Cytomegalovirus (CMV) remains an important cause of morbidity and mortality after allogeneic stem cell transplantation (SCT), but cytotoxic T lymphocytes (CTL) may play a critical role in controlling CMV reactivation. Fluorescent HLA-peptide tetramers containing immunodominant peptides from CMV were used to prospectively monitor the recovery of CMV CTL in recipients of allogeneic transplants from siblings (n = 13) or unrelated donors (n = 11). In patients given allografts from a sibling when both the patient and donor were seropositive for CMV before SCT, recovery of CMV-specific CTL was rapid and reached up to 21% of all CD8+ T cells. Early reconstitution of CMV-specific immunity was not observed if either the donor or recipient was seronegative for CMV. In recipients of transplants from volunteer unrelated donors, recovery of CMV-specific CTL was delayed in comparison to that in recipients of transplants from siblings and no CTL were observed within the first 100 days after SCT. CTL numbers were increased after episodes of CMV reactivation but were suppressed by prednisolone therapy. Recovery of CMV-specific CTL to levels greater than 10 × 106/L was associated with protection from CMV disease. It was concluded that use of HLA-peptide tetramers to quantify CMV CTL is valuable for studying T-cell responses after allogeneic SCT. It should allow prediction of CMV reactivation in individual patients and assist in the development of adoptive T-cell immunotherapy.
Introduction
Cytomegalovirus (CMV) disease continues to cause morbidity and mortality after allogeneic stem cell transplantation (SCT), despite the introduction of routine screening and antiviral agents.1 Major risk factors for CMV disease include recipient seropositivity, acute graft-versus-host disease (GVHD), T-cell depletion of the graft, and the use of unrelated or mismatched donors.2,3 Prophylactic administration of ganciclovir to CMV-seropositive recipients of allogeneic marrow transplants reduces the incidence and severity of CMV infection but is associated with an increased risk of neutropenia and bacterial and fungal infections.4,5 Indeed, the superiority of prophylactic compared with pre-emptive ganciclovir after transplantation has been difficult to prove.6,7 Although prophylactic ganciclovir offers a significant reduction in human CMV disease during the first 100 days after transplantation, there is an increasing recognition that late presentation of CMV disease can occur, and this has a high mortality rate.8 Prospective monitoring with CMV polymerase chain reaction (PCR) evaluation or CMV antigenemia assessment to direct pre-emptive antiviral therapy can reduce the incidence of CMV disease and CMV-associated mortality, but the high sensitivity of these assays leads to treatment of many patients who would never have progression to disease.9Novel criteria to help direct antiviral treatment after allogeneic SCT would be of great value.
Evidence from studies in animals10 indicates that virus-specific CD8+ cytotoxic T lymphocytes (CTL) have a predominant role in protection against CMV disease. This has led to the development of protocols for reconstituting CMV-specific CTL immunity in transplant recipients by means of adoptive transfer of cloned CTL against the CMV structural protein pp65.11,12 Standard methods for detecting antigen-specific T-cell responses were based on the detection of a functional response in the presence of antigen. The introduction of HLA-peptide tetramers has allowed direct visualization of T cells specific for any given peptide, based on the generation of fluorescent HLA-peptide complexes.13 This technique is both highly specific and sensitive and has been applied to many areas of both human and murine immunology.14-16 In this study, we generated tetramers containing immunodominant peptides from the CMV matrix peptide pp65 and used them to assess the recovery of CMV CTL in patients after allogeneic sibling or unrelated SCT.
Patients, materials, and methods
Patient population
Patients who received an allogeneic SCT and were HLA-A*0201 or HLA-B*0702 positive were eligible for study entry. Patients seronegative for CMV who had CMV-seronegative donors were excluded from the study. Twelve patients received transplants from HLA-identical siblings, 1 patient was given a transplant from a locus-HLA–mismatched related donor, and 11 received transplants from an HLA-matched volunteer unrelated donor (MUD). Selection of unrelated donors was based on the serologic identity of class I antigens (including splits by isoelectric focusing) and DNA typing for DRB1 alleles. In addition, for MUD SCT, the frequency of alloreactive CTL precursors in the donor's peripheral blood was assayed.17 Detailed information on the patients is shown in Table1. All patients achieved sustained engraftment and survived for more than 120 days after transplantation. Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Characteristics of patients and transplant protocols
| Variable . | HLA-identical sibling transplant recipients . | MUD transplant recipients . |
|---|---|---|
| Patient/donor CMV serologic status | ||
| +/+ | 9 | 3 |
| +/− | 2 | 3 |
| −/+ | 2 | 5 |
| Patient/donor sex | ||
| M/M | 1 | 7 |
| M/F | 3 | 3 |
| F/M | 4 | 1 |
| F/F | 5 | 0 |
| HLA tetramer selected for analysis | ||
| HLA-*A0201 | 10* | 5 |
| HLA-*B0702 | 2 | 1 |
| HLA-*A0201 + HLA-*B0702 | 1 | 5 |
| Median (range) age of patient, y | 42 (26-57) | 31 (14-51) |
| Underlying disease | ||
| CML-CP | 8 | 9 |
| CML-AP/2nd CP | 1 | 1 |
| MPD | 1 | 0 |
| AML | 3 | 1 |
| Source of stem cells | ||
| Bone marrow | 9* | 11 |
| PBSC | 4 | 0 |
| Total-body irradiation dose (cGy) | ||
| 1320 | 11 | 0 |
| 1440 | 2* | 11 |
| Time of T-cell depletion with Campath 1H† | ||
| Day − 10 to day − 6 | 0 | 6 |
| Day − 5 to day + 4 | 0 | 4 |
| Day − 5 to day − 1 | 1* | 1 |
| Ganciclovir use after SCT | ||
| Prophylactic | 0 | 6 |
| Pre-emptive | 3 | 5 |
| Acute GVHD | ||
| Grade 0-II | 11* | 10 |
| Grade III-IV | 2 | 1 |
| Chronic GVHD | ||
| None to limited | 12* | 8 |
| Severe | 1 | 3 |
| Median (range) day to engraftment | ||
| Neutrophils > 0.5 × 109/L | 25 (14-33) | 26 (13-35) |
| Platelets > 50 × 109/L | 26 (12-91) | 27 (19-> 90) |
| Median (range) dose of BM cells infused (× 108 MNC/kg) | 3.39 (1.75-4.65) | 3.63 (1.93-5.17) |
| Median (range) dose of PBSC cells infused (× 106CD34/kg) | 7.42 (4.4-10.98) | N/A |
| Variable . | HLA-identical sibling transplant recipients . | MUD transplant recipients . |
|---|---|---|
| Patient/donor CMV serologic status | ||
| +/+ | 9 | 3 |
| +/− | 2 | 3 |
| −/+ | 2 | 5 |
| Patient/donor sex | ||
| M/M | 1 | 7 |
| M/F | 3 | 3 |
| F/M | 4 | 1 |
| F/F | 5 | 0 |
| HLA tetramer selected for analysis | ||
| HLA-*A0201 | 10* | 5 |
| HLA-*B0702 | 2 | 1 |
| HLA-*A0201 + HLA-*B0702 | 1 | 5 |
| Median (range) age of patient, y | 42 (26-57) | 31 (14-51) |
| Underlying disease | ||
| CML-CP | 8 | 9 |
| CML-AP/2nd CP | 1 | 1 |
| MPD | 1 | 0 |
| AML | 3 | 1 |
| Source of stem cells | ||
| Bone marrow | 9* | 11 |
| PBSC | 4 | 0 |
| Total-body irradiation dose (cGy) | ||
| 1320 | 11 | 0 |
| 1440 | 2* | 11 |
| Time of T-cell depletion with Campath 1H† | ||
| Day − 10 to day − 6 | 0 | 6 |
| Day − 5 to day + 4 | 0 | 4 |
| Day − 5 to day − 1 | 1* | 1 |
| Ganciclovir use after SCT | ||
| Prophylactic | 0 | 6 |
| Pre-emptive | 3 | 5 |
| Acute GVHD | ||
| Grade 0-II | 11* | 10 |
| Grade III-IV | 2 | 1 |
| Chronic GVHD | ||
| None to limited | 12* | 8 |
| Severe | 1 | 3 |
| Median (range) day to engraftment | ||
| Neutrophils > 0.5 × 109/L | 25 (14-33) | 26 (13-35) |
| Platelets > 50 × 109/L | 26 (12-91) | 27 (19-> 90) |
| Median (range) dose of BM cells infused (× 108 MNC/kg) | 3.39 (1.75-4.65) | 3.63 (1.93-5.17) |
| Median (range) dose of PBSC cells infused (× 106CD34/kg) | 7.42 (4.4-10.98) | N/A |
MUD indicates volunteer unrelated donor; CMV, cytomegalovirus; CML, chronic myeloid leukemia; CP, chronic phase; AP, accelerated phase; MPD, myeloproliferative disease; AML, acute myelogenous leukemia; PBSC, peripheral blood stem cells; SCT, stem cell transplantation; GVHD, graft-versus-host disease; BM, bone marrow; MNC, mononuclear cells; and N/A, not applicable.
There was one related donor who was locus-HLA mismatched.
Minus signs indicate days before transplantation; and plus signs, days after transplantation.
Transplantation regimens
All patients received cyclophosphamide and total-body irradiation as pretransplantation conditioning (Table 1). GVHD prophylaxis consisted of conventional cyclosporine–methotrexate therapy18 in all patients. In addition, recipients of a MUD transplant received intravenous anti-CD52 monoclonal antibody (Campath 1H) for 4 days before SCT. Acute GVHD was diagnosed by using clinical criteria described previously.19 Acyclovir prophylaxis (800 mg/day orally) was given to all patients, beginning on day 7 before SCT and continuing through day 35 afterward. Fluconazole (100 mg/day) and ciprofloxacin (1 g/day) were administered from day 7 before SCT until the time of engraftment. Trimethoprim-sulfamethoxazole was started when neutrophil engraftment (absolute neutrophil count, > 0.5 × 109/L on 3 consecutive days) was achieved.
Monitoring for CMV infection
Serum samples from allograft recipients and donors were analyzed before SCT for CMV-specific IgG antibodies by using enzyme-linked immunosorbent assays. Patients who were seronegative for CMV received only CMV-seronegative, leukocyte-depleted blood products. All patients were tested weekly for CMV antigenemia or by CMV PCR analysis until day 90 (HLA-identical sibling transplant recipients) or day 180 after SCT (MUD transplant recipients). CMV antigenemia assays and qualitative plasma CMV PCR assays were done according to methods described previously.20 On detection of CMV reactivation (antigenemia assessment results, > 1 CMV antigen cell/50 000 peripheral blood mononuclear cells [PBMC] or positive results on 2 consecutive PCRs done 3 days apart), ganciclovir therapy was begun (5 mg/kg twice daily given intravenously for 14 consecutive days, followed by 5 mg/kg given once daily 5 days a week until day 90 after SCT). All seropositive recipients of a MUD transplant received prophylactic ganciclovir (5 mg/kg daily given intravenously 7 days a week) from the time of neutrophil engraftment to day 90 after SCT.
CMV disease
CMV disease was defined as recovery of the virus from a visceral site (lung or gastrointestinal tract) or from bronchoalveolar lavage in patients who had signs and symptoms consistent with CMV infection.
Blood samples for tetramer analysis
Ten milliliters of heparin-treated blood was collected from bone marrow recipients before and at time points up to 540 days after SCT. If possible, blood was also obtained from the donor before SCT. PBMC were isolated by using Ficoll-Isopaque density gradient centrifugation. If possible, samples were collected from patients every 2 to 4 weeks after allogeneic SCT. Initial samples were obtained when evidence of marrow engraftment (defined as a neutrophil count of > 0.5 × 109/L) was obtained and when the lymphocyte count exceeded 0.2 × 109.
Tetramer production
Soluble major histocompatibility complex (MHC)–peptide tetramers were produced by using standard approaches.13Briefly, recombinant HLA-A*0201 and HLA-B*0702 heavy chain and β2-microglobulin protein were produced inEscherichia coli cells transformed with the relevant expression vectors. Expression of the HLA heavy chain was limited to the extracellular domain, and the C-terminus of this domain was modified by the addition of a substrate sequence for the biotinylating enzyme BirA. The peptide epitopes were the HLA-A*0201–restricted NLVPMVATV epitope (amino acids 495-503 of the lower matrix protein, pp65) and the HLA-B*0702 epitope, TPRVTGGGAM (amino acids 417-426 of the lower matrix protein pp65). Monomeric HLA-peptide complexes were folded in vitro by adding HLA protein to β2-microglobulin in the presence of the appropriate peptide. The MHC complexes were biotinylated by using purified recombinant BirA enzyme and were then purified by gel filtration and anion exchange chromatography. HLA-peptide tetramers were made by mixing the biotinylated protein complex with streptavidin-phycoerythrin (PE) (Leinco, St Louis, MO) at a molar ratio of 4:1. Tetramers were purified by gel filtration on a Sephadex S-200 column (Amersham Pharmacia, St Albans, United Kingdom).
Detection of CMV-specific CTL using HLA class I–pp65 tetramer complexes
PBMC (2 × 106) were surface stained for 1 hour at 4°C with CD45RO–fluorescein isothiocyanate, conjugated (FITC); PE-labeled tetrameric complex; and tricolor-conjugated antibody against CD8 in phosphate-buffered saline containing 1% bovine serum albumin. Cells were washed and fixed with 1% paraformaldehyde before analysis with a fluorescence-activated cell-sorter flow cytometer. The limit of detection by tetramers was 0.02% of CD8+ T cells. The peripheral blood lymphocyte count was used to determine the absolute number of CMV-specific CTL.
CD45RO or CD45RA expression was determined by 3-color fluorescent analysis using FITC-conjugated anti-CD45R isoform antibodies (Serotec, United Kingdom). Expression of CD45R isoforms on tetramer-staining cells was calculated after gating on CD8+ cells.
Statistical methods
The following factors were analyzed in a univariate analysis for their effect on CMV-specific CTL reconstitution: donor (sibling versus MUD), T-cell depletion, serologic status of donor and recipient, recipient/donor serologic status combination, graft type (bone marrow versus peripheral blood), ganciclovir prophylaxis, episode of CMV reactivation, and high-dose steroid use. Variables found to be significant (P value < .20) in univariate analysis were then included in a multivariate logistic regression analysis. Results from all 39 patients were analyzed. In a second analysis, seropositive patients who had seropositive donors were excluded.
Results
Specificity of staining with HLA-peptide tetramers
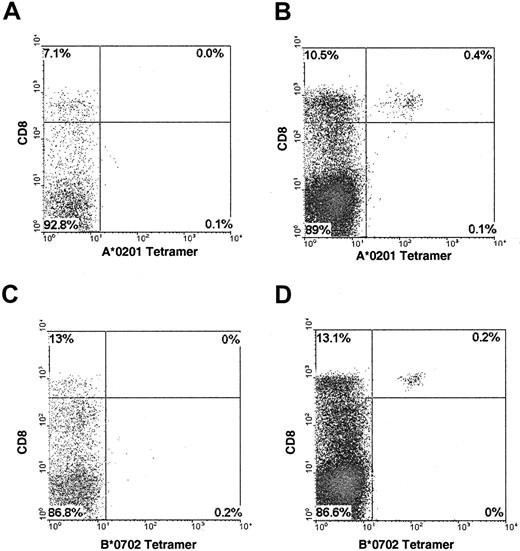
Both the HLA-A*0201:495-503 and HLA-B*0702:417-426 tetramers were specific and sensitive in their staining of CMV-specific CTL (Figure1). The pp65-specific CTL lines showed a high fluorescence level after staining with the appropriate tetramer, whereas control CTL were not stained (data not shown). PBMC from CMV-seronegative marrow donors and patients (n = 6) given a transplant from a CMV-seronegative donor did not show staining with the tetramers.
Staining of CMV-specific CTL with HLA-peptide tetramers.
PBMC from a CMV-seropositive HLA-A*0201 negative (A) or positive (B) donor were stained with the HLA-A*0201:495-503 tetramer; 0.4% of PBMC from donor B stained with the tetramer, equivalent to 3.7% of the CD8+ population. PBMC from a CMV-seropositive HLA-B*0702 negative (C) or positive (D) donor were stained with the HLA-B*0702:417-426 tetramer; 0.2% of PBMC from donor D stained with the tetramer, equivalent to 1.5% of the CD8+population.
Staining of CMV-specific CTL with HLA-peptide tetramers.
PBMC from a CMV-seropositive HLA-A*0201 negative (A) or positive (B) donor were stained with the HLA-A*0201:495-503 tetramer; 0.4% of PBMC from donor B stained with the tetramer, equivalent to 3.7% of the CD8+ population. PBMC from a CMV-seropositive HLA-B*0702 negative (C) or positive (D) donor were stained with the HLA-B*0702:417-426 tetramer; 0.2% of PBMC from donor D stained with the tetramer, equivalent to 1.5% of the CD8+population.
Prospective monitoring of CMV-specific CTL reconstitution in recipients of sibling allografts
To determine the kinetics of recovery of CMV-specific CTL, PBMC were isolated, when possible, every 2 or 4 weeks after SCT. The time the initial sample was obtained was determined by the rate of lymphocyte reconstitution. The median number of tests per patient was 7 (range, 3-16). Patients were placed into 1 of 3 groups according to the serologic status of the recipient and donor. In group 1 (n = 9), both the patients and donors were seropositive for CMV. In group 2 (n = 2), the patients were CMV seropositive but the donors were seronegative. In group 3 (n = 2), only the donors were CMV seropositive (Table 2). Patients were studied for up to 18 months after SCT.
Patients in whom CMV-specific cytotoxic T lymphocytes (CTL) were studied prospectively after allografting
| Group/patient no. . | Donor . | HLA allele . | CMV-specific CTL . | CMV reactivation (days after SCT) . | GVHD grade . | Follow-up (days after SCT) . | ||
|---|---|---|---|---|---|---|---|---|
| Day first detected . | As % of CD8+ count at peak . | Peak absolute cell count (× 105 cells) . | ||||||
| Group 1 (recipient +/donor + for CMV) | ||||||||
| 1 | S | A2 | 32 | 12 | 250 | 82 | III | 214 |
| 2 | S | A2 | 31 | 1.5 | 53 | ND | 0 | 143 |
| 3 | S | A2 | 31 | 0.7 | 2.1 | ND | I | 120 |
| 4 | S | A2 | 48 | 3.3 | 369 | ND | II | 531 |
| 5 | S | B7 | 43 | 10.1 | 409 | ND | II | 351 |
| 6 | S | A2 | 21 | 21.0 | 162 | ND | III | 90 (90†) |
| 7 | S | B7 | 55* | 3.2 | 195 | 47 & 139 | III | 338 |
| 8 | S | A2 | 77* | 0.18 | ND | ND | 0 | 122 |
| 9 | S | A2 | 81* | 4.6 | 310 | 52 | II | 516 |
| 10 | V | B7 | 116 | 1.2-5 | 64 | 56 & 126 | II | 521 |
| 11 | V | A2 | 112 | 5.9 | 20 | 20 & 150 | III | 337 |
| 12 | V | A2; B7 | 100 | 0.1; 2.9 | 8.6; 112 | 27 | 0 | 210 (286†) |
| Group 2 (recipient +/donor − for CMV) | ||||||||
| 13 | S | A2 | ND | ND | ND | ND | 0 | 363 |
| 14 | S | A2 | ND | ND | ND | ND | 0 | 193 |
| 15 | V | A2 | ND | ND | ND | ND | II | 353 |
| 16 | V | A2 | ND | ND | ND | ND | 0 | 161 |
| 17 | V | A2 | 149 | 0.6 | ND | 33 & 114 | III | 208 |
| Group 3 (recipient −/donor + for CMV) | ||||||||
| 18 | S | B7 | ND | ND | ND | ND | 0 | 120 |
| 19 | 1 antigen m/m | A2 | ND | ND | ND | ND | 0 | 296 |
| 20 | V | B7 | 160 | 0.8 | 4 | ND | II | 173 (201†) |
| 21 | V | A2; B7 | ND | ND | ND | ND | 0 | 177 (232†) |
| 22 | V | A2; B7 | ND | ND | ND | ND | 0 | 451 |
| 23 | V | A2 | ND | ND | ND | ND | 0 | 392 |
| 24 | V | A2 | ND | ND | ND | ND | 0 | 180 |
| Group/patient no. . | Donor . | HLA allele . | CMV-specific CTL . | CMV reactivation (days after SCT) . | GVHD grade . | Follow-up (days after SCT) . | ||
|---|---|---|---|---|---|---|---|---|
| Day first detected . | As % of CD8+ count at peak . | Peak absolute cell count (× 105 cells) . | ||||||
| Group 1 (recipient +/donor + for CMV) | ||||||||
| 1 | S | A2 | 32 | 12 | 250 | 82 | III | 214 |
| 2 | S | A2 | 31 | 1.5 | 53 | ND | 0 | 143 |
| 3 | S | A2 | 31 | 0.7 | 2.1 | ND | I | 120 |
| 4 | S | A2 | 48 | 3.3 | 369 | ND | II | 531 |
| 5 | S | B7 | 43 | 10.1 | 409 | ND | II | 351 |
| 6 | S | A2 | 21 | 21.0 | 162 | ND | III | 90 (90†) |
| 7 | S | B7 | 55* | 3.2 | 195 | 47 & 139 | III | 338 |
| 8 | S | A2 | 77* | 0.18 | ND | ND | 0 | 122 |
| 9 | S | A2 | 81* | 4.6 | 310 | 52 | II | 516 |
| 10 | V | B7 | 116 | 1.2-5 | 64 | 56 & 126 | II | 521 |
| 11 | V | A2 | 112 | 5.9 | 20 | 20 & 150 | III | 337 |
| 12 | V | A2; B7 | 100 | 0.1; 2.9 | 8.6; 112 | 27 | 0 | 210 (286†) |
| Group 2 (recipient +/donor − for CMV) | ||||||||
| 13 | S | A2 | ND | ND | ND | ND | 0 | 363 |
| 14 | S | A2 | ND | ND | ND | ND | 0 | 193 |
| 15 | V | A2 | ND | ND | ND | ND | II | 353 |
| 16 | V | A2 | ND | ND | ND | ND | 0 | 161 |
| 17 | V | A2 | 149 | 0.6 | ND | 33 & 114 | III | 208 |
| Group 3 (recipient −/donor + for CMV) | ||||||||
| 18 | S | B7 | ND | ND | ND | ND | 0 | 120 |
| 19 | 1 antigen m/m | A2 | ND | ND | ND | ND | 0 | 296 |
| 20 | V | B7 | 160 | 0.8 | 4 | ND | II | 173 (201†) |
| 21 | V | A2; B7 | ND | ND | ND | ND | 0 | 177 (232†) |
| 22 | V | A2; B7 | ND | ND | ND | ND | 0 | 451 |
| 23 | V | A2 | ND | ND | ND | ND | 0 | 392 |
| 24 | V | A2 | ND | ND | ND | ND | 0 | 180 |
For patients who were positive for both HLA A*0201 and HLA B*0702, the results for the HLA A*0201 tetramer are shown first, followed by the results for the B*0702 tetramer.
S indicates sibling; V, volunteer unrelated donor; ND, not detected; and 1 HLA antigen mismatch.
First sample studied.
Day of death.
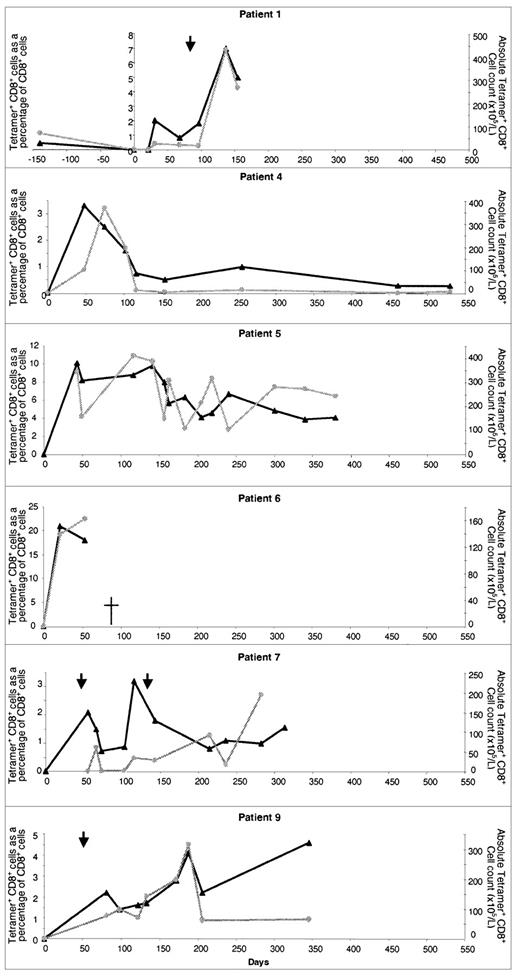
CMV-specific CTL were detected in all patients in group 1, either within the first 50 days (n = 7) or at the first time point in the study (Figure 2). Analysis was delayed in patients 7, 8, and 9, from whom samples were first collected on day 55, day 77, and day 81, respectively, after SCT. In 2 patients, CTL reconstitution was extremely marked. In patient 5, more than 10% of all CD8+ lymphocytes stained with the tetramer on day 43, representing an absolute CMV-specific CTL count of 409 × 105/L. In patient 6, 21% of CD8+ T cells stained with the tetramer on day 21 (absolute count, 139 × 105/L). CMV-specific CTL continued to be detected for the whole study period and CMV did not develop in any patient in group 1. CMV reactivation was detected in patients 1, 7, and 9 and was treated with ganciclovir. In patient 1, this period of reactivation was followed by the development of very high levels of CMV-specific CTL.
Reconstitution of CMV-specific CTL in CMV-seropositive patients given transplants from CMV-seropositive sibling donors.
Results in 6 individual patients are shown. The number of days after transplantation is shown on the x-axis. The percentage of CD8+ T cells binding HLA-peptide tetramer (▴) and the absolute CMV-specific CTL count () are shown on the y-axes. Times of CMV reactivation are shown by arrows.
Reconstitution of CMV-specific CTL in CMV-seropositive patients given transplants from CMV-seropositive sibling donors.
Results in 6 individual patients are shown. The number of days after transplantation is shown on the x-axis. The percentage of CD8+ T cells binding HLA-peptide tetramer (▴) and the absolute CMV-specific CTL count () are shown on the y-axes. Times of CMV reactivation are shown by arrows.
CMV-specific CTL were analyzed in the donors for patients 5, 6, 7, and 9 and were found to be 0.01%, 3%, 1.9%, and 0.15%, respectively, of CD8+ T cells. Because only 4 donors were studied, it was not possible to make any correlation between the percentage of CMV-specific CTL in the donor and subsequent reconstitution after SCT. CMV-specific CTL were not detectable in prospective monitoring of patients in group 2 or 3, and no CMV reactivations or primary infections were observed in this group.
Prospective monitoring of CMV-specific CTL reconstitution in recipients of MUD allografts
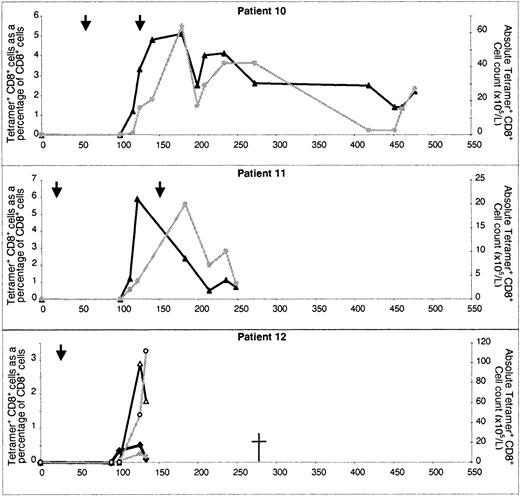
In all recipients of MUD transplants (n = 11), the recovery of CMV CTL was profoundly delayed compared with that in recipients of sibling allografts. Moreover, no tetramer-binding cells were detectable before day 100 after SCT (Table 2). Three recipients (patients 10, 11, and 12) were in group 1 and all had evidence of CMV reactivation or CMV disease before day 60, despite antiviral prophylaxis. CMV-specific CTL recovery was evident beginning on day 100 (Figure3). CMV reactivation was observed in patient 10 on days 56 and 126 after allografting and required foscarnet and ganciclovir therapy. At 126 days, this patient's CMV-specific CTL count was 16 × 105/L. A brief course of ganciclovir therapy was given, and since day 141, the patient has had no evidence of CMV infection and CTL numbers have exceeded 20 × 105/L. In patient 11, interstitial pneumonitis developed on day 20 after SCT and was treated successfully with ganciclovir and anti-CMV immunoglobulin. Ganciclovir was stopped on day 110 and CTL became detectable on day 122 (5.9% of CD8+ T cells; absolute count, 3.8 × 105/L). There was further evidence of CMV reactivation 150 days after allografting and ganciclovir therapy was begun again. The patient continued to take steroids for treatment of GVHD (grade III) until death due to bacterial sepsis occurred on day 345. On no occasion did CTL values in this patient exceed 20 × 105/L.
Reconstitution of CMV-specific CTL in CMV-seropositive patients given transplants from CMV-seropositive MUDs.
Results in individual patients are shown. The number of days after transplantation is shown on the x-axis. The percentage of CD8+ T cells binding HLA-peptide tetramer (▴) and the absolute CMV-specific CTL count () are shown on the y-axes. In the graph for patient 12, the results for both the A*0201 tetramer (solid points) and the B*0702 tetramer (open points) are shown. Times of CMV reactivation are shown by arrows.
Reconstitution of CMV-specific CTL in CMV-seropositive patients given transplants from CMV-seropositive MUDs.
Results in individual patients are shown. The number of days after transplantation is shown on the x-axis. The percentage of CD8+ T cells binding HLA-peptide tetramer (▴) and the absolute CMV-specific CTL count () are shown on the y-axes. In the graph for patient 12, the results for both the A*0201 tetramer (solid points) and the B*0702 tetramer (open points) are shown. Times of CMV reactivation are shown by arrows.
Of the 8 patients with a MUD in group 2 or 3, CMV-specific CTL were detected in only 2 and the levels were low. CTL became detectable in patient 20 after the infusion of “top-up” T-cell–depleted marrow (2 × 103 CD3+ cells/kg) on day 112. CMV-specific CTL were first detected 48 days after the infusion of top-up stem cells and rose to a peak of only 4 × 105/L. This patient was monitored by means of CMV-antigenemia assessments after SCT, but because the patient was neutropenic, the possibility that CMV reactivation occurred cannot be excluded. Patient 17 had 2 episodes of CMV reactivation, 33 and 114 days after allografting, and CMV-specific CTL became detectable on discontinuation of ganciclovir therapy 149 days after SCT. The patient continued to take steroids to treat intestinal GVHD (grade III) throughout this period (beginning 20 days after SCT).
Levels of CMV-specific CTL in long-term survivors after allografting
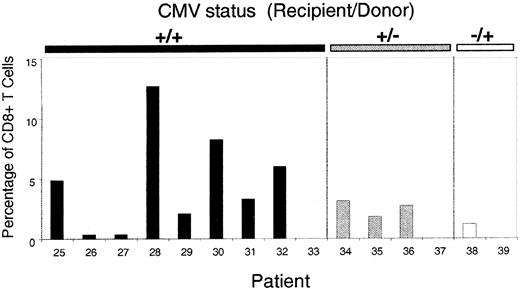
After the prospective study of CMV-specific CTL reconstitution, 15 additional patients were studied at one time point at least 6 months after allografting (Table 3). Nine of these patients were CMV seropositive before SCT and had received a transplant from a CMV-seropositive donor. Eight of the patients had CMV-specific CTL populations in peripheral blood. The highest level was in patient 28, in whom more than 12% of CD8+ T cells bound tetramer (Figure 4). Three of 4 patients in group 2 had detectable CMV-specific CTL, and all these patients had an episode of CMV reactivation after SCT. Similarly, the one patient in group 3 in whom CMV-specific CTL were detectable had an episode of primary CMV infection before analysis. No CTL were detected in 3 patients, 2 of whom had no history of CMV infection or reactivation. Patient 33 did not have detectable CTL, despite a documented episode of CMV reactivation on day 52, and it is possible that the CMV-specific CTL responsible for controlling viral reactivation were directed at peptide epitopes not covered by the tetramers used in this study. These observations suggest that in vivo viral antigen stimulation is required for the development of CMV-specific reconstitution in recipients whose donors have not had priming to the CMV antigen. Indeed, in univariate analysis, CMV reactivation was a significant predictor of CMV CTL reconstitution (P = .00259).
Patients in whom CMV-specific CTL were measured at one time point at least 6 months after allografting
| Group/patient no. . | Donor . | HLA allele . | CMV-specific CTL . | CMV reactivation (days after SCT) . | GVHD grade . | Follow-up (days after SCT) . | |
|---|---|---|---|---|---|---|---|
| As % of CD8+ count at peak . | Peak absolute cell count (× 105) . | ||||||
| Group 1 (recipient +/donor + for CMV) | |||||||
| 25 | V | A2 | 4.9 | 47 | 82 | III | 550 |
| 26 | S | B7 | 0.4 | 7 | ND | I | 1030 |
| 27 | S | A2; B7 | 0; 0.4 | 0; 28 | ND | I | 247 |
| 28 | S | B7 | 12.7 | 1525 | ND | II | 1029 |
| 29 | S | A2; B7 | 0.1; 1.1 | 1.6; 22 | ND | II | 1395 |
| 30 | S | A2; B7 | 1.3; 8 | 72; 442 | 42 | III | 688 |
| 31 | S | A2 | 2.5 | 117 | 47 & 139 | III | 338 |
| 32 | S | A2 | 6 | 300 | 31 | 0 | 280 |
| 33 | S | A2; B7 | ND | ND | 52 | II | 516 |
| Group 2 (recipient +/donor − for CMV) | |||||||
| 34 | V | A2; B7 | 0.1; 3.1 | 44; 1463 | 85 & 122 | II | 990 |
| 35 | S | A2 | 1.8 | 89 | 87 | 0 | 1260 |
| 36 | V | A2 | 2.7 | 25 | 55 | II | 247 |
| 37 | S | A2 | ND | ND | ND | 0 | 732 |
| Group 3 (recipient −/donor + for CMV) | |||||||
| 38 | S | A2; B7 | 1.2; 1.5 | 82; 102 | 56 & 120 | II | 337 |
| 39 | S | A2 | ND | ND | ND | 0 | 392 |
| Group/patient no. . | Donor . | HLA allele . | CMV-specific CTL . | CMV reactivation (days after SCT) . | GVHD grade . | Follow-up (days after SCT) . | |
|---|---|---|---|---|---|---|---|
| As % of CD8+ count at peak . | Peak absolute cell count (× 105) . | ||||||
| Group 1 (recipient +/donor + for CMV) | |||||||
| 25 | V | A2 | 4.9 | 47 | 82 | III | 550 |
| 26 | S | B7 | 0.4 | 7 | ND | I | 1030 |
| 27 | S | A2; B7 | 0; 0.4 | 0; 28 | ND | I | 247 |
| 28 | S | B7 | 12.7 | 1525 | ND | II | 1029 |
| 29 | S | A2; B7 | 0.1; 1.1 | 1.6; 22 | ND | II | 1395 |
| 30 | S | A2; B7 | 1.3; 8 | 72; 442 | 42 | III | 688 |
| 31 | S | A2 | 2.5 | 117 | 47 & 139 | III | 338 |
| 32 | S | A2 | 6 | 300 | 31 | 0 | 280 |
| 33 | S | A2; B7 | ND | ND | 52 | II | 516 |
| Group 2 (recipient +/donor − for CMV) | |||||||
| 34 | V | A2; B7 | 0.1; 3.1 | 44; 1463 | 85 & 122 | II | 990 |
| 35 | S | A2 | 1.8 | 89 | 87 | 0 | 1260 |
| 36 | V | A2 | 2.7 | 25 | 55 | II | 247 |
| 37 | S | A2 | ND | ND | ND | 0 | 732 |
| Group 3 (recipient −/donor + for CMV) | |||||||
| 38 | S | A2; B7 | 1.2; 1.5 | 82; 102 | 56 & 120 | II | 337 |
| 39 | S | A2 | ND | ND | ND | 0 | 392 |
For patients who were positive for both HLA A*0201 and HLA B*0702, the results for the HLA A*0201 tetramer are shown first, followed by the results for the B*0702 tetramer.
CMV-specific CTL at single time points in 15 patients more than 6 months after allografting.
Patients 25 to 33 were in group 1 (both donor and recipient positive for CMV), patients 34 to 37 were in group 2 (recipient positive and donor negative for CMV), and patients 38 and 39 were in group 3 (recipient negative and donor positive for CMV). The y-axis represents tetramer-binding cells as a fraction of CD8+ T cells.
CMV-specific CTL at single time points in 15 patients more than 6 months after allografting.
Patients 25 to 33 were in group 1 (both donor and recipient positive for CMV), patients 34 to 37 were in group 2 (recipient positive and donor negative for CMV), and patients 38 and 39 were in group 3 (recipient negative and donor positive for CMV). The y-axis represents tetramer-binding cells as a fraction of CD8+ T cells.
Influence of drug therapy on CMV-specific CTL reconstitution
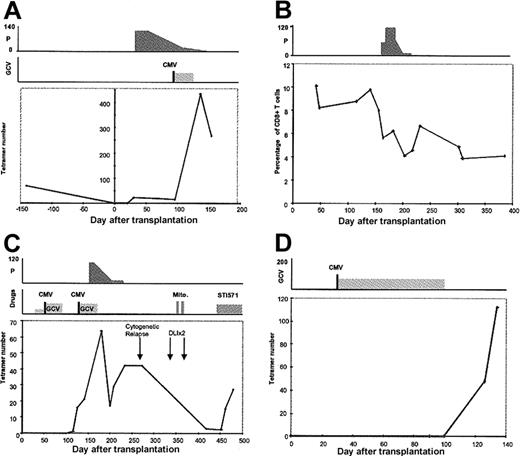
The factors that affect reconstitution of CMV CTL in patients given allografts are likely to be multifactorial. Patients given transplants from MUDs received in vivo T-cell depletion with Campath antibody as part of prophylaxis strategy against GVHD. CMV-seropositive recipients of a MUD transplant received ganciclovir prophylaxis as well as T-cell depletion. In 3 patients (1, 10, and 12), high levels of CTL developed after discontinuation of ganciclovir (Figure 5). However, neither T-cell depletion nor ganciclovir prophylaxis were found to be significantly associated with CMV-specific CTL reconstitution (P = .17 and P = .85, respectively).
Examples of CMV-specific immune reconstitution in individual patients.
(A) In patient 1, CMV-specific CTL were detected on day 33 but were at low levels during prolonged treatment with prednisolone. After an episode of CMV reactivation on day 82 and a short course of ganciclovir therapy, the number of CMV-specific CTL increased to a high level. A CTL assay done 4 months before transplantation had shown that CTL numbers were considerably lower than those observed after recovery from reactivation. (B) Patient 5 had early and sustained immune reconstitution, but the percentage of tetramer-binding CTL fell from 9.8% of CD8+ T cells to 5.7% after the introduction of prednisolone for control of GVHD. A similar reduction in CTL numbers was also observed (data not shown). (C) Patient 10 received stem cells from an unrelated donor, and no CTL were detectable until day 116. After a second episode of CMV reactivation on day 126, CTL increased to levels of 70 × 105/L. A course of prednisolone therapy suppressed CTL numbers and, following recovery, CTL were virtually eliminated after unsuccessful donor leukocyte infusions and chemotherapy for cytogenetic relapse of chronic myeloid leukemia. Introduction of the tyrosine kinase inhibitor STI571 was associated with recovery of CTL numbers. (D) Patient 12 also received stem cells from an unrelated donor. After an early period of CMV reactivation, the patient was given a prolonged course of ganciclovir therapy. CMV-specific CTL were detected only after discontinuation of the drug. P indicates prednisolone; GCV, ganciclovir; vertical lines with CMV Ag+, episode of CMV reactivation; mito, mitoxantrone; and STI571, tyrosine kinase inhibitor. The x-axis represents days after transplantation. All drug doses are in milligrams. Cell numbers on the y-axis are × 105/L cells.
Examples of CMV-specific immune reconstitution in individual patients.
(A) In patient 1, CMV-specific CTL were detected on day 33 but were at low levels during prolonged treatment with prednisolone. After an episode of CMV reactivation on day 82 and a short course of ganciclovir therapy, the number of CMV-specific CTL increased to a high level. A CTL assay done 4 months before transplantation had shown that CTL numbers were considerably lower than those observed after recovery from reactivation. (B) Patient 5 had early and sustained immune reconstitution, but the percentage of tetramer-binding CTL fell from 9.8% of CD8+ T cells to 5.7% after the introduction of prednisolone for control of GVHD. A similar reduction in CTL numbers was also observed (data not shown). (C) Patient 10 received stem cells from an unrelated donor, and no CTL were detectable until day 116. After a second episode of CMV reactivation on day 126, CTL increased to levels of 70 × 105/L. A course of prednisolone therapy suppressed CTL numbers and, following recovery, CTL were virtually eliminated after unsuccessful donor leukocyte infusions and chemotherapy for cytogenetic relapse of chronic myeloid leukemia. Introduction of the tyrosine kinase inhibitor STI571 was associated with recovery of CTL numbers. (D) Patient 12 also received stem cells from an unrelated donor. After an early period of CMV reactivation, the patient was given a prolonged course of ganciclovir therapy. CMV-specific CTL were detected only after discontinuation of the drug. P indicates prednisolone; GCV, ganciclovir; vertical lines with CMV Ag+, episode of CMV reactivation; mito, mitoxantrone; and STI571, tyrosine kinase inhibitor. The x-axis represents days after transplantation. All drug doses are in milligrams. Cell numbers on the y-axis are × 105/L cells.
Prednisolone therapy is a recognized risk factor for CMV reactivation. In univariate analysis, we found that high doses of prednisolone were associated with a significant decrease in both the percentage and absolute number of CMV-specific CTL (P = .015; Figure5).
Multivariate analysis results
Although our univariate analysis found that several factors were significantly associated with CMV-specific CTL, including CMV reactivation (P = .00259), use of high-dose steroids (P = .015), recipient CMV status (P = .0052), donor CMV status (P = .048), and recipient/donor serologic status combination (P = .00006), only the last variable was found to be significant in the final logistic analysis model. In an analysis of the patients in groups 2 and 3, CMV reactivation was also found to be significantly associated with CMV-specific CTL (Fisher exact test, P = .0016).
CD45R isoform expression of CMV-specific CTL
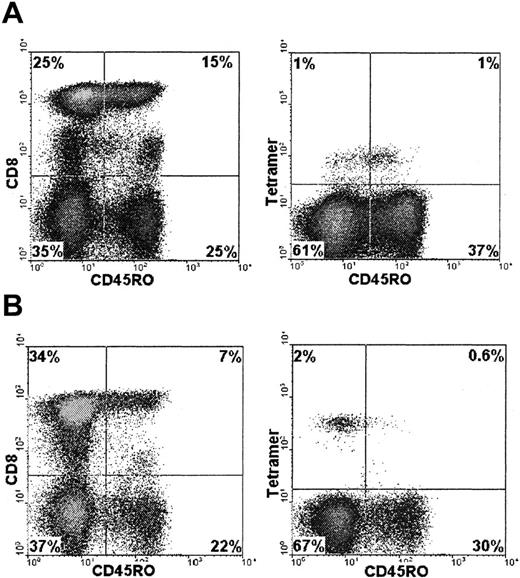
CD45 is a leukocyte common molecule associated with T-cell signal transduction. Several isoforms are differentially spliced from messenger RNA and are associated with the differentiation state of the T cell.21,22 CD45RA is typically a marker of naı̈ve T cells, whereas CD45RO is expressed on antigen-experienced cells. However, effector and memory CTL have been observed within the CD45RA population23 and CD45RA+ tetramer-binding cells have been reported.24 Thymus-independent regeneration of CD45RA+ CD8+ T cells has been observed after transplantation.25 We determined CD45 isoform expression on CMV-specific CTL identified in patients during prospective monitoring. CD45R expression was studied in 8 donors in whom serial samples (n = 33) were available at different time points. Expression of CD45RO was low on CMV-specific CTL (Figure6) in comparison with the total CD8+ population. On CD8+ T cells, the average percentage of CD45RO+ cells was 54%, whereas in tetramer-positive CD8+ cells, it was 32% (P < .001).
Expression of the CD45R0 isoform on CMV-specific CTL.
CD45R0 expression was determined in the total CD8+ T-cell population or the tetramer-binding CD8+ T cells by using flow cytometry. Examples shown are from patient 5 (A) and patient 1 (B). CD45RO expression is indicated on the x-axis. The y-axis represents CD8+ staining of the total PBMC population or tetramer staining of CD8+ gated cells.
Expression of the CD45R0 isoform on CMV-specific CTL.
CD45R0 expression was determined in the total CD8+ T-cell population or the tetramer-binding CD8+ T cells by using flow cytometry. Examples shown are from patient 5 (A) and patient 1 (B). CD45RO expression is indicated on the x-axis. The y-axis represents CD8+ staining of the total PBMC population or tetramer staining of CD8+ gated cells.
Relation between the number of CMV-specific CTL and protection from reactivation of CMV
Direct visualization of CMV-specific CTL provides the opportunity to determine whether there is a direct relation between the absolute number of CTL in peripheral blood and protection from CMV reactivation or disease. CTL in blood only represent a subset of virus-specific CTL, since CMV-specific CTL also home to secondary lymphoid organs and other tissues. Nevertheless, in our study, no patient with a CMV-specific CTL count of 100 × 105 cells/L or greater had a subsequent documented episode of CMV disease. In many patients, CTL counts higher than this developed. Patient 28, 3 years after receiving a sibling allograft, had an absolute count of 1.5 × 108 CTL/L binding the HLA-A*0201 tetramer, equivalent to approximately 10% of an average total lymphocyte count.
Discussion
CMV remains an important cause of morbidity and mortality after allogeneic SCT, despite antiviral therapy. Studies have confirmed that CMV seropositivity is an independent risk factor for mortality in patients given either T-cell–depleted sibling transplants or unrelated-donor transplants.1,7 The development of an effective CMV-specific immune response is important in the prevention of CMV disease, and cytotoxic cells appear to be the predominant effector mechanism. However, relatively little is known about the kinetics, magnitude, and specificity of the CD8+ T-cell response to CMV after transplantation. HLA-peptide tetramers, which allow direct visualization of antigen-specific CTL, have been widely applied to the study of human and murine immune responses. Initial studies with tetramers showed that the CTL response to CMV was among the strongest immune response studied.26 CTL specific for individual immunodominant CMV epitopes typically represent about 1% of all CD8+ T cells in immunocompetent donors, but levels are particularly elevated in the setting of immunosuppression such as that occurring after solid-organ transplantation.27 28 We applied tetramers incorporating immunodominant peptides from the CMV protein pp65 to study CMV-specific CD8+ T-cell reconstitution after SCT.
A striking observation was the high percentage of CMV-specific CTL in some patients. In patient 6, 21% of CD8+ T cells stained with the tetramer 3 weeks after SCT. In patients 1 and 5, 10% and 12% of CD8+ T cells stained with the tetramer. These percentages are among the highest ever observed for any human antigen other than in cases of acute viral infection and emphasize the potency of CMV in stimulating virus-specific CTL. Our findings are compatible with those of many studies in recipients of stem cells that found CMV seropositivity to be associated with greater and more rapid CD8+ T-cell reconstitution than CMV seronegativity.29-31 Whether this marked CD8+reconstitution is actually CMV specific has been unclear, but the data presented here indicate that this is likely to be the case. Exactly how CMV can stimulate such high levels of CTL is not known. One factor may be that the immunodominant pp65 protein is expressed at high levels in cells of the monocyte lineage, which mature into antigen-presenting cells. This study included only 4 patients who received peripheral blood stem cell allografts, and we could not find a difference in immune reconstitution between this group of patients and those given bone marrow (P = .89).
In seropositive patients given sibling allografts, CMV-specific immune reconstitution occurred early (median, 37 days; range, 25-55 days) but only in those who received transplants from a CMV-seropositive donor. These data concur with earlier findings that the CMV serologic status of the donor has an important influence on the early detection of CMV-specific T-cell cytotoxicity or proliferation.8,32 One study found that CMV-seropositive patients with CMV-seronegative donors were up to 50 times more likely to have a fatal CMV infection after sibling allogeneic transplantation than those with a seropositive donor.33 However, the study addressed only T-cell–depleted allografts, and other investigations have not found a significant association between CMV disease and donor serologic status.34,35 In patients with seronegative donors, virus-specific memory cells are not present in the stem cell graft and must therefore be derived either from residual recipient cells that have survived conditioning or from donor T cells as a result of primary immune stimulation with viral peptides. It is likely that patients in this group will require in vivo viral reactivation in order to generate CMV-specific immune responses,11 as has been reported previously for other viruses, including varicella zoster.36 Among 10 seropositive recipients who received stem cells from seronegative donors, CMV CTL were detectable only in the 4 patients who had episodes of CMV reactivation after transplantation. Patients in this subgroup have the highest peak viral loads37 and they may be at increased risk of viral disease, although no CMV disease occurred in our cohort.
Our analysis also suggests that the emergence and persistence of CMV-specific CTL in CMV-seronegative recipients with CMV-seropositive donors requires an episode of primary viral infection. Although CMV is likely to be transferred in the donor stem cell graft, the fact that early virus-specific immune reconstitution was not observed in this group is likely to have reflected a low viral load and therefore less immune stimulation. This group is at low risk of viral disease, and indeed, none occurred in our study. In contrast to results in pairs in which either the recipient or donor was CMV seronegative, CMV-specific reconstitution did not depend on viral reactivation in sibling transplantations in which both the patient and donor were CMV seropositive. However, levels of CMV reactivation may have been below the limit of the detection methods used.
Compared with the early reconstitution of CMV-specific immunity in recipients of HLA-identical transplants, the recovery of CMV-specific CTL in all CMV-positive recipients of MUD transplants was profoundly delayed and no tetramer-binding cells were detectable before day 100. Several factors may explain this observation. Recipients of MUD transplants were treated with Campath-1H in vivo as part of their GVHD prophylaxis regimen. The Campath antibody is extremely effective at depleting T cells and can lead to marked lymphopenia after transplantation, even when given before the procedure. In addition, patients who received MUD transplants were given ganciclovir prophylaxis, and a strong correlation between ganciclovir prophylaxis and failure to recover CMV-specific T-cell responses in the first 90 days after transplantation has been reported.8 This is likely a reflection of a reduction in viral synthesis and consequent impaired priming of the immune system.
Despite the relative delay in immune reconstitution in the MUD group, when CMV-specific CTL do become detectable, they can represent a considerable proportion (up to 6%) of the CD8+ population, although we found that the absolute number of CMV-specific CTL was lower than that in patients who received a sibling allograft.
We also evaluated CD45RO isoform expression on CMV-specific CTL in this study. Although expression of this marker was once regarded as a stable marker of antigen-experienced T cells, it is now clear that effector CTL can revert to a CD45RA+RO− phenotype. In the transplant patients, CD45RO was expressed on a minority of tetramer-positive cells, indicating a high degree of reversion to CD45RA expression. Because antigen-primed CD45RA+CD8+ cells express high levels of perforin and show high cytolytic activity without prestimulation in vitro, they are thought to represent an effector rather than a memory phenotype.38 Cell numbers in our study were limited, so we did not undertake a comprehensive study of CD45RA+expression on CTL, although reciprocal expression of high levels of CD45RO and CD45RA was found in a subset of patients (data not shown). Our findings support a model in which high levels of effector CTL are boosted in allogeneic transplant recipients as a result of viral replication in vivo.
Quantitative evaluation of the CMV-specific immune response allowed us to examine the influence of viral reactivation and concurrent drug therapy on the level of antigen-specific CTL. There were several examples of viral reactivation leading to an increase in CMV-specific CTL numbers. In patient 1, CMV reactivation was followed by a 25-fold increase in CMV-specific CTL from the time of CMV reactivation on day 96 to recovery on day 136. In contrast, ganciclovir was previously associated with impaired cellular immunity to CMV and the existence of this relation was supported by our observations in patient 7, in whom levels of CMV-specific CTL were low until ganciclovir treatment was stopped. A reduction in a patient's overall exposure to ganciclovir should facilitate a more rapid reconstitution of the immune system and here tetramers may be useful in identifying patients with adequate CMV-specific immunity.
The current study provides some indication of the level of CMV-specific CTL that protects against CMV disease. Only 2 tetramers were used in this study, and they measured only a component of the total CMV-specific T-cell immune response. Nevertheless, patients in whom a CMV-specific CD8+ T-cell response of 107cells/L or greater developed were protected from CMV reactivation. This finding correlates well with results in immunocompetent donors with no evidence of CMV reactivation, in whom the tetramers used in this study stained an average of 1% to 2% of CD8+ T cells. Assuming a total lymphocyte count of 2 × 109/L and an average CD8+ T-cell percentage of 20%, this is equal to a CMV-specific CD8+ T-cell count of 0.4 to 0.8 × 107/L. CTL numbers in patients who underwent SCT were higher than this, and a similar pattern of CTL “overshooting” was observed previously in patients recovering from solid-organ allografting.27 28
In this study, we could only address CD8+ T-cell reconstitution, but it is likely that CMV-specific CD4+ T cells are also important for protection against disease. Persistent CD4+ lymphopenia is a poor prognostic factor for patients with CMV disease,39 and impaired CMV-specific T-cell proliferation identifies patients at risk of late onset (> 120 days) CMV disease40 or interstitial pneumonia.41 In addition, the persistence of adoptively transferred, donor-derived, CMV-specific CD8+ T-cell clones was impaired in patients who did not have recovery of CMV-specific T-helper responses.11
The development of fluorescent HLA-peptide tetramers offers considerable potential in the study of antigen-specific immune reconstitution after SCT. The ability to make a rapid assessment of antigen-specific immune reconstitution should be of value in both the development of novel transplant protocols and, possibly, in the clinical management of individual cases. In addition, tetrameric staining of donor lymphocytes followed by selection will allow development of antigen-specific adoptive transfer directly from SCT donor to recipient.
M.C. holds an MRC Clinical Training Fellowship.
Supported by grants from the Leukaemia Research Fund and the Kay Kendall Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
P.A.H. Moss, CRC Institute for Cancer Studies, University of Birmingham, Birmingham B15 2TA, United Kingdom; e-mail:p.moss@bham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal