Abstract
Erythropoietin (EPO) specifically activates the Janus kinase JAK2 and the transcription factor signal transducer and activator of transcription-5 (STAT5). All members of the STAT family are tyrosine phosphorylated in response to cytokine stimulation at a conserved carboxy-terminal tyrosine, Y694, in the case of STAT5. To determine structural features important for STAT signaling, we generated an activation-specific STAT5 antibody using a phosphopeptide containing amino acids 687 to 698 of STAT5 as antigen. This antibody specifically recognizes tyrosine- phosphorylated STAT5 but not nonphosphorylated STAT5. In immunoprecipitation reactions from cell lines and primary erythroblasts, 2 distinct polyclonal activation-specific STAT5 antibodies selectively immunoprecipitate the tyrosine phosphorylated EPO receptor (EPO-R) in addition to STAT5 under native and denaturing conditions. We propose that the activation-specific STAT5 antibody recognizes the 2 substrates to which the STAT5 SH2 domain interacts, namely, the tyrosine- phosphorylated EPO-R and STAT5 itself. Several studies have implicated EPO-R Y343, Y401, Y431, and Y479 in the recruitment of STAT5. Using a series of EPO-R tyrosine mutants expressed in Ba/F3 cells, we have shown that the activation-specific STAT5 antibody immunoprecipitates an EPO-R containing only 2 tyrosines at positions 343 and 401, confirming the importance of these tyrosines in STAT5 recruitment. These data uncover a novel aspect of STAT SH2 domain recognition and demonstrate the utility of activation-specific antibodies for examining the specificity of STAT–cytokine receptor interactions.
Introduction
Erythropoietin (EPO), the primary cytokine regulator of erythropoiesis, exerts its biological function by binding to its cognate receptor, a 66-kd single transmembrane receptor.1 Despite undergoing ligand-dependent tyrosine phosphorylation, the EPO receptor (EPO-R) and other members of the cytokine receptor family do not contain a tyrosine kinase catalytic domain within their cytoplasmic regions. The identification of the Janus family of tyrosine kinases has revealed a mechanism by which hematopoietic cytokines activate intracellular tyrosine phosphorylation. Several studies have shown that EPO specifically activates Janus kinase-2 (JAK2).2,3 The critical importance of EPO,4 EPO-R,4,5 and JAK2 6 7 in erythropoiesis have been demonstrated through gene targeting strategies; deletion of any of these genes gives rise to embryonic lethality because of an inability of the mice to successfully undergo the transition from primitive to definitive erythropoiesis.
Stimulation of JAK2 catalytic activity results in the tyrosine phosphorylation of several tyrosine residues of the EPO-R cytoplasmic tail.8 After the EPO-R is tyrosine phosphorylated, SH2 domain–containing proteins such as signal transducer and activator of transcription-5 (STAT5),9-15 Ship1,16Shp1,17 Shp2,18,19 and the 85-kd subunit of phosphatidylinositol 3′ kinase20-22 are recruited to specific sites.
Elegant studies, first performed in the interferon signaling system, proposed the following mechanism of STAT activation.23 In resting cells, STAT proteins are cytosolic and are not normally phosphorylated. Cytokine-dependent JAK activation results in tyrosine phosphorylation of several cytoplasmic tyrosine residues of the cytokine receptor, some of which may represent consensus binding sites for the SH2 domain of particular STAT proteins. Specific STAT proteins are recruited to the cytokine receptor in an SH2-dependent fashion. When in the proximity of the active JAK kinase, the STAT protein becomes tyrosine phosphorylated at a conserved position. Through an unknown mechanism, the STAT protein disassociates from the cytokine receptor, undergoes reciprocal SH2-mediated homodimerization, and shuttles rapidly to the nucleus.
Several studies have shown that EPO-dependent JAK2 activation results in tyrosine phosphorylation of STAT5.9-15 Using a variety of techniques including analysis of EPO-R deletion mutants, EPO-R tyrosine mutants, and phosphopeptide competitions in electrophoretic mobility shift assays, the interaction sites of STAT5 on the EPO-R were proposed to include Y343, Y401, Y431, and Y479.9,12-14 However, more careful mutagenesis studies have suggested that Y343 and Y401 are the major STAT5 binding sites.13,14 EPO-R Y343 is sufficient for STAT5 tyrosine phosphorylation.9
Phosphopeptide analysis demonstrated that STAT1 becomes tyrosine phosphorylated at a unique position, Y701.24 Indeed, all STAT proteins contain a conserved tyrosine located carboxy-terminal to the SH2 domain, which becomes tyrosine phosphorylated upon cytokine stimulation and participates in SH2 domain–mediated homodimerization or heterodimerization (Y694 for STAT5A, Y699 for STAT5B).25 To examine the importance of this motif for STAT5 signaling, we synthesized a tyrosine-phosphorylated peptide and generated an antibody that specifically recognizes tyrosine-phosphorylated but not unactivated STAT5. Herein, we show that this antibody recognizes tyrosine-phosphorylated STAT5 after interleukin-3 (IL-3) or EPO stimulation in Western blotting analysis. The activation-specific STAT5 antibody also faithfully immunoprecipitates tyrosine-phosphorylated STAT5. Significantly, the activation-specific STAT5 antibody also immunoprecipitates the tyrosine-phosphorylated EPO-R. We hypothesized that this antibody may selectively recognize the 2 substrates of the STAT5 SH2 domain, namely, the tyrosine-phosphorylated EPO-R and STAT5 itself. The ability of the activation-specific STAT5 antibody to immunoprecipitate several EPO-R tyrosine mutants was examined. These experiments revealed that the activation-specific antibody was capable of immunoprecipitating an EPO-R containing only Y343 and Y401.
Materials and methods
Reagents
Murine IL-3 (R&D Systems, Minneapolis, MN), EPO (Ortho, Toronto, ON), and murine interferon-α (IFN-α; Gibco, Gaithersburg, MD) were used at the indicated concentrations. A polyclonal activation-specific STAT5 antibody was graciously provided by Dr Sarah Guadagno at Zymed Laboratories (San Francisco, CA). A STAT5 polyclonal antibody was generously provided by Dr J. N. Ihle (Memphis, TN).
Cells and cell culture
Ba/F3 and DA-3 were maintained as described in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum containing 100 pg/mL murine IL-3 and 50 μM β-mercaptoethanol. HCD-57 cells were maintained in Iscoves modified Dulbecco medium, 20% fetal calf serum, 0.1 U/mL EPO, and 50 μM β-mercaptoethanol.
Various EPO-R constructs were electroporated into Ba/F3 cells. Individual G418-resistant subclones were isolated by limiting dilution. The expression of EPO-R was confirmed by Western blotting using a peptide-specific EPO-R antibody (Santa Cruz, Santa Cruz, CA), and the EPO-dependent growth characteristics of each subclone were examined by performing an XTT assay as described.26
Generation of phenylhydrazine-primed splenic erythroblasts
C57Bl/6 mice were injected intraperitoneally on days 1 and 2 with a sterile solution of phenylhydrazine hydrochloride (6 mg/mL) in alpha–minimal essential medium to achieve a dose of 60 mg/kg.27 The mice were killed on day 5 by cervical dislocation. The spleen was removed, and a single-cell suspension was generated. The cells were incubated in alpha–minimal essential medium containing 2% fetal calf serum and 50 μM β-mercaptoethanol for 4 hours. Cells were stimulated with 50 U/mL EPO for various periods, and lysates were generated as described previously.3
Generation of EPO-R mutants
EPO-R tyrosine mutants were generated via overlap extension polymerase chain reaction. These included a series of single tyrosine mutants in which phenylalanine was substituted for different tyrosine residues in the EPO-R and a series of add-back mutants to an EPO-R devoid of tyrosine residues. Oligonucleotide primers were selected that produced a phenylalanine at amino acid positions 343, 401, 431, or 479. Polymerase chain reaction was performed using pBluescript SK–EPO-R or selected pBluescript SK–EPO-R tyrosine mutants to generate toSphI-EcoRI fragment in pCR-Script. The fidelity of all constructs was confirmed by sequencing both strands of the 440–base pair fragment. Each SphI-EcoRI fragment was subcloned into SphI-EcoRI–digested pBluescript SK–EPO-R. The full-length EPO-R cDNA was then subcloned into pCDNA3 using KpnI and EcoRI.
Antibody generation
A peptide was synthesized that contained amino acids 687 to 698 of ovine STAT5. A cysteine residue was added to the N-terminus of the peptide and was used to conjugate the peptide to bovine serum albumin using a succinimide ester. Rabbits were immunized with the peptide–bovine serum albumin conjugate. A second activation-specific STAT5 antibody (71-6900) was generously provided by Dr Sarah Guadagno.
Immunoprecipitation and Western blotting
The indicated cell lines were incubated in the absence of cytokine for 4 hours and then stimulated with the corresponding cytokine: 10 ng/mL murine IL-3, 50 U/mL human EPO, 5 ng/mL murine interferon-α. Cell lysis, immunoprecipitation, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting was carried out as described.3
Immunoblots were performed with the activation-specific STAT5 antibody at 1:5000 dilution followed by horseradish peroxidase (HRP)–conjugated protein A (1:5000). For detection of tyrosine phosphorylation, the monoclonal antiphosphotyrosine 4G10 antibody was used (1 μg/mL) followed by HRP-sheep antimouse immunoglobulin G (1:5000). For detection of STAT5 protein, antibody to STAT5 (1:5000) was incubated followed by an incubation with the appropriate HRP–protein A. Immunoreactive proteins were stripped from nitrocellulose membranes by incubation in 62.5 mM Tris-HCl (pH 6.8), 2% (wt/vol) SDS, and 100 mM β-mercaptoethanol at 50°C for 30 minutes.
Immunoprecipitations were performed on 2 × 107 cell equivalents of each protein lysate. Cells were lysed in a buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 10 mM Na4P2O7, 10 mM NaF, 1 mM Na3VO4, 5 mM ethylenediaminetetraacetic acid (EDTA), 2 μg/mL aprotinin, 2 μg/mL leupeptin, 2 μg/mL pepstatin A, and 1 mM phenylmethylsulfonyl fluoride (PMSF) (buffer A) for preparation of nondenaturing lysates. The samples were incubated on ice for 5 minutes and then centrifuged at 13 000g for 5 minutes. For generation of denatured extracts, 2 × 107cells were boiled for 5 minutes in 250 μL of a buffer containing 50 mM Tris-HCl (pH 8.0), 0.5% SDS, 10 mM Na4P2O7, 10 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 2 μg/mL pepstatin A, and 1 mM PMSF. Then, 750 μL of 50 mM TrisHCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 1.0% Triton X-100 containing the above phosphatase, and protease inhibitors were added. The samples were rotated for 5 minutes and then centrifuged at 14 000g for 15 minutes.
The activation-specific STAT5 antibody was added to either lysate, and an immunoprecipitation was performed. Samples were resolved by SDS-PAGE, and the identity of tyrosine-phosphorylated proteins and STAT proteins were analyzed by immunoblotting as described above.
Electrophoretic mobility shift assays
Nuclear extracts were prepared from Ba/F3–EPO-R subclones stimulated in the presence or absence of 10 ng/mL IL-3 or 50 U/mL EPO. Cell pellets were resuspended in 1.0 mL buffer B (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 1 mM PMSF, 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 5 μg/mL pepstatin A) and allowed to swell on ice for 10 minutes. Cells were then vortexed for 10 seconds, centrifuged at 10 000g for 10 seconds, and the supernatant was discarded. The pellet was washed once in buffer B, and then nuclear proteins were resuspended in an appropriate volume of buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 1 mM PMSF, 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 5 μg/mL pepstatin A).
Gel shift experiments were performed as described28 with a double-stranded 32P-labeled oligonucleotide derived from the β-casein promoter (AGATTTCTAGGAATTCAAATC) and its complement. A total of 2 μg nuclear extract was mixed with 0.25 ng32P-labeled oligonucleotide in 20 μL binding buffer (13 mM HEPES [pH 7.9], 65 mM NaCl, 1 mM dithiothreitol, 0.15 mM EDTA, 8% glycerol, 0.8 μg/mL poly[dI-dC]) for 20 minutes at 4°C. For competition experiments, a 50-fold excess of either the unlabeled β-casein oligonucleotide (specific) or an oligonucleotide derived from the DUB-1 promoter29 (nonspecific) was added to the binding reaction. For supershifting experiments, a peptide-specific STAT5 antibody (generously provided by Dr J. N. Ihle) was added at the completion of the binding reaction and incubated for an additional 20 minutes at 4°C.
Results
Activation-specific STAT5 antibody identifies tyrosine- phosphorylated STAT5 in a variety of hematopoietic cell lines
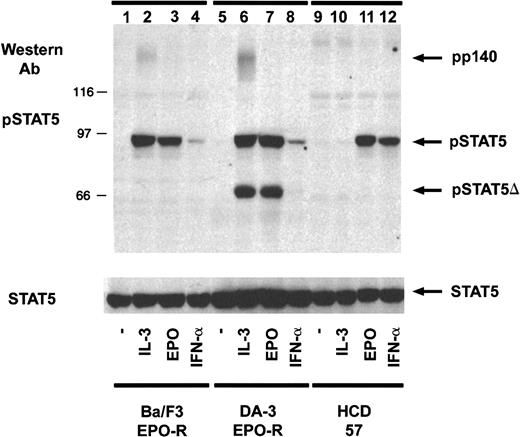
To characterize the specificity of the activation-specific STAT5 antibody, Western blotting was performed on lysates from several cytokine-dependent hematopoietic cell lines. Cells were depleted of cytokine for 4 hours and then stimulated for 10 minutes and analyzed by Western blotting (Figure 1). IL-3 and EPO stimulation of either Ba/F3–EPO-R (lanes 2 and 3) or DA-3–EPO-R (lanes 6 and 7) cells and EPO stimulation of HCD-57 cells (lane 11) resulted in the phosphorylation of a 95-kd phosphoprotein detected by the activation-specific STAT5 antibody. All 3 cell lines respond to IFN-α and IFN-γ (data not shown); however, at this exposure STAT5 phosphorylation in response to IFN-α could only be detected in HCD-57 cells (lane 12). Long exposures revealed a weak IFN-α–stimulated tyrosine phosphorylation of STAT5 (data not shown). The activation-specific STAT5 antibody also weakly recognized a 140-kd phosphoprotein after IL-3 stimulation, likely the tyrosine-phosphorylated IL-3 receptor (lanes 2 and 6) and a truncated form of STAT5 expressed in DA-3–EPO-R cells (lanes 5 and 6). Truncated versions of STAT5 have been previously been described in myeloid cell lines including DA-3,30 32D,31FDC-P1,32,33 and WEHI-3 33,34 as well as primary monocytes.35 No other cytokine-stimulated phosphoproteins were detected using the activation-specific STAT5 antibody. The identity of the 95-kd phosphoprotein was confirmed by stripping and reprobing the blot with a peptide-specific STAT5 antibody (lower panel).
An activation-specific STAT5 antibody recognizes tyrosine-phosphorylated STAT5.
Ba/F3–EPO-R, DA-3–EPO-R, and HCD-57 cells were incubated for 4 hours in the absence of cytokine and then stimulated with various cytokines as shown; 100 μg protein lysate was resolved via SDS-PAGE and transferred to nitrocellulose. The membrane was incubated with an activation-specific STAT5 antibody (1 μg/mL) followed by HRP–protein A (1:5000) (top). Immunoreactive proteins were detected by enhanced chemiluminescence. The membrane was stripped and reprobed with an antibody that recognizes total STAT5 (1:5000) (bottom). pSTAT5 indicates phosphorylated STAT5; Ab, antibody.
An activation-specific STAT5 antibody recognizes tyrosine-phosphorylated STAT5.
Ba/F3–EPO-R, DA-3–EPO-R, and HCD-57 cells were incubated for 4 hours in the absence of cytokine and then stimulated with various cytokines as shown; 100 μg protein lysate was resolved via SDS-PAGE and transferred to nitrocellulose. The membrane was incubated with an activation-specific STAT5 antibody (1 μg/mL) followed by HRP–protein A (1:5000) (top). Immunoreactive proteins were detected by enhanced chemiluminescence. The membrane was stripped and reprobed with an antibody that recognizes total STAT5 (1:5000) (bottom). pSTAT5 indicates phosphorylated STAT5; Ab, antibody.
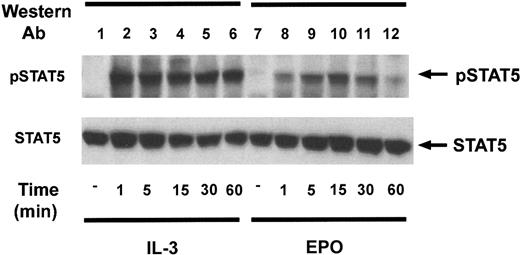
The kinetics of activation of STAT5 was analyzed in Ba/F3–EPO-R cells (Figure 2). IL-3 and EPO led to the rapid induction of a 95-kd phosphoprotein as detected by immunoblot analysis with the activation-specific STAT5 antibody. The EPO-dependent tyrosine phosphorylation of STAT5 was maximal at 15 minutes (lane 10).
Kinetics of STAT5 tyrosine phosphorylation in Ba/F3–EPO-R cells.
Ba/F3–EPO-R cells were incubated for 4 hours in the absence of cytokine and then stimulated with either 10 ng/mL murine IL-3 (lanes 2-6) or 50 U/mL human EPO (lanes 7-12) from 1 to 60 minutes as shown. Activation-specific STAT5 (top) and STAT5 antibodies (bottom) were used in immunoblotting experiments as described in the legend to Figure 1.
Kinetics of STAT5 tyrosine phosphorylation in Ba/F3–EPO-R cells.
Ba/F3–EPO-R cells were incubated for 4 hours in the absence of cytokine and then stimulated with either 10 ng/mL murine IL-3 (lanes 2-6) or 50 U/mL human EPO (lanes 7-12) from 1 to 60 minutes as shown. Activation-specific STAT5 (top) and STAT5 antibodies (bottom) were used in immunoblotting experiments as described in the legend to Figure 1.
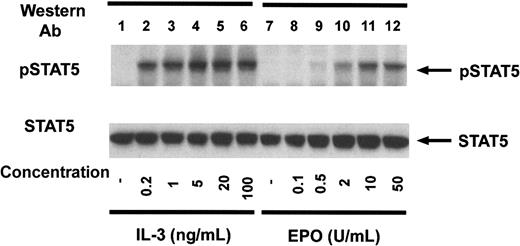
Ba/F3–EPO-R cells were incubated with increasing concentrations of either murine IL-3 or human EPO to examine the concentration dependence of STAT5 tyrosine phosphorylation (Figure3). Concentrations as low as 200 pg/mL IL-3 (lane 2) or 0.5 U/mL EPO (lane 9) resulted in tyrosine phosphorylation of STAT5 consistent with the biologically active concentrations of these cytokines.
Dose response of STAT5 tyrosine phosphorylation in Ba/F3–EPO-R cells.
Ba/F3–EPO-R cells were incubated for 4 hours in the absence of cytokine and then stimulated with various concentrations of either murine IL-3 (lanes 2-6) or human EPO (lanes 7-12) for 10 minutes as shown. Activation-specific STAT5 (top) and STAT5 antibodies (bottom) were used in immunoblotting experiments as described in the legend to Figure 1.
Dose response of STAT5 tyrosine phosphorylation in Ba/F3–EPO-R cells.
Ba/F3–EPO-R cells were incubated for 4 hours in the absence of cytokine and then stimulated with various concentrations of either murine IL-3 (lanes 2-6) or human EPO (lanes 7-12) for 10 minutes as shown. Activation-specific STAT5 (top) and STAT5 antibodies (bottom) were used in immunoblotting experiments as described in the legend to Figure 1.
A common epitope is shared by STAT5 and the EPO-R in EPO-stimulated cells
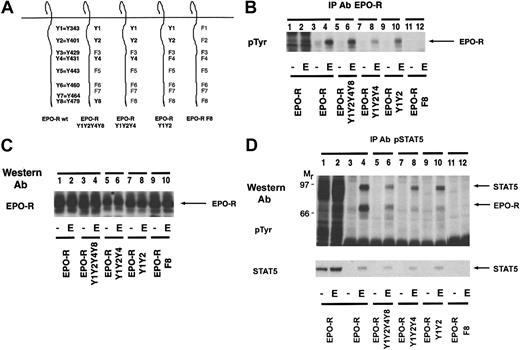
To determine whether proteins other than STAT5 contain a similar phosphotyrosine motif after EPO stimulation, an immunoprecipitation experiment was performed (Figure 4A). Lysates were prepared under native and denaturing conditions, and immunoprecipitations were performed with 2 distinct activation-specific STAT5 polyclonal antibodies to exclude the possibility of unique reactivity of a single antiserum (see “Materials and methods”). Precipitating proteins were initially analyzed by antiphosphotyrosine Western blotting. Immunoprecipitations performed with 2 distinct activation-specific STAT5 antibodies revealed that each antibody immunoprecipitated STAT5 (lanes 4 and 6). However, an additional 72-kd phosphoprotein, the EPO-R, was immunoprecipitated by both activation-specific STAT5 antibodies (lanes 4 and 6) from nondenatured lysates. To determine whether the activation-specific STAT5 antibodies could directly immunoprecipitate the EPO-R, experiments were also performed from denatured lysates. The EPO-R was directly immunoprecipitated from these lysates using both activation-specific antibodies (lanes 10 and 12). Importantly, a peptide-specific antibody that recognized total STAT5 did not immunoprecipitate the EPO-R from either lysate preparation (lanes 2 and 8). No other cytokine-inducible phosphoproteins were detected in this experiment.
Activation-specific STAT5 antibodies selectively immunoprecipitate tyrosine-phosphorylated STAT5 and EPO-R.
(A) Ba/F3–EPO-R cells were incubated for 8 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Lysates (Lys) were prepared under nondenaturing conditions (lanes 1-6) or denaturing conditions (lanes 7-12). Immunoprecipitations were performed with the following STAT5 antibodies: total STAT5, activation-specific polyclonal phosphorylated STAT5 (pSTAT5) (David A. Frank [DAF]), and activation-specific polyclonal pSTAT5 (Zymed). A Western blot was performed with the antiphosphotyrosine antibody, 4G10. The mobility of STAT5 and EPO-R are indicated. (B) Splenic erythroblasts were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Immunoprecipitation (IP) and Western blotting were performed as described above. pTyr indicates phosphotyrosine.
Activation-specific STAT5 antibodies selectively immunoprecipitate tyrosine-phosphorylated STAT5 and EPO-R.
(A) Ba/F3–EPO-R cells were incubated for 8 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Lysates (Lys) were prepared under nondenaturing conditions (lanes 1-6) or denaturing conditions (lanes 7-12). Immunoprecipitations were performed with the following STAT5 antibodies: total STAT5, activation-specific polyclonal phosphorylated STAT5 (pSTAT5) (David A. Frank [DAF]), and activation-specific polyclonal pSTAT5 (Zymed). A Western blot was performed with the antiphosphotyrosine antibody, 4G10. The mobility of STAT5 and EPO-R are indicated. (B) Splenic erythroblasts were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Immunoprecipitation (IP) and Western blotting were performed as described above. pTyr indicates phosphotyrosine.
The ability of the activation-specific STAT5 antibody to immunoprecipitate STAT and the EPO-R from primary erythroblasts was examined (Figure 4B). Phenylhydrazine causes an anemia when administered in vivo, which results in an elevation in splenic EPO-responsive erythroblasts.27 As illustrated above, the activation-specific STAT5 antibody selectively immunoprecipitates EPO-R and STAT5 after EPO stimulation of the primary cells (lanes 2 and 4).
These experiments indicate that 2 distinct activation-specific STAT5 antibodies are capable of immunoprecipitating both EPO-R and STAT5 after EPO stimulation. This suggested that either the EPO-R was being coprecipitated in a complex with STAT5 or that an epitope on EPO-R is shared with the tyrosine phosphorylation site of STAT5. Coprecipitation of STAT5 with the EPO-R has been demonstrated; however, the fraction of tyrosine-phosphorylated EPO-R shown to associate with STAT5 is relatively small, in keeping with the transient nature of STAT association with cytokine receptors.36 37 Given that the activation-specific STAT5 antibodies selectively recognized tyrosine phosphorylated STAT5 and EPO-R but no other EPO-dependent substrates, we became intrigued with the possibility that this antibody selectively recognizes the 2 substrates to which the STAT5 SH2 domain binds, STAT5 and EPO-R. Alternatively, a small fraction of EPO-R and STAT5 could associate, which would account for these observations.
Data from studies using various EPO-R deletion and tyrosine substitutions suggest that there is considerable redundancy in the ability of EPO-R to recruit and activate STAT5. As many as 4 distinct tyrosines have been implicated in STAT5 binding, including EPO-R Y343 (Y1), Y401 (Y2), Y431 (Y4), and Y479 (Y8). Some of this evidence was garnered by the addition of specific phosphopeptides to electrophoretic mobility shift assays. More careful mutagenesis approaches revealed that EPO-dependent tyrosine phosphorylation of Y343 and Y401 accounts for most STAT5 binding.13 14
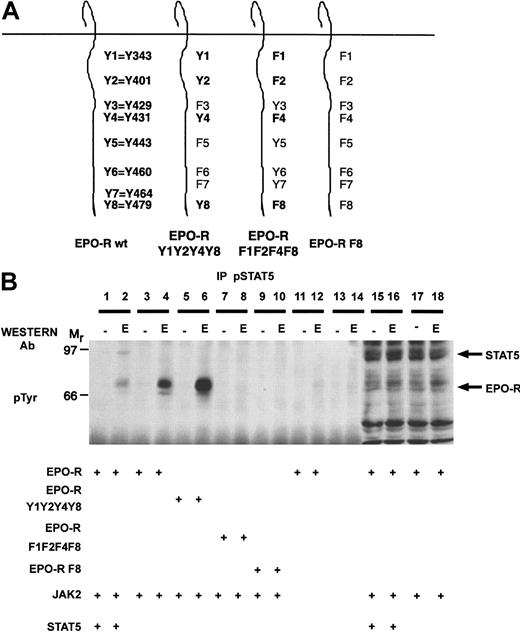
To examine whether the activation-specific STAT5 antibody could immunoprecipitate the tyrosine-phosphorylated EPO-R in the absence of transfected STAT5, reconstitution experiments were performed in 293T cells (Figure 5). For this experiment, we used wild-type EPO-R, EPO-R containing STAT5 binding sites (EPO-R Y1Y2Y4Y8), a complementary mutant containing tyrosine to phenylalanine substitutions (EPO-R F1F2F4F8), and a full-length EPO-R construct devoid of cytoplasmic tyrosine residues (EPO-R F8). Transient transfections were performed with the various EPO-R constructs in the presence or absence of JAK2 and/or STAT5. The cells were depleted of serum 24 hours after transfection and then stimulated in the presence or absence of EPO. Lysates were prepared, and immunoprecipitations were performed with the activation-specific STAT5 antibody. EPO-dependent signaling was established in this transient assay because EPO could stimulate the tyrosine phosphorylation of EPO-R and STAT5 (lane 2). The activation-specific STAT5 antibody immunoprecipitated both STAT5 and the wild-type EPO-R after EPO stimulation (lane 2). Importantly, the activation-specific antibody selectively immunoprecipitated the EPO-R in the absence of exogenous STAT5 (lane 4). EPO-R Y1Y2Y4Y8 (lane 6) was immunoprecipitated by the antibody, whereas EPO-R F1F2F4F8 (lane 8) and EPO-R F8 (lane 10) were not recognized by the activation-specific STAT5 antibody after EPO stimulation. JAK2 was required for EPO-R tyrosine phosphorylation (lane 12), and no tyrosine-phosphorylated proteins were observed in mock transfected cells (lanes 13 and 14). This experiment clearly showed that the activation-specific STAT5 antibody could directly immunoprecipitate the EPO-R in the absence of transfected STAT5. Similar results were obtained for the Zymed activation-specific STAT5 antibody (data not shown).
The activation-specific STAT5 antibody specifically immunoprecipitates EPO-R.
(A) Selected EPO-R tyrosine to phenylalanine mutants were generated. The nomenclature is indicated for the wild-type (wt) EPO-R. (B) 293T cells were transiently transfected with the indicated plasmids as shown. Twenty-four hours after transfection, cells were depleted of serum for 18 hours and then stimulated in the presence or absence of 50 U/mL EPO for 10 minutes. Lysates were prepared, and immunoprecipitations (IP) were performed with the activation-specific STAT5 antibody. Tyrosine-phosphorylated proteins were detected by immunoblotting with the monoclonal 4G10 antiphosphotyrosine antibody. The mobility of STAT5 and EPO-R are indicated.
The activation-specific STAT5 antibody specifically immunoprecipitates EPO-R.
(A) Selected EPO-R tyrosine to phenylalanine mutants were generated. The nomenclature is indicated for the wild-type (wt) EPO-R. (B) 293T cells were transiently transfected with the indicated plasmids as shown. Twenty-four hours after transfection, cells were depleted of serum for 18 hours and then stimulated in the presence or absence of 50 U/mL EPO for 10 minutes. Lysates were prepared, and immunoprecipitations (IP) were performed with the activation-specific STAT5 antibody. Tyrosine-phosphorylated proteins were detected by immunoblotting with the monoclonal 4G10 antiphosphotyrosine antibody. The mobility of STAT5 and EPO-R are indicated.
We were interested in identification of the epitope on EPO-R that is shared with the tyrosine phosphorylation site of STAT5. Having mapped the association site in the previous experiment to EPO-R Y1, Y2, Y4, or Y8, we selected 4 distinct mutants: EPO-R Y1Y2Y4Y8, EPO-R Y1Y2Y4, EPO-R Y1Y2, and EPO-R F8 (Figure 6A).
The activation-specific STAT5 antibody selectively recognizes the STAT5 docking site on the EPO-R.
(A) Selected EPO-R tyrosine to phenylalanine mutants were generated. The nomenclature is indicated for the wild-type EPO-R. (B) Cell lines expressing each EPO-R construct were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. An immunoprecipitation (IP) was performed with a peptide-specific EPO-R antibody. The extent of tyrosine phosphorylation was detected by Western blotting using the monoclonal antiphosphotyrosine antibody, 4G10. The mobility of the EPO-R is indicated. (C) One hundred micrograms of lysate was resolved via SDS-PAGE, and a Western blot was performed using a peptide-specific EPO-R antibody. The mobility of the EPO-R is indicated. (D) The identical cell lines were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Lysates were prepared under denaturing conditions, and immunoprecipitations were performed with an activation-specific STAT5 antibody. A Western blot was then performed with the monoclonal antiphosphotyrosine antibody, 4G10 (top). The membrane was stripped and reprobed with a peptide-specific STAT5 antibody (bottom). The mobility of STAT5 and EPO-R are indicated.
The activation-specific STAT5 antibody selectively recognizes the STAT5 docking site on the EPO-R.
(A) Selected EPO-R tyrosine to phenylalanine mutants were generated. The nomenclature is indicated for the wild-type EPO-R. (B) Cell lines expressing each EPO-R construct were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. An immunoprecipitation (IP) was performed with a peptide-specific EPO-R antibody. The extent of tyrosine phosphorylation was detected by Western blotting using the monoclonal antiphosphotyrosine antibody, 4G10. The mobility of the EPO-R is indicated. (C) One hundred micrograms of lysate was resolved via SDS-PAGE, and a Western blot was performed using a peptide-specific EPO-R antibody. The mobility of the EPO-R is indicated. (D) The identical cell lines were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Lysates were prepared under denaturing conditions, and immunoprecipitations were performed with an activation-specific STAT5 antibody. A Western blot was then performed with the monoclonal antiphosphotyrosine antibody, 4G10 (top). The membrane was stripped and reprobed with a peptide-specific STAT5 antibody (bottom). The mobility of STAT5 and EPO-R are indicated.
We examined the tyrosine phosphorylation of the EPO-R in the various cell lines by performing an immunoprecipitation with a peptide-specific EPO-R antibody followed by antiphosphotyrosine immunoblotting (Figure6B). All of the EPO-R tyrosine mutants, with the exception of EPO-R F8, were tyrosine phosphorylated after EPO stimulation. The level of phosphorylation of EPO-R Y1Y2Y4 was slightly lower (lane 8), which probably reflects lower expression of the EPO-R in this particular subclone.
To examine the expression of the EPO-R in the selected subclones, an EPO-R Western blot was performed using lysates from each cell line (Figure 6C). Comparable expression of the EPO-R was observed in each lane, with slightly lower expression of EPO-R Y1Y2Y4 (lanes 5 and 6). XTT assays revealed that all EPO-R mutants with the exception of EPO-R F8 displayed EPO-dependent proliferation (data not shown).
We next examined whether these mutations disrupted the epitope shared with tyrosine-phosphorylated STAT5. Each cell line was stimulated in the presence or absence of EPO, lysates were made, and immunoprecipitations were performed with the activation-specific STAT5 antibody (Figure 6D). STAT5 was tyrosine phosphorylated in response to EPO in cells expressing all of the EPO-R constructs with the exception of EPO-R F8. The identity of STAT5 was confirmed by stripping and reprobing the blot with a peptide-specific STAT5 antibody. Furthermore, the 72-kd EPO-R was observed in all of the activation-specific STAT5 immunoprecipitations with the exception of EPO-R F8. This confirms that tyrosine phosphorylation of the EPO-R at Y1 or Y2 is sufficient for STAT5 activation, as previously reported.13,14 The activation-specific STAT5 antibody is very selective because it can immunoprecipitate an EPO-R containing tyrosines at Y1 (Y343) and Y2 (Y401), which represent the optimal STAT5 recruitment sites.13 14
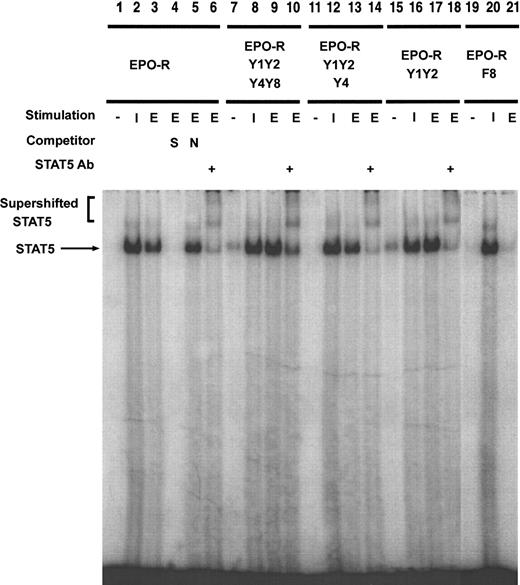
To confirm that EPO-dependent STAT5 tyrosine phosphorylation correlated with DNA binding, an electrophoretic mobility shift assay was performed (Figure 7). Nuclear extracts were prepared from each cell line stimulated in the absence of cytokine or in the presence of IL-3 or EPO. A complex was observed after stimulation of Ba/F3–EPO-R cells with either IL-3 (lane 2) or EPO (lane 3). This oligonucleotide-DNA complex could be competed with an excess of unlabeled oligonucleotide (lane 4) but not a nonspecific oligonucleotide (lane 5). The presence of STAT5 in the complex was confirmed by the addition of a peptide-specific STAT5 antibody, which resulted in supershifting of the band to a higher mobility. Ba/F3–EPO-R Y1Y2Y4Y8, Ba/F3–EPO-R Y1Y2Y4, and Ba/F3–EPO-R Y1Y2 cells all stimulated a STAT5-specific complex after EPO stimulation. There was a slight increase in EPO-dependent STAT5 activation in Ba/F3–EPO-R F8, similar to previous reports.14
EPO-dependent STAT5 DNA binding is activated in several EPO-R tyrosine mutants.
Nuclear extracts were prepared from Ba/F3–EPO-R (lanes 1-6), Ba/F3 EPO-R Y1Y2Y4Y8 (lanes 7-10), Ba/F3 Y1Y2Y4 (lanes 11-14), Ba/F3 Y1Y2 (lanes 15-18), and Ba/F3 EPO-R F8 (lanes 19-21) cells stimulated in the presence or absence of IL-3 (I) or EPO (E). Electrophoretic mobility shift assays were performed as described in “Materials and methods.” Complexes were resolved on a 5% native polyacrylamide gel. The specificity of DNA binding was determined by the addition of unlabeled β-casein oligonucleotide (S), a nonspecific oligonucleotide from the DUB-1 promoter (N), and by incubation with a peptide-specific STAT5 antibody. Complexes were analyzed via PhosphorImager detection.
EPO-dependent STAT5 DNA binding is activated in several EPO-R tyrosine mutants.
Nuclear extracts were prepared from Ba/F3–EPO-R (lanes 1-6), Ba/F3 EPO-R Y1Y2Y4Y8 (lanes 7-10), Ba/F3 Y1Y2Y4 (lanes 11-14), Ba/F3 Y1Y2 (lanes 15-18), and Ba/F3 EPO-R F8 (lanes 19-21) cells stimulated in the presence or absence of IL-3 (I) or EPO (E). Electrophoretic mobility shift assays were performed as described in “Materials and methods.” Complexes were resolved on a 5% native polyacrylamide gel. The specificity of DNA binding was determined by the addition of unlabeled β-casein oligonucleotide (S), a nonspecific oligonucleotide from the DUB-1 promoter (N), and by incubation with a peptide-specific STAT5 antibody. Complexes were analyzed via PhosphorImager detection.
Discussion
Several studies have shown that EPO activates tyrosine phosphorylation and DNA binding of STAT5 in a time- and dose-dependent manner.9-15 Transformation by Friend spleen focus-forming virus has also been shown to correlate with constitutive activation of STAT5.38 STAT proteins are cytosolic and unphosphorylated under resting conditions.39 EPO-dependent JAK2 activation initiates a cascade of tyrosine phosphorylation that results in phosphorylation of the EPO-R and several critical intracellular substrates. Phosphorylation of specific motifs on the EPO-R results in the recruitment of STAT5 to the EPO-R and subsequent tyrosine phosphorylation by JAK2. Tyrosine phosphorylation allows STAT5 to disassociate from the EPO-R and undergo a reciprocal SH2-mediated dimerization. The STAT5 dimer is shuttled to the nucleus via an unknown mechanism and has been shown to transcriptionally regulate oncostatin M,40 Cis,41 and cyclin D1.42
The binding specificity of several SH2 proteins was first described by an elegant affinity chromatography approach. Using this method it was shown that SH2 domains bound to distinct phosphotyrosine sequences and the specificity was conferred by amino acids located immediately on the carboxyl-terminal side of the phosphotyrosine.43 44Unfortunately, the cytosolic transcription factors, STATs, are refractory to this analysis, so the identification of STAT SH2 binding sites has evolved through the mutagenesis of tyrosine sequences found in various cytokine receptors.
In this study, we have shown that polyclonal antibodies raised against a phosphorylated peptide corresponding to the STAT5 tyrosine phosphorylation site recognize the tyrosine-phosphorylated STAT5 but not the unphosphorylated STAT5. In addition, 2 distinct polyclonal antibody preparations displayed the unexpected finding of also recognizing the tyrosine-phosphorylated EPO-R in cell lines and primary splenic-derived erythroblasts in immunoprecipitation experiments. The activation-specific STAT5 antibodies did not recognize any other EPO-stimulated phosphoproteins in Western blotting experiments. Thus, the activation-specific STAT5 antibodies appear to recapitulate the selectivity of the STAT5 SH2 binding sites, namely, the EPO-R and STAT5 itself. Neither antibody appears to function as a nonspecific antiphosphotyrosine antibody because other EPO-dependent tyrosine-phosphorylated substrates such as phospholipase Cγ1,45 Ship1,16,46 JAK2,2,3Cbl,47-49 Shp2,18,19,50 and Shc50-52 are not recognized.
Previous studies have shown that EPO activates the tyrosine phosphorylation of STAT5 in transfected cell lines including Ba/F3–EPO-R,9,14 32D–EPO-R,53DA-3–EPO-R,9,12,53 and FDCP–EPO-R,13and in erythroid cell lines including HCD-57,36,54UT-7,11,55,56 and human colony-forming units–erythroid.57 Phenylhydrazine administration generates a population of splenic erythroblasts that accurately represents erythroid differentiation as monitored by the expression of EPO-R, GATA-1, EKLF, NF-E2, δ-aminolevulinic acid synthase, α-globin, and β-major globin.58 In this study, we have shown that phenylhydrazine-primed erythroblasts are EPO-responsive and the EPO-R is immunoprecipitated by the activation-specific STAT5 antibody.
Several cytokines, including EPO,9-15thrombopoietin,59,60 IL-3,53,61IL-5,61 granulocyte-macrophage colony-stimulating factor,55,61 IL-2,62,63 growth hormone,55,64-66 and prolactin,55 all confer activation of STAT5. The ability of such a wide range of cytokines to induce the tyrosine phosphorylation of STAT5 relies on the expression and tyrosine phosphorylation of the appropriate binding motifs in all of these receptors. In addition to the aforementioned studies completed on EPO, mutagenesis experiments of the IL-2Rβ chain67and the IL-3Rβc receptor68 indicate that the consensus binding motif for STAT5 is pY-L1-X2-(I/L/V)3. The activation-specific STAT5 antibody also immunoprecipitates the IL-3Rβc after IL-3 stimulation (data not shown). After tyrosine phosphorylation, the STAT5 SH2 domain of one STAT5 monomer interacts with the tyrosine-phosphorylated sequence of the corresponding STAT5 (pYVKP) to form a dimeric complex. Thus, there appears to be little similarity between the ligand specificity of the unphosphorylated and phosphorylated forms of STAT5. Earlier studies have shown that an activation-specific STAT1 antibody69also recognizes Bcr-Abl.70
The crystal structures of STAT1 71 and STAT3β 72 have been solved and have revealed unanticipated information regarding the mechanism of SH2-mediated dimerization. In the case of STAT1, the region of the phosphopeptide from one monomer that contacts the SH2 domain of the dimer showed that positions +1, +3, +5, +6, and +7 of Y701 made important contacts with a hydrophobic pocket of the SH2 domain. In particular, Leu706 (+5) formed a hydrophobic binding site containing Ala641 and Val642. Importantly, the amino acids found at the corresponding region of the STAT5A proteins are distinct (Ile653 and Arg654), indicating that this region could mediate binding specificity of the STAT5 phosphopeptide.
The significant finding of this study was the ability of 2 unique activation-specific STAT5 antibodies to selectively recognize the tyrosine-phosphorylated EPO-R and STAT5 in immunoprecipitation experiments. Despite a lack of obvious sequence similarity between the STAT5 SH2 substrates found on the EPO-R and STAT5, there is strong recognition of both proteins by the antibody after EPO stimulation. Perhaps tyrosine phosphorylation of STAT5 exerts a conformational change within the SH2 domain of STAT5. This may account for why the unphosphorylated STAT5 molecule would have a distinct binding specificity as compared with when STAT5 is tyrosine phosphorylated. This hypothesis must await the solution of monomeric, unactivated, and dimeric tyrosine-phosphorylated STAT5 crystal structures.
We thank Eleanor Fish for the gift of murine IFN-α and Sonya Penfold and members of the Barber laboratory for helpful comments on the manuscript.
Supported by grants from the Medical Research Council of Canada (D.L.B.), University of Toronto Connaught New Staff Grant (D.L.B.), National Institutes of Health DK 50693 (B.G.N.), F32 DK 09465 (M.Y.), and CA 79547 (D.A.F.). D.A.F. received funding from the Brent Leahey Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dwayne L. Barber, Ontario Cancer Institute, Division of Cellular and Molecular Biology, 610 University Ave, Toronto, ON, M5G 2M9, Canada; e-mail: dbarber@oci.utoronto.ca.

![Fig. 4. Activation-specific STAT5 antibodies selectively immunoprecipitate tyrosine-phosphorylated STAT5 and EPO-R. / (A) Ba/F3–EPO-R cells were incubated for 8 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Lysates (Lys) were prepared under nondenaturing conditions (lanes 1-6) or denaturing conditions (lanes 7-12). Immunoprecipitations were performed with the following STAT5 antibodies: total STAT5, activation-specific polyclonal phosphorylated STAT5 (pSTAT5) (David A. Frank [DAF]), and activation-specific polyclonal pSTAT5 (Zymed). A Western blot was performed with the antiphosphotyrosine antibody, 4G10. The mobility of STAT5 and EPO-R are indicated. (B) Splenic erythroblasts were incubated for 4 hours in the absence of cytokine and then stimulated with 50 U/mL human EPO for 10 minutes. Immunoprecipitation (IP) and Western blotting were performed as described above. pTyr indicates phosphotyrosine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2230/5/m_h80810883004.jpeg?Expires=1767788333&Signature=H2N1d9qkIafraYZYdV75kyiYPz0bwDNy~4T~SPgHY174Gq~cUrNZrAOHFOnP9Fyi2TOv-8bT1FFw5m8TvmzhMyXtL4iZ9u6CQ2IdVgnU2z0QLUkTDCQbk2sx2PgKFZFn1FxXvdJV8H8It5aTRdsSVWZBUmg84HQNpumO9w1xmimMblfYbH69Tmksf1A0knjHlUXAb9NlboMtTLvb7tTHHmke64FDGUIq6v4MGqYjQ96Wk6ANQ~W5oQ5iHKcqrvfwhVatyVfHmwpbyt~IiB5S1-ZxS~BCwsbukH5C8oBNLDHooy-IbkDUIjI~mCZm8ovVYi3ZFpZLYHfwTg0zq5clag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal