Immunostimulatory cytosine-phophate-guanosine (CpG)–containing motifs in bacterial DNA are potent immune system activators. Depending on the bases flanking the CpG motif and on the DNA backbone, CpG oligodeoxynucleotides (ODNs) can induce relatively more B-cell activation or relatively more natural killer (NK)–cell activation. To evaluate their antitumor activities, an NK-optimized ODN (1585) and 2 B-cell–optimized ODNs (1826 and 2006) were compared for their ability to protect naive mice against a lethal acute myelogenous leukemia (AML) challenge. CpG 2006, but not CpG 1585, administered 2 days before the AML challenge, allowed mice to survive more than 100 times a lethal tumor dose. Cell depletion studies showed that protection did not require T or B cells but depended on NK cells and also on an NK-independent mechanism. CpG 2006 protected against AML challenge in both syngeneic and allogeneic bone marrow transplant (BMT) recipients at both early and late time points after transplantation. Although CpG 1585 had no protective effect on its own, it showed a striking synergy with CpG 2006 to induce prolonged survival to AML challenge in allogeneic recipients of T-cell–depleted marrow grafts, exceeding the survival benefit of donor lymphocyte infusion (DLI). When combined with DLI, a synergistic effect was observed in recipients of CpG2006 or 2006 + 1585 with 88% of mice surviving long-term. These data are the first to indicate that the systemic administration of CpG ODNs is a potent means of inducing therapeutic anti-AML innate immune responses in naive and BMT recipients.
Introduction
The innate immune system is geared toward providing a rapid response to foreign pathogens by pattern recognition receptors that distinguish prokaryotic from eukaryotic DNA.1 These receptors specifically bind to unmethylated cytosine-phosphate-guanosine (CpG) dinucleotides, enabling bacteria and other pathogens to stimulate the innate immune system.2-6Synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs mimic bacterial DNA and can activate immune responses.2-5 CpG administration results in high T helper 1 (Th1) activity and lower toxicity than complete Freund adjuvant.7-10 CpG ODNs directly stimulate B lymphocytes to produce interleukin-6 (IL-6) and IL-103; natural killer (NK) cells to produce interferon γ (IFNγ); and monocytes and dendritic cells (DCs) to produce IL-6, IL-12, IL-18, tumor necrosis factor α (TNFα), and IFNα.11 These soluble factors may activate innate immune cells such as NK cells and also may function to stimulate the adaptive immune system. Stimulation of the adaptive immune system can result in B-cell secretion of immunoglobulin, DC expression of major histocompatibility complex (MHC) class II antigens and costimulatory molecules and T-cell activation from either soluble factors or from binding to activated B cells, DCs, or macrophages.12-14 Thus, CpG motifs not only engage the innate immune system but also are able to recruit effector cells that comprise the adaptive immune system. As such, CpG ODNs may be exploited as a means of stimulating both the innate and the adaptive immune system to recognize and respond to foreign antigens, as would occur with a viral infection or neoantigens expressed on tumor cells.
Acute myelogenous leukemia (AML) is the most common form of acute leukemia in adults. Intensive chemotherapy regimens have led to remission induction in 70% to 85% of patients. Autologous bone marrow transplantation (BMT) can result in long-term, disease-free survival, although such therapy can be associated with morbidity/mortality, and postremission relapses are frequent.15-17 Although a graft-versus-leukemia (GVL) effect for AML has been observed in allogeneic bone marrow transplant (alloBMT) recipients and in recipients given delayed lymphocyte infusions (DLIs) to treat relapse after alloBMT,18 19 the graft-versus-host (GVH) effect that often accompanies the GVL effect is a significant cause of morbidity and mortality. For patients not undergoing BMT or for those receiving autologous BMT, alloresponses as a means to achieving remission are not an option. In these instances, the generation of tumor-specific immune responses requires the tumor cell or an antigen-presenting cell (APC) to present tumor antigens to autologous immune-competent T cells. Tumors usually are poor stimulators of immune responses. Because endogenous T-cell responses are not sufficiently potent as to protect against AML recurrence and alloresponses are associated with graft-versus-host disease (GVHD), alternative approaches to preventing AML recurrence are needed. CpG ODNs are attractive candidates for such purposes.
In autologous and especially alloBMT recipients, T cells generated after BMT may not be fully immunocompetent because of defects in the thymic microenvironment and other types of injury.20 In contrast to the prolonged period of time that can be required for T-cell immune reconstitution, NK-cell recovery after BMT is rapid.21-23 NK cells present early after BMT have been shown to be potent effectors against myeloid leukemias.23Because CpG ODNs can be potent stimulators of innate immune system effector cells, we reasoned that CpG ODNs might provide a means of achieving an anti-AML response in mice early after BMT prior to reconstitution of a fully functional adaptive immune system. Our data demonstrate that CpG ODNs are highly effective in inducing an anti-AML immune response in naive mice as well as in BMT recipients of either autologous or T-cell–depleted (TCD) allogeneic bone marrow when CpG ODNs are given as early as 2 weeks after BMT, a time period when T-cell reconstitution is poor. The combined administration of CpG ODNs along with mature donor lymphocytes (DLI) completely prevented AML-induced lethality. These data have important clinical ramifications.
Materials and methods
Mice
C57BL/6 (termed B6:H2b), B10.BR/SgSnJ (H2k), and B6-severe combined immune deficient (B6-SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were bred and housed in a specific pathogen-free facility in microisolator cages. Mice were used at 8 to 10 weeks of age.
CpG ODNs
Phosphorothioate-modified ODNs and chimeric ODNs were provided by the Coley Pharmaceutical Group (Wellesley, MA). Chimeric ODNs have phosphorothioate linkages between the first 2 bases on the 5′ end and the last 5 bases on the 3′ end with central bases connected by phosphodiester linkages. This arrangement of 5′ and 3′ terminal base modifications previously has been shown to be optimal for in vitro immune stimulation.24 Pharmacokinetic studies have demonstrated that in vivo such chimeric ODNs containing internal phosphodiester bonds undergo degradation and are cleared from the plasma approximately 10 times faster than corresponding phosphorothioate ODNs.25 ODN had undetectable endotoxin levels using the Limulus amoebocyte lysate (LAL) assay. ODNs were diluted in Tris-EDTA buffer and further diluted in phosphate-buffered saline for in vivo use. The ODN sequences are CpG 1585, GGGGTCAACGTTGAGGGGGG; CpG 1826, TCCATGACGTTCCTGACGTT; control 1982, TCCAGGACTTCTCTCAGGTT; CpG 2006, TCGTCGTTTTGTCGTTTTGTCGTT; and control 2118, GGGGTCAAGCTTGAGGGGGG. ODNs were injected at 100 μg/dose intraperitoneally once (CpG 1826 or CpG 2006) or twice weekly (CpG 1585). This dose was determined to be optimal for the induction of antitumor responses in several systems involving comparisons of 30, 100, and 300 μg/injection. CpG ODN 1585 was given twice weekly because this ODN has a shorter half-life than the others.24,25 Approximately 4% of the fully phosphorothioate-modified ODN dose is present in the spleen during the first 24 hours after injection,26 resulting in a splenic concentration of 4 μg/mL. Because chimeric ODNs such as 1585 are eliminated from the plasma approximately 10 times as fast as fully phosphorothioate-modified ODNs, the spleen concentration is unlikely to exceed 0.4 μg/mL. In one experiment, complete Freund adjuvant was homogenized and given at a dose of 0.2 mL subcutaneously on day −2.
AML challenge
C1498, derived as a spontaneous tumor line from a B6 mouse, was grown in RPMI 1640 (Gibco, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (Hyclone, Ogden, UT) as described.24C1498 is an MHC class I+II− AML line.27 C1498 cells were given intravenously at doses ranging from 2 × 103 to 2 × 106/mouse. Necropsies were performed at the time of death. AML tumor involvement was found in all mice, unless otherwise noted.
Anti-AML responses in naive mice
CpG ODNs were given beginning on day −2 or as late as day 6 until day 26 relative to the injection of C1498 cells (range, 2 × 105 to 2 × 106). In some model systems, administration of CpG ODN must precede challenge with infectious pathogens by 2 days for optimal effect,28 but, in other model systems, treatment can be delayed for a week or longer.29 The duration of the CpG ODN immune effects is 2 weeks or more after the last injection.28 To determine the effectors responsible for the CpG-induced immune response, mice were treated with irrelevant rat immunoglobulin G (IgG) or anti–NK 1.1 (clone PK136)– or anti-CD4 (clone GK1.5)– and/or anti-CD8 (clone 2.43)–depleting monoclonal antibodies (mAbs) given intraperitoneally at a dose of 400 μg each from days −1 and weekly for 4 weeks that routinely results in more than 95% depletion of the targeted cell population for more than 1 week beyond discontinuing of mAb administration.
Anti-AML responses in BMT recipients
To generate syngeneic BMT recipients, B6 mice were irradiated with 8.0 Gy total body irradiation (TBI) on day −1 and reconstituted with 5 × 106 B6 bone marrow cells on day 0. CpG ODNs were administered beginning on day 63 and ending on day 90 (CpG 1585) or day 92 (CpG 2006) after BMT. C1498 was given intravenously on day 80 after BMT. Cohorts received 2 subcutaneous vaccines of irradiated C1498 cells (107) given on days 66 and 73 or no immunizations.
To determine the effects of CpG ODNs given later after alloBMT, B6 recipients were irradiated with 8.0 Gy TBI on day −1, reconstituted with B10.BR TCD bone marrow (2 × 107). CpG ODNs were administered during the time period of days 72 to 112, and C1498 cells (2 × 105) were given on day 86 after BMT. To assess the effects of CpG ODNs early after BMT, CpG ODNs were injected from days 20 to 62 after BMT, and C1498 cells (2 × 105) were given to recipients on day 36 after BMT. As a more rigorous test, CpG ODNs were injected from days 15 to 42 after BMT, and C1498 cells (2 × 105) were given on day 17 after BMT. AlloBMT recipients were monitored daily for survival and clinical appearance and weighed twice weekly. All available animals were examined by necropsy for evidence of GVHD and tumor.30
To assess the effects of CpG ODNs on DLI-mediated GVHD and GVL, B6 recipients were given B10.BR TCD bone marrow as described above. On day 17 after BMT, C1498 cells (2 × 105) were given under the cover of CpG ODNs that were administered from days 15 to 42 after BMT. On day 21 after BMT, splenocytes (25 × 106) from B10.BR donors30 were infused.
Flow cytometry
Cells were incubated with anti-FcR mAb and then mAb directed toward CD4 or CD8, CD19, and Mac1. Cells were analyzed by 2- or 3-color flow cytometry by using fluorescein isothiocyanate-, phycoerythrin-, or biotin-conjugated (along with SA-peridinin chlorophyll protein) mAb (Pharmingen). Irrelevant mAb control values were subtracted from values obtained with relevant values, using a FACScalibur (Becton Dickinson, Mountain View, CA). Forward and side scatter settings were gated to exclude red cells and debris, and 104 cells were analyzed for each determination.
Cytokine analysis
B6 splenocytes (106/mL) were plated in RPMI 1640 with 10% fetal bovine sera. ODNs were added at 0.3 to 3.0 μg/mL. Supernatants were collected for enzyme-linked immunosorbent assay analysis (IL-6, IL-12, IFNγ, TNFα: all sensitivities < 50 pg/mL).
Statistical analyses
Group comparisons of continuous data were made by Studentt test. Survival data were analyzed by life table methods, using the Mantel-Peto-Cox summary of chi-square. Actuarial survival and relapse rates are shown. P values ≤ .05 were considered significant.
Results
Delineation of ODN with distinct profiles of immune activation
In previous studies we reported that ODNs containing particular arrangements of CpG motifs with a phosphodiester backbone flanked by polyG motifs with a nuclease-resistant phosphorothioate backbone had dramatically enhanced NK-stimulating effects compared with ODNs with all-thioate backbones.31 We have recently reported that the optimal CpG motif for activating murine B cells is GACGTT, whereas that for activating human B cells is GTCGTT.32 33 To investigate the immune-stimulatory effects, in vitro assays were performed using representative ODNs from these 3 classes: NK-optimized ODN 1585, mouse B-cell stimulating-optimized ODN 1826, and human-stimulating ODN 2006. Each ODN stimulated splenic production of proinflammatory cytokines (TNFα, IFNγ, IL-6, and IL-12) (Table1). Although IL-12 was induced to a similar degree by all 3 ODNs at high ODN concentrations, IL-12 production at lower ODN concentrations showed the following hierarchy: CpG 1826 > 2006 > 1585. On the basis of achievable in vivo concentrations, relevant comparisons would be CpG 1585 at 0.3 μg/mL versus CpG 2006 at 3.0 μg/mL. CpG 1585 was a more potent inducer of IFNγ and TNFα production than CpG 1826 or 2006. Consistent with IFNγ and TNFα expression, CpG 1585 is a more potent inducer of NK-cell function than the other ODNs (Z. Ballas, personal communication, 2001). In contrast, CpG 1826 and 2006 were more potent inducers of IL-6 production as compared with CpG 1585. B-cell proliferation roughly correlated with induction of IL-6 expression (data not shown). These data all were reproduced fully in a second experiment. We conclude that CpG 2006 is a more potent inducer of the proinflammatory cytokines IL-12, IL-6, and IFNγ at biologically achievable doses as compared with CpG 1585.
Effect of cytosine-phosphate-guanosine oligodeoxynucleotides on proinflammatory cytokine production
| CpG ODN . | Concentration (μg/mL) . | IFNγ . | IL-6 . | IL-12 . | TNFα . |
|---|---|---|---|---|---|
| 1585 | 0.3 | 281 ± 66 | 205 ± 68 | 878 ± 64 | < 50 |
| 1585 | 1.0 | 627 ± 106 | 2 580 ± 136 | 6 531 ± 123 | 767 ± 218 |
| 1585 | 3.0 | 1 674 ± 368 | 5 501 ± 592 | 11 487 ± 507 | 3 486 ± 837 |
| 1826 | 0.3 | 323 ± 36 | 6 174 ± 1 056 | 9 811 ± 65 | 308 ± 237 |
| 1826 | 1.0 | 423 ± 72 | 16 105 ± 3 144 | 10 041 ± 963 | 868 ± 186 |
| 1826 | 3.0 | 884 ± 131 | 17 163 ± 1 918 | 7 476 ± 1 639 | 1 985 ± 594 |
| 2006 | 0.3 | 233 ± 108 | 829 ± 26 | 3 605 ± 407 | 252 ± 418 |
| 2006 | 1.0 | 337 ± 124 | 5 033 ± 242 | 7 755 ± 734 | 243 ± 113 |
| 2006 | 3.0 | 684 ± 253 | 10 846 ± 587 | 7 895 ± 1 413 | 1 404 ± 367 |
| 1982 | 0.3 | 475 ± 175 | 423 ± 620 | 395 ± 33 | < 50 |
| 1982 | 1.0 | 191 ± 7 | 316 ± 116 | 130 ± 152 | 51 ± 258 |
| 1982 | 3.0 | 381 ± 44 | 554 ± 179 | 3 082 ± 297 | 1 468 ± 630 |
| 2118 | 0.3 | 500 ± 9 | 283 ± 77 | 546 ± 37 | < 50 |
| 2118 | 1.0 | 243 ± 46 | 324 ± 61 | 454 ± 14 | < 50 |
| 2118 | 3.0 | 434 ± 36 | 731 ± 116 | 386 ± 155 | 897 ± 152 |
| CpG ODN . | Concentration (μg/mL) . | IFNγ . | IL-6 . | IL-12 . | TNFα . |
|---|---|---|---|---|---|
| 1585 | 0.3 | 281 ± 66 | 205 ± 68 | 878 ± 64 | < 50 |
| 1585 | 1.0 | 627 ± 106 | 2 580 ± 136 | 6 531 ± 123 | 767 ± 218 |
| 1585 | 3.0 | 1 674 ± 368 | 5 501 ± 592 | 11 487 ± 507 | 3 486 ± 837 |
| 1826 | 0.3 | 323 ± 36 | 6 174 ± 1 056 | 9 811 ± 65 | 308 ± 237 |
| 1826 | 1.0 | 423 ± 72 | 16 105 ± 3 144 | 10 041 ± 963 | 868 ± 186 |
| 1826 | 3.0 | 884 ± 131 | 17 163 ± 1 918 | 7 476 ± 1 639 | 1 985 ± 594 |
| 2006 | 0.3 | 233 ± 108 | 829 ± 26 | 3 605 ± 407 | 252 ± 418 |
| 2006 | 1.0 | 337 ± 124 | 5 033 ± 242 | 7 755 ± 734 | 243 ± 113 |
| 2006 | 3.0 | 684 ± 253 | 10 846 ± 587 | 7 895 ± 1 413 | 1 404 ± 367 |
| 1982 | 0.3 | 475 ± 175 | 423 ± 620 | 395 ± 33 | < 50 |
| 1982 | 1.0 | 191 ± 7 | 316 ± 116 | 130 ± 152 | 51 ± 258 |
| 1982 | 3.0 | 381 ± 44 | 554 ± 179 | 3 082 ± 297 | 1 468 ± 630 |
| 2118 | 0.3 | 500 ± 9 | 283 ± 77 | 546 ± 37 | < 50 |
| 2118 | 1.0 | 243 ± 46 | 324 ± 61 | 454 ± 14 | < 50 |
| 2118 | 3.0 | 434 ± 36 | 731 ± 116 | 386 ± 155 | 897 ± 152 |
B6 splenocytes were incubated for 24 hours with the indicated ODNs. After 24 hours, supernatant was collected for cytokine analysis by enzyme-linked immunosorbent assay as described in “Materials and methods.” Triplicate samples were analyzed. Cytokine values are in pg/mL. Data are expressed as mean ± 1 SD. A value of < 50 pg/mL indicates that the mean value was at the limits of detection of the assay. These data were reproduced in a replicate experiment.
Underlined values indicate cytokine values that are significantly (P < .05) higher than those observed with CpG 1585 at the corresponding ODN concentration. Values in italics indicate cytokine values that are significantly (P < .05) lower than those observed with CpG 1585 at the corresponding ODN concentration.
CpG indicates cytosine-phosphate-guanosine; ODN, oligodeoxynucleotide; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Administration of CpG ODNs in vivo is highly effective in stimulating immune responses to AML cells
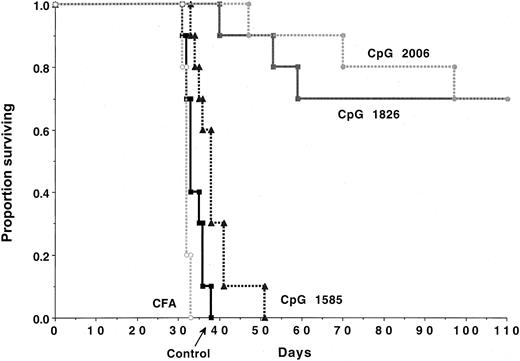
The immune response to a low burden of C1498 cells (≤ 3 × 104) is dependent on NK cells, whereas resistance to a higher tumor burden (≥ 105) is T-cell dependent.27 To determine whether CpG ODNs might stimulate an immune response to a higher tumor burden, naive B6 mice were given CpG ODNs selected as potent stimulators of NK-cell function (CpG 1585) or as potent stimulators of B-cell and DC function (CpG 1826 or CpG 2006). In vitro, C1498 proliferation was not impaired by exposure to high concentrations (100 μg/mL) of CpG 1585 or CpG 2006 ODNs (CpG 1826 was not tested) (not shown). B6 recipients challenged with C1498 cells (2 × 105/mouse) succumbed within 5.5 weeks to tumor (Figure 1). Complete Freund adjuvant did not significantly affect survival. Recipients given CpG 1585 had a significant (P < .008) extension in survival, although all succumbed to tumor by 7.5 weeks after the challenge. In contrast, CpG 1826 or CpG 2006 given beginning on day −2 before C1498 challenge protected 70% of mice from a lethal AML dose (Figure 1).
CpG ODN administration can protect naive mice against AML-induced lethality.
B6 mice (n = 10 per group) were treated with the indicated CpG ODN (100 μg/dose) intraperitoneally beginning on day −2 prior to intravenous C1498 (2 × 105 cells/mouse) infusion according to the schedule described in “Materials and methods.” CpG ODN-treated mice had a significant (P < .008) increase in survival rates as compared with nontreated or complete Freund adjuvant-treated controls. Recipients of CpG 1826 or 2006 ODNs had comparable survival that was superior (P ≤ .001) to CpG 1585-treated recipients.
CpG ODN administration can protect naive mice against AML-induced lethality.
B6 mice (n = 10 per group) were treated with the indicated CpG ODN (100 μg/dose) intraperitoneally beginning on day −2 prior to intravenous C1498 (2 × 105 cells/mouse) infusion according to the schedule described in “Materials and methods.” CpG ODN-treated mice had a significant (P < .008) increase in survival rates as compared with nontreated or complete Freund adjuvant-treated controls. Recipients of CpG 1826 or 2006 ODNs had comparable survival that was superior (P ≤ .001) to CpG 1585-treated recipients.
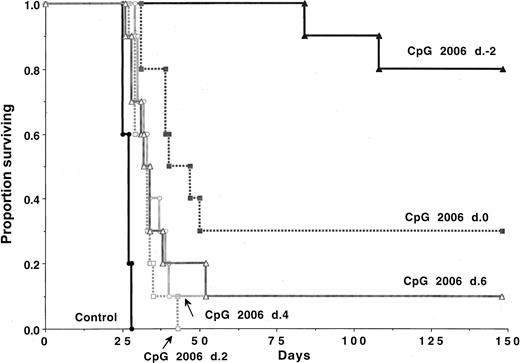
CpG 2006 rather than CpG 1826 was selected for further study because a similar effect was observed with these 2 ODNs, and CpG 2006 is immune stimulatory in humans and mice.32 Although all controls died within 4 weeks of C1498 challenge (2 × 105 cells), 80% of the recipients treated with CpG 2006 beginning on day −2 survived the 5-month period (Figure 2). CpG 2006 given on day 0 of the C1498 challenge rescued 30% of mice. A delay in CpG administration of 2, 4, or 6 days after the C1498 challenge significantly (P ≤ .001) prolonged survival as compared with controls, although 10% or less survived long-term. A greater degree of protection required that CpG ODNs were administered before or with the C1498 challenge.
CpG 2006 ODN is more effective when administration is initiated before AML challenge.
B6 mice (n = 10 per group) were treated with weekly CpG 2006 ODN initiated as indicated, relative to C1498 (2 × 105cells/mouse) infusion. CpG ODN-treated recipients survived significantly (P < .003) longer than nontreated controls, regardless of the schedule used. Mice receiving CpG 2006 beginning on day −2 survived significantly (P < .001) longer than all other groups; those that received CpG 2006 beginning on day 0 survived significantly (P < .05) longer than all groups except the day −2 group.
CpG 2006 ODN is more effective when administration is initiated before AML challenge.
B6 mice (n = 10 per group) were treated with weekly CpG 2006 ODN initiated as indicated, relative to C1498 (2 × 105cells/mouse) infusion. CpG ODN-treated recipients survived significantly (P < .003) longer than nontreated controls, regardless of the schedule used. Mice receiving CpG 2006 beginning on day −2 survived significantly (P < .001) longer than all other groups; those that received CpG 2006 beginning on day 0 survived significantly (P < .05) longer than all groups except the day −2 group.
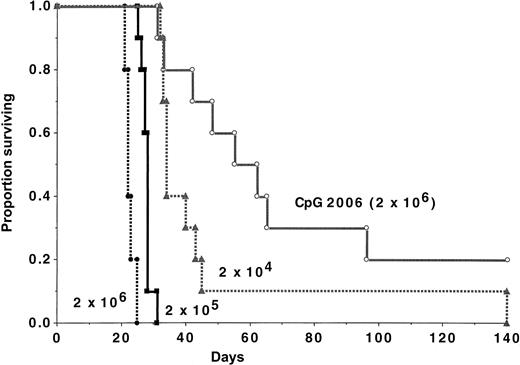
To quantify the magnitude of the CpG 2006 effect, recipients were challenged with various C1498 doses, ranging from 2 × 104 to 2 × 106 cells (Figure3). A cohort was given CpG 2006 and 2 × 106 cells. In controls, a dose-response effect of C1498 cells was evident, as measured by time to mortality. None of the mice survived a challenge with 2 × 104 cells or 2 × 105 cells. In contrast, 20% of CpG 2006-treated recipients of 2 × 106 C1498 cells survived long-term, significantly (P = .002) higher than recipients of 100-fold fewer cells.
CpG 2006 ODN administration reduces AML tumorigenicity by more than 100-fold.
B6 mice (n = 10 per group) were challenged with various C1498 doses, as indicated. A cohort of mice was treated with CpG 2006 ODN, beginning on day −2. Mice receiving CpG 2006 survived significantly (P ≤ .03) longer than all groups, including those that received 100-fold fewer C1498 cells (2 × 104cells/mouse).
CpG 2006 ODN administration reduces AML tumorigenicity by more than 100-fold.
B6 mice (n = 10 per group) were challenged with various C1498 doses, as indicated. A cohort of mice was treated with CpG 2006 ODN, beginning on day −2. Mice receiving CpG 2006 survived significantly (P ≤ .03) longer than all groups, including those that received 100-fold fewer C1498 cells (2 × 104cells/mouse).
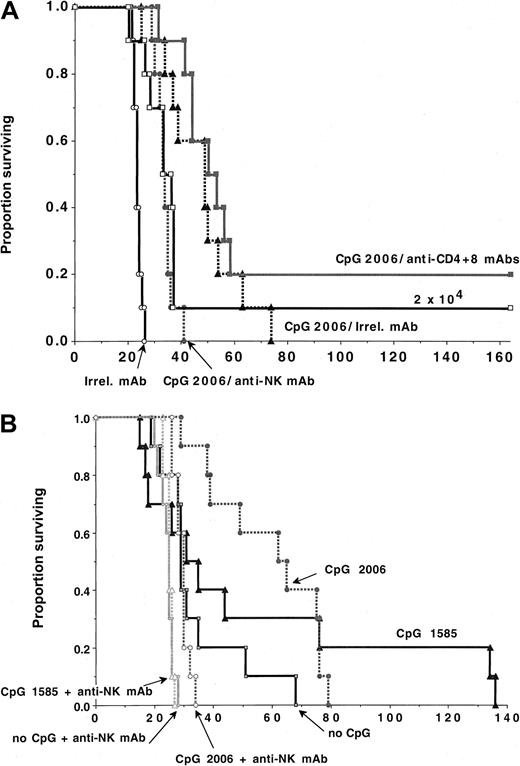
To determine which effector cells were required for the antitumor effects mediated by CpG administration, CpG 2006-treated B6 recipients of C1498 cells (6 × 105) were given irrelevant mAb or mAbs to deplete T cells or NK cells (Figure4A). In contrast to irrelevant mAb-treated controls that died of tumor by 25 days after infusion, CpG treatment significantly delayed tumor mortality by 50 days with all succumbing to tumor by 75 days after infusion (P = .008). CpG-treated mice receiving 6 × 105 C1498 cells had a modest but not statistically significant longer mean survival time (P = .10) as compared with controls receiving 30-fold fewer C1498 cells, indicating a high level of antitumor activity conferred by CpG administration. Depletion of NK cells significantly impaired survival in CpG-treated recipients (P = .0054), although CpG-treated, NK-depleted mice survived longer than controls (P = .0006). In contrast, pan-TCD did not adversely affect survival (P = .14). Additionally, CpG-treated recipients depleted of both NK and T cells had similar survival to CpG-treated recipients depleted of NK cells alone (not shown). These data were reproduced in other experiments using a lower tumor cell dose (2 × 105) (not shown). Together, these studies indicate that the tumor protection conferred by CpG administration is mediated primarily but not exclusively by NK cells.
NK-cells but neither T nor B cells are obligatory for optimal CpG ODN-induced anti-AML responses.
B6 (A) or B6-SCID (B) mice (n = 10 per group) were treated with CpG 1585 or 2006, beginning on day −2 prior to the infusion of C1498 cell (A: 6 × 105 cells/mouse unless otherwise indicated; B: 2 × 105 cells/mouse). Cohorts of mice were given irrelevant, anti-NK or anti-CD4+CD8 mAbs, as indicated, according to “Materials and methods.” (A) CpG 2006 administration reduced tumorigenicity as compared with controls (P = .0008). Depletion of NK cells (P = .0054) but not T cells (P = .14) significantly reduced the anti-AML efficacy of CpG 2006, although CpG-treated NK-depleted recipients survived significantly (P = .0006) longer than non–CpG-treated controls. (B) B6-SCID mice treated with CpG 2006 but not CpG 1585 survived significantly longer after C1498 challenge than controls (P = .00 and .16, respectively). NK-depleted B6-SCID mice treated with CpG 2006 but not CpG 1585 survived significantly longer after C1498 challenge than controls (P = .0008 and .36, respectively).
NK-cells but neither T nor B cells are obligatory for optimal CpG ODN-induced anti-AML responses.
B6 (A) or B6-SCID (B) mice (n = 10 per group) were treated with CpG 1585 or 2006, beginning on day −2 prior to the infusion of C1498 cell (A: 6 × 105 cells/mouse unless otherwise indicated; B: 2 × 105 cells/mouse). Cohorts of mice were given irrelevant, anti-NK or anti-CD4+CD8 mAbs, as indicated, according to “Materials and methods.” (A) CpG 2006 administration reduced tumorigenicity as compared with controls (P = .0008). Depletion of NK cells (P = .0054) but not T cells (P = .14) significantly reduced the anti-AML efficacy of CpG 2006, although CpG-treated NK-depleted recipients survived significantly (P = .0006) longer than non–CpG-treated controls. (B) B6-SCID mice treated with CpG 2006 but not CpG 1585 survived significantly longer after C1498 challenge than controls (P = .00 and .16, respectively). NK-depleted B6-SCID mice treated with CpG 2006 but not CpG 1585 survived significantly longer after C1498 challenge than controls (P = .0008 and .36, respectively).
Long-term CpG 2006-treated B6 survivors of C1498 challenge (110-148 days) were rechallenged with C1498 cells at the same dose as used for the initial challenge (2 × 105 cells). Despite the potent CpG 2006-induced anti-AML effects, pooled data in 2 experiments revealed that all 12 CpG 2006-treated recipients succumbed to AML lethality within 32 days after rechallenge, a time course similar to that observed with the initial challenge and with concurrent controls (not shown). Subsequent experiments will be designed to determine whether CpG given at AML rechallenge will be as effective as treatment at the time of initial AML challenge. Together, these data indicate a lack of memory-cell responses in CpG-treated recipients and are consistent with NK cells as the major effector cells in CpG 2006-mediated anti- AML responses.
Studies were performed in CpG-treated B6-SCID mice to determine whether CpG ODNs could provide an anti-AML effect in T- and B-cell–deficient mice. B6-SCID mice were treated with CpG 2006 or CpG 1585 and challenged with C1498 cells (2 × 105). In B6-SCID mice, known to have heightened NK activity, CpG 2006 but not CpG 1585 significantly delayed the time to AML-induced lethality (Figure 4B). NK-cell depletion of B6-SCID mice reduced the time to AML-induced lethality as compared with non–NK-depleted controls (P = .006), indicating that endogenous NK function in B6-SCID mice provided some anti-AML immune responses (Figure 4B). NK-cell depletion completely abrogated the effect of CpG 1585 in B6-SCID mice. In contrast, although NK-cell depletion largely eliminated the anti-AML effect of CpG 2006, NK-cell depleted, CpG 2006-treated B6-SCID mice survived significantly longer than NK-cell–depleted controls (P = .0008), suggesting that cell types other than T, B, or NK cells stimulated by CpG 2006 contributed to the anti-AML response. The most likely candidate populations are DCs and monocytes, known to be activated by CpG 2006.
CpG 2006 but not CpG 1585 induces anti-AML responses in syngeneic BMT recipients
Immunotherapy may be most useful in the setting of minimal residual disease as might occur after BMT. However, after BMT, the adaptive immune system is slow to be restored. Initial studies were performed in syngeneic BMT recipients that were challenged with C1498 (105) cells on day 80 after BMT (Figure5A). Cohorts of mice were treated with CpG 2006 or CpG 1585, beginning on day 64 after BMT or were immunized with irradiated C1498 cells (107 on days 66 and 73 after BMT) prior to AML challenge. Another control group received a lower C1498 dose (104), which is nonlethal to non-BMT recipients. Consistent with post-BMT immune dysfunction, recipients challenged with a typically nonlethal dose of 104 C1498 cells succumbed to leukemia within 2 months after the challenge. BMT recipients challenged with 105 cells died faster, succumbing by 1.5 months after challenge. In contrast, 50% of CpG 2006-treated recipients survived the C1498 cell (105) challenge long-term (> 4.5 months after challenge), indicating a more than 10-fold reduction in AML-induced lethality. Irradiated cellular vaccines were highly effective in inducing AML resistance with 90% of BMT recipients surviving long-term (P = .023 versus CpG 2006).
CpG 2006 treatment prolongs the survival of syngenic BMT recipients challenged with AML cells 2.5 months after BMT but does not induce a memory cell response.
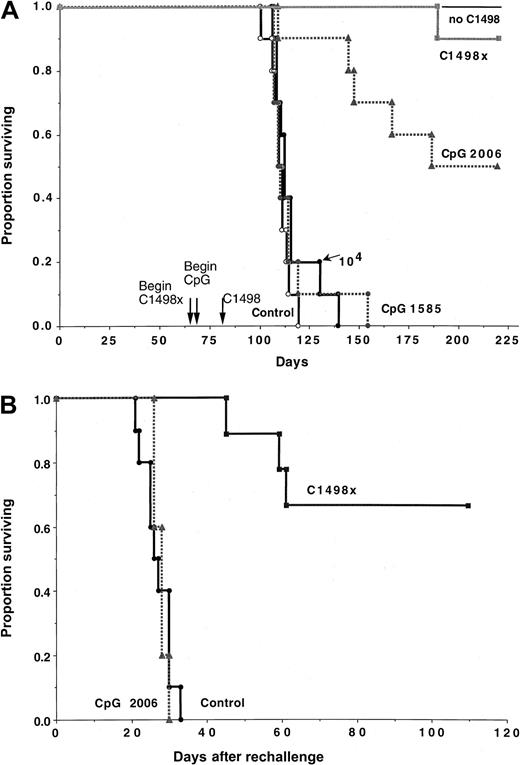
Lethally irradiated B6 recipients were reconstituted with B6 bone marrow cells (n = 10 per group). Cohorts of mice, as indicated, received CpG 1585 or 2006 ODNs beginning on day 64 and continuing through day 90 (CpG 1585) or day 92 (CpG 2006) after BMT. A separate cohort received irradiated tumor vaccines (termed C1498x) given subcutaneously on days 66 and 73 after BMT. Mice were challenged with C1498 (105/mouse) or, as indicated, no C1498 cells or C1498 (104/mouse). All surviving mice in the indicated groups were challenged with C1498 cells (105/mouse) on day 219 after BMT to assess memory cell response. (A) Although CpG 1585-treated recipients did not protect mice against C1498 challenge (P = .21 versus control), CpG 2006 was effective (P = .00053 versus control), resulting in more than a 10-fold reduction in tumorigenicity when comparing outcome to recipients of C1498 at a dose of 104 cells/mouse (P = .00074). (B) C1498x but not CpG 2006 induced a memory cell response as compared with control BMT recipients not previously challenged with C1498 cells (P = .00015 and .46, respectively).
CpG 2006 treatment prolongs the survival of syngenic BMT recipients challenged with AML cells 2.5 months after BMT but does not induce a memory cell response.
Lethally irradiated B6 recipients were reconstituted with B6 bone marrow cells (n = 10 per group). Cohorts of mice, as indicated, received CpG 1585 or 2006 ODNs beginning on day 64 and continuing through day 90 (CpG 1585) or day 92 (CpG 2006) after BMT. A separate cohort received irradiated tumor vaccines (termed C1498x) given subcutaneously on days 66 and 73 after BMT. Mice were challenged with C1498 (105/mouse) or, as indicated, no C1498 cells or C1498 (104/mouse). All surviving mice in the indicated groups were challenged with C1498 cells (105/mouse) on day 219 after BMT to assess memory cell response. (A) Although CpG 1585-treated recipients did not protect mice against C1498 challenge (P = .21 versus control), CpG 2006 was effective (P = .00053 versus control), resulting in more than a 10-fold reduction in tumorigenicity when comparing outcome to recipients of C1498 at a dose of 104 cells/mouse (P = .00074). (B) C1498x but not CpG 2006 induced a memory cell response as compared with control BMT recipients not previously challenged with C1498 cells (P = .00015 and .46, respectively).
Surviving recipients were challenged with C1498 cells (105) on day 219 after BMT and were compared with a group of concurrently challenged BMT controls that had not received prior exposure to C1498 cells (Figure 5B). Long-term survivors initially treated with CpG 2006 had no evidence of a memory cell response, as these recipients died at an identical rate as concurrently challenged BMT controls. In contrast, 67% of recipients immunized with irradiated cellular vaccines survived more than 3.5 months after rechallenge. Thus, irradiated cellular vaccines but not CpG 2006 treatment is able to induce a demonstrable anti-AML memory cell response in syngeneic BMT recipients.
To determine whether CpG 2006 could induce anti-AML responses at early times after BMT, syngeneic BMT recipients were challenged on day 14 after BMT with C1498 cells at doses varying from 2 × 103to 105 (Figure 6). Cohorts received CpG 2006 beginning on day 12 after BMT. At that time, splenic analyses were performed on representative mice (n = 5 per group) to determine the status of immune reconstitution and whether CpG 2006 had influenced these parameters by the day of C1498 challenge. Comparable splenic cellularity and very low but similar absolute numbers of T cells (range, 1.0-1.6 × 106 in individual mice) were noted in these 2 groups, consistent with peripheral T-cell deficiency. Recipients challenged with 105 C1498 cells succumbed to leukemia within 1 month after challenge. Although CpG 2006 delayed the time to mortality in recipients of 105 cells as compared with nontreated controls (P = .002), all succumbed to leukemia by 8 weeks after challenge. Fifty percent of recipients of 104 cells survived long-term and there was no mortality in recipients of 2 × 103 cells. Eighty percent of CpG 2006-treated recipients of 104 cells survived long-term (P = .13 versus controls). Because recipients of 104 cells had a superior survival to CpG-treated recipients of 105 cells (P = .046) and because recipients of 2 × 103 cells had a superior survival to CpG-treated recipients of 104 cells (P = .040), these data are consistent with a less than the 10-fold reduction observed when CpG 2006 was administered later after BMT. Regardless of the degree in reduction in the tumorigenicity of C1498, it is clear that CpG 2006 can augment the anti-AML response of syngeneic BMT recipients before the adaptive immune system can be fully reestablished.
CpG 2006 treatment prolongs the survival of syngenic BMT recipients challenged with AML cells early (day 17) after BMT.
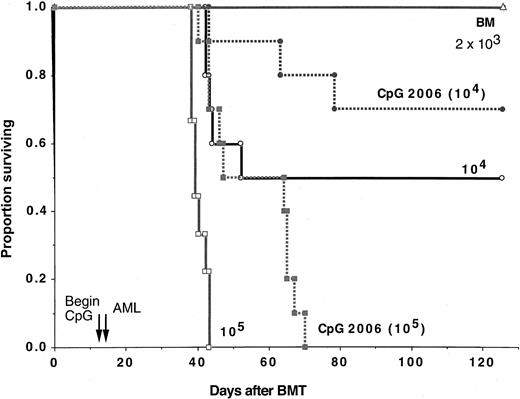
Lethally irradiated B6 recipients were reconstituted with B6 bone marrow cells (n = 9-10 per group). Cohorts of mice, as indicated, received CpG 2006 ODNs beginning on day 12 and continuing through day 45 after BMT. C1498 cells were infused at the indicated cell doses on day 14 after BMT. A separate cohort received no C1498 cells (termed BM). CpG 2006-treated recipients of C1498 cells (105/mouse) survived longer than controls (P = .0025), whereas those that received C1498 cells (104/mouse) had a similar survival rate as compared with controls (70% versus 50%;P = .13). The decrease in tumorigenicity induced by CpG 2006 was less than 10-fold because CpG 2006-treated recipients of C1498 cells at 105 had a poorer survival than control recipients of 104 cells (P = .046). In addition, CpG 2006-treated recipients of C1498 cells at 104 had a poorer survival than control recipients of 2 × 103 cells (P = .04).
CpG 2006 treatment prolongs the survival of syngenic BMT recipients challenged with AML cells early (day 17) after BMT.
Lethally irradiated B6 recipients were reconstituted with B6 bone marrow cells (n = 9-10 per group). Cohorts of mice, as indicated, received CpG 2006 ODNs beginning on day 12 and continuing through day 45 after BMT. C1498 cells were infused at the indicated cell doses on day 14 after BMT. A separate cohort received no C1498 cells (termed BM). CpG 2006-treated recipients of C1498 cells (105/mouse) survived longer than controls (P = .0025), whereas those that received C1498 cells (104/mouse) had a similar survival rate as compared with controls (70% versus 50%;P = .13). The decrease in tumorigenicity induced by CpG 2006 was less than 10-fold because CpG 2006-treated recipients of C1498 cells at 105 had a poorer survival than control recipients of 104 cells (P = .046). In addition, CpG 2006-treated recipients of C1498 cells at 104 had a poorer survival than control recipients of 2 × 103 cells (P = .04).
AlloBMT recipients of full MHC-disparate TCD bone marrow require longer periods of time for functional immune reconstitution as compared with syngeneic BMT recipients. To determine whether the beneficial effects of CpG ODN early after syngeneic BMT would translate into an alloBMT setting, irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow and then challenged with C1498 (2 × 105cells) on day 36 after BMT (Figure 7). Cohorts received no CpG ODN, CpG 1585, or CpG 2006 beginning day 20 after BMT. A cohort of nontreated controls received a lower C1498 dose (2 × 104 cells). All recipients of 2 × 105 C1498 cells died within 4 weeks, and 90% of recipients of 10-fold lower cells died within 5.5 weeks after AML infusion. As compared with controls, CpG 1585-treated recipients of 2 × 105 C1498 cells had a slight increase in survival (P = .045). However, all recipients succumbed within 6 weeks of challenge, and survival was shorter than controls given 2 × 104 cells (P = .049). As compared with controls, CpG 2006-treated recipients challenged with 2 × 105 cells survived longer (P = .001) with all mice succumbing approximately 5 weeks after tumor challenge. The degree of tumor reduction was estimated to be about 10-fold because survival of CpG 2006-treated recipients given 2 × 105cells was similar to controls given 2 × 104 cells (P = .34).
CpG 2006 treatment prolongs the survival of recipients of allogeneic TCD bone marrow challenged with AML cells early (day 36) after BMT.
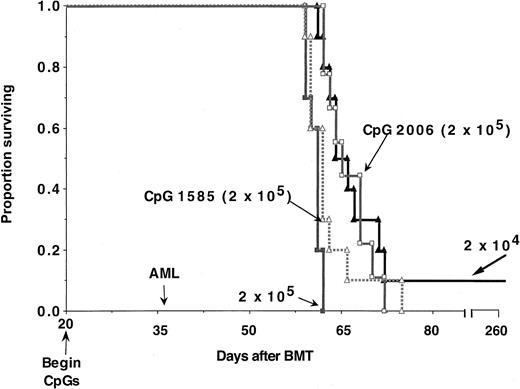
Lethally irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow (n = 9-10 per group). Cohorts of mice received no treatment, CpG 1585, or CpG 2006 ODNs. ODN treatment was initiated on day 20 and continued through day 65. On day 36, recipients were given C1498 cells at the indicated cell dose. Recipients treated with CpG 1585 or CpG 2006 had a prolonged survival compared with controls receiving 2 × 105 cells (P = .045 and .001, respectively). Recipients given CpG 1585 and C1498 cells at a dose of 2 × 105 cells survived for a shorter duration, whereas those receiving CpG 2006 survived for a comparable duration than controls receiving 10-fold fewer C1498 cells (2 × 104) (P = .049 and .34, respectively). On the basis of these data, CpG 2006 resulted in an approximate 10-fold reduction in tumorigenicity.
CpG 2006 treatment prolongs the survival of recipients of allogeneic TCD bone marrow challenged with AML cells early (day 36) after BMT.
Lethally irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow (n = 9-10 per group). Cohorts of mice received no treatment, CpG 1585, or CpG 2006 ODNs. ODN treatment was initiated on day 20 and continued through day 65. On day 36, recipients were given C1498 cells at the indicated cell dose. Recipients treated with CpG 1585 or CpG 2006 had a prolonged survival compared with controls receiving 2 × 105 cells (P = .045 and .001, respectively). Recipients given CpG 1585 and C1498 cells at a dose of 2 × 105 cells survived for a shorter duration, whereas those receiving CpG 2006 survived for a comparable duration than controls receiving 10-fold fewer C1498 cells (2 × 104) (P = .049 and .34, respectively). On the basis of these data, CpG 2006 resulted in an approximate 10-fold reduction in tumorigenicity.
Studies have shown that the innate system returns earlier than the adaptive immune system in recipients of allogeneic TCD bone marrow grafts.20,23 A significant correlation between return of IL-2–activated NK cells with antimyeloid leukemia cytolytic potential by 3 weeks after BMT and the reduced risk of relapse has been reported.23 Because CpG ODNs can activate the innate immune system, we modified the model to analyze the effects of CpG ODNs on anti-AML immune responses early after alloBMT prior to reconstitution of the adaptive immune system. Irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow. Recipients were challenged with 2 × 105 C1498 cells on day 17 after BMT. Cohorts were given no CpG ODNs, CpG 1585, CpG 2006, or both CpGs (Figure 8A). Additional cohorts received a single DLI dose (25 × 106 cells) on day 21, the typical day of DLI administration in this model,28 alone or in combination with CpG ODNs (Figure 8B). Control and CpG 1585-treated recipients of C1498 died at a similar rate with all succumbing within 31 to 38 days after challenge (Figure 8A). In contrast, CpG 2006-treated recipients survived significantly longer than controls (P = .015), requiring 6 months after challenge for uniform lethality. Three (33%) of 9 recipients that were autopsied had no gross evidence of tumor. Combined administration of CpG 1585 + 2006 ODNs resulted in 20% long-term survival (> 8 months after challenge), which was significantly (P = .001) superior to controls but ultimately statistically similar (P = .07) to CpG 2006 alone. Two (22%) of 9 mice that were autopsied had no evidence of tumor.
Combined treatment with CpG 1585 + 2006 and DLI markedly prolongs the survival of recipients of allogeneic TCD bone marrow challenged with AML cells early (day 17) after BMT.
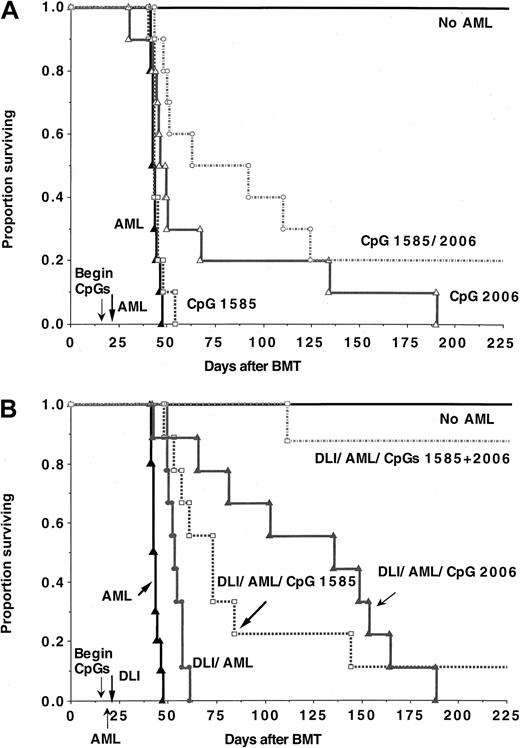
Lethally irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow (n = 8-10 per group). On day 17, recipients were given C1498 cells (2 × 105). An additional control group received no C1498 cells. Cohorts of mice received no treatment, CpG 1585, CpG 2006, or both ODNs. ODNs treatment were initiated on day 15 and continued through day 43 (A). Other cohorts received DLI (B10.BR splenocytes, 25 × 106/mouse) on day 21 alone or with CpG ODNs (B). (A) As compared with controls, CpG 1585 treatment did not significantly prolong survival (P = .08), whereas CpG 2006 alone or CpG 1585 + 2006 significantly prolonged survival (P = .015 and .001, respectively). (B) As compared with controls, all groups receiving DLI had a significantly prolongation in survival (P ≤ .0006). The inclusion of CpG 1585, 2006, or both ODNs along with DLI resulted in an equivalent or in most cases a significant increase in survival as compared with either DLI alone (P = .01, .004, and .0001, respectively) or the comparable CpG ODN groups alone (P = .001, .10, or .002, respectively).
Combined treatment with CpG 1585 + 2006 and DLI markedly prolongs the survival of recipients of allogeneic TCD bone marrow challenged with AML cells early (day 17) after BMT.
Lethally irradiated B6 recipients were reconstituted with B10.BR TCD bone marrow (n = 8-10 per group). On day 17, recipients were given C1498 cells (2 × 105). An additional control group received no C1498 cells. Cohorts of mice received no treatment, CpG 1585, CpG 2006, or both ODNs. ODNs treatment were initiated on day 15 and continued through day 43 (A). Other cohorts received DLI (B10.BR splenocytes, 25 × 106/mouse) on day 21 alone or with CpG ODNs (B). (A) As compared with controls, CpG 1585 treatment did not significantly prolong survival (P = .08), whereas CpG 2006 alone or CpG 1585 + 2006 significantly prolonged survival (P = .015 and .001, respectively). (B) As compared with controls, all groups receiving DLI had a significantly prolongation in survival (P ≤ .0006). The inclusion of CpG 1585, 2006, or both ODNs along with DLI resulted in an equivalent or in most cases a significant increase in survival as compared with either DLI alone (P = .01, .004, and .0001, respectively) or the comparable CpG ODN groups alone (P = .001, .10, or .002, respectively).
As compared with nontreated controls, DLI administered 4 days after C1498 challenge extended survival (P = .0006). However, all died within 6.5 weeks after AML cell injection. DLI was similarly effective as CpG 2006 administration at this period after BMT (P > .50) (Figures 8A versus 8B). Mean weight curves in DLI-treated groups were modestly but not significantly lower (P = .08) than the comparable non–DLI-treated groups (weight curves are not shown). When DLI was combined with CpG 1585, survival was extended greater than with CpG 1585 (P = .001) or DLI (P = .01) alone (Figure8B), and 4 (44%) of 9 mice died without evidence of tumor in contrast to the uniform detection of tumor in the other 2 groups. Mean weight curves in the DLI and DLI + CpG 1585 groups were comparable (P = .96). When DLI was combined with CpG 2006, survival was modestly but not significantly longer than with CpG 2006 alone (P = .10) and was superior to DLI alone (P = .004). Moreover, 5 (63%) of 8 mice that were autopsied had no gross evidence of tumor. DLI-induced GVH was enhanced by the inclusion of CpG 2006 with DLI as indicated by the clinical appearance (ruffled fur, hunched posture, cachexia) and lower mean weight curves in DLI + CpG 2006 with or without CpG 1585 as compared with DLI (P ≤ .01) or CpG ODNs alone (P < .001). In contrast to 10% or less survival rate in recipients given DLI and either CpG ODN, the combination of DLI and both CpG ODNs resulted in 88% long-term survival (P = .0001 versus DLI; P = .002 versus CpG 1585 + 2006) without gross evidence of tumor at necropsy. Thus, the combined administration of DLI and CpG ODN is a highly potent strategy to stimulate anti-AML immune responses in alloBMT recipients of TCD bone marrow grafts at time periods prior to complete immune reconstitution.
Discussion
Several major findings can be derived from our study. First, CpG 2006 is a potent inducer of anti-AML responses in naive as well as syngeneic BMT recipients. In the setting of alloBMT, CpG 2006 also induces an anti-AML response that is bolstered by the inclusion of CpG 1585 and especially by combining both CpG ODNs with DLI. DLI-induced GVHD responses were increased by CpG 2006 administration. Thus, CpG ODNs are potent antileukemia agents that are effective in naive and BMT recipients with minimal residual disease even in the setting in which mice do not have a fully functional adaptive immune system.
We have shown that CpG 2006 but not CpG 1585 ODN pretreatment can reduce tumorigenicity by more than 100-fold in naive mice. CpG ODN was more effective when given 2 days before the AML challenge than concurrently with or following the challenge. To date, the published literature indicates that systemic CpG ODN administration alone may delay but not prevent tumor growth.7,34-40 Because CpG ODNs each have distinct functions on the immune system, extrapolation to studies using CpG ODNs other than the ones used here is not possible. Results with CpG 2006 for the induction of antitumor immune responses have not been reported, although recent unpublished data (Z. Ballas, A.M.K., 2001) indicate that our findings with systemic administration can be observed in other tumor models. Because CpG ODNs in general stimulate the innate immune system, antitumor studies have focused on developing CpG ODNs as adjuvants for tumor-associated antigens or cellular vaccinations. We have shown that systemic CpG ODNs can induce an antilymphoma immune response when combined with fusion proteins consisting of tumor idiotype protein conjugated to either a neoantigen or granulocyte-macrophage colony-stimulating factor or with an antitumor mAb.7,34,35 Others have shown that systemic CpG ODNs can enhance the survival of mice that receive peptide or protein vaccines followed by a melanoma cell challenge.38Local CpG ODN injection in the vicinity of or at the tumor bed site eradicated tumor and/or led to long-term survival.36 37 In both instances, CpG ODN generated a memory cell response as assessed by rechallenge experiments.
In our experiments, NK cells were required for the anti-AML responses provided by CpG 1585 or 2006 ODNs as demonstrated by mAb depletion studies and the use of T- and B-cell–deficient SCID recipients. NK-cell depletion did not completely eliminate the protective effect of CpG 2006 ODN in either wild-type or SCID recipients, and TCD did not adversely affect the antitumor immune responses of CpG 2006-treated wild-type mice. Our studies demonstrated a requirement for NK cells in the optimal AML resistance of mice treated with CpG ODN. T cells were not obligatory for the CpG ODN-facilitated tumor resistance. Consistent with these findings, we could not uncover a memory cell response to AML rechallenge in CpG-treated mice resisting initial AML challenge. Because CpG ODNs are known to stimulate the function of monocytes and DCs, the most likely interpretation of these results is that NK cells are required for optimal antitumor responses, and monocyte/DCs participate by either augmenting indirectly31 or directly41 stimulating NK function or by monocyte/DC-mediated antitumor cytotoxicity.42
CpG ODN 2006 had the greatest antitumor activity in this model, despite being optimized for activation of human rather than murine cells. This unexpected observation points to the relative conservation in the immune recognition of CpG DNA motifs between different species. Although bacterial and synthetic ODN can induce NK cells to produce IFNγ,43 IFNγ is neither directly cytotoxic nor cytostatic to the growth of this tumor cell line (not shown). A more attractive candidate protein induced by CpG ODNs is IL-12.12,28,35 In general, CpG ODNs with phosphorothioate-modified backbones are not as efficient at directly inducing NK-cell function but are more efficient in inducing IL-12 than those with nonmodified backbones.2,31 32 CpG 2006 is known to be a more potent inducer of IL-12 than CpG 1585 in B6 mice at the biological concentrations likely achieved in this study and is more effective in inducing anti-AML resistance than CpG 1585 in our model. Although IL-12 does affect C1498 growth in vitro, C1498 IL-12 transductants are markedly less tumorigenic than control-transduced cells (unpublished data, 2001). We hypothesize that the superiority of 2006 in our tumor models may relate to its ability to induce relatively strong IL-12 production compared with the other CpG ODNs. Future studies will be required to fully elucidate the responsible mechanisms operative in vivo.
Immature DCs treated with CpG ODN and cocultured with irradiated tumor cells have been shown to provide protection against tumor challenge in vaccinated mice.39 CpG ODNs are known to activate APCs and to enhance the capacity of APCs to stimulate both CD4+ and CD8+ T-cell responses.35 44 We hypothesize that APC stimulation induced by CpG 2006 contributed to the antitumor effects. Although CpG 1585 is a potent stimulator of NK function, it is possible that CpG 2006 stimulation of APCs is responsible for the generation of NK effectors and activated monocytes/DCs that work in concert to resist AML. Consistent with this hypothesis, preliminary data indicate that NK-depleted flt3L knockout mice that have a defect in DC numbers were more susceptible to AML-induced lethality than NK-depleted wild-type mice (unpublished data).
An important aspect of our studies was the finding that CpG 2006 ODN administered to syngeneic or allo-TCD bone marrow recipients at early time periods after BMT could augment an anti-AML response. As compared with controls, syngeneic recipients with severe T-cell immune deficiency had a significant survival advantage when given CpG 2006 ODN and challenged with AML cells on day 14 after BMT and when rechallenged later after BMT (day 80). AlloBMT recipients treated with CpG 2006 ODN and challenged on day 36 after BMT also had a significant survival advantage and combined CpG 1585 + 2006 ODNs resulted in 20% long-term survival, even though 2 months or more are required for substantial T-cell immune reconstitution in this setting. In preclinical lymphoma studies, anti-idiotypic mAb given along with a single dose of CpG ODN resulted in 70% versus 40% survival when mAb was combined with 3 doses of IL-2.31 In naive mice, high-dose IL-2 is not particularly effective in inducing AML resistance,24 in contrast to CpG 2006 ODN. If proven to be well tolerated in humans, CpG ODNs would represent an attractive alternative to a continuous infusion of low-dose IL-2.
A mainstay of the treatment of leukemia relapse after BMT is the use of DLI. However, DLI is far less effective in acute than chronic leukemia. In our preclinical studies, alloBMT recipients of TCD bone marrow challenged with AML cells on day 17 and then treated on day 21 with DLI had an extended survival, although all died with leukemia. The inclusion of CpG 1585 or CpG 2006 ODNs provided a more potent DLI-facilitated GVL effect and the coadministration of both CpG ODNs with DLI resulted in 88% long-term survival. A potential explanation is the possibility that these CpG ODNs stimulate DLI-derived T-cell and NK-cell effectors, possibly in concert with bone marrow–derived effector cells present at this early time period after BMT. Consistent with the potential effects of CpG ODNs on DLI-derived T cells, recipients given DLI + CpG ODNs had a more severe GVHD reaction than either DLI- or CpG ODN-treated controls. Future studies will be required to determine whether CpG ODNs + DLI differentially affect particular GVHD target organs. Combined administration of DLI + CpG ODNs represents a new approach potentially useful in situations in which DLI alone is incompletely effective in tumor cell reduction.
Th1-like responses dominate the rapid response of the innate immune system to foreign antigens. Immunostimulatory bacterial DNA and synthetic CpG ODNs result in Th1 responses.6 Although Th1 responses are desirable for cancer immunotherapy, proinflammatory Th1 responses could be detrimental early after BMT. Studies are in progress to explore the effects of CpG ODNs on GVHD and graft rejection. Regardless, CpG 2006 ODNs given with DLI are highly effective in treating AML. Although mice had clinical GVHD, the GVHD was not of sufficient severity to cause lethality in most recipients. Combined CpG ODN and DLI could be especially useful in patients that have acute leukemia typically refractory to DLI.
Supported in part by grants R01 CA-72669, R01 HL63452, and P01 CA66579 from the National Institutes of Health, by a Career Development Award from the Department of Veterans Affairs, by a grant from the Leukemia Task Force, and by a grant from the Coley Pharmaceutical Group (A.M.K., B.R.B.).
A.M.K. has declared a financial interest in the Coley Pharmaceutical Group, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, University of Minnesota Hospital, Box 109 Mayo Bldg, 420 SE Delaware St, Minneapolis, MN 55455; e-mail: blaza001@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal