19jcbZgrGzCgT2~zzIN3jaIEQGWYKX1moFWPogUcUiok9a5HP8KPxsUpafD7pun7F0FQ-dGo48arSN8AVhp1rkuQH-MXF7WHgF8vzCL8fE6x~48W9PYMzRTIi8JupA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal