Langerhans cell histiocytosis (LCH) consists of lesions composed of cells with a dendritic Langerhans cell (LC) phenotype. The clinical course of LCH ranges from spontaneous resolution to a chronic and sometimes lethal disease. We studied 25 patients with various clinical forms of the disease. In bone and chronic lesions, LCH cells had immature phenotype and function. They coexpressed LC antigens CD1a and Langerin together with monocyte antigens CD68 and CD14. Class II antigens were intracellular and LCH cells almost never expressed CD83 or CD86 or dendritic cell (DC)–Lamp, despite their CD40 expression. Consistently, LCH cells sorted from bone lesions (eosinophilic granuloma) poorly stimulated allogeneic T-cell proliferation in vitro. Strikingly, however, in vitro treatment with CD40L induced the expression of membrane class II and CD86 and strongly increased LCH cell allostimulatory activity to a level similar to that of mature DCs. Numerous interleukin-10–positive (IL-10+), Langerin−, and CD68+ macrophages were found within bone and lymph node lesions. In patients with self-healing and/or isolated cutaneous disease, LCH cells had a more mature phenotype. LCH cells were frequently CD14− and CD86+, and macrophages were rare or absent, as were IL-10–expressing cells. We conclude that LCH cells in the bone and/or chronic forms of the disease accumulate within the tissues in an immature state and that most probably result from extrinsic signals and may be induced to differentiate toward mature DCs after CD40 triggering. Drugs that enhance the in vivo maturation of these immature DCs, or that induce their death, may be of therapeutic benefit.
Introduction
Langerhans cell histiocytosis (LCH) affects mainly young children and features accumulation of CD1a+ Birbeck granule+ cells within the epidermis and dermis, the bones, and occasionally lymphoid organs, lungs, and digestive tract.1-4 A frequent clinical feature is a skin eruption in the first months or days after birth. It may spontaneously resolve (Hashimoto-Pritzker syndrome) or be part of a widespread disease (Letterer-Siwe syndrome).5-8 In the older child, chronic/granulomatous forms are more frequent (eosinophilic granuloma, Hand-Schuller-Christian disease).9 Eosinophilic granuloma, found in 50% to 80% of all patients with LCH,9 consists of chronic lytic bone lesions that may be unifocal or multifocal and may associate with the involvement of smooth tissue. It may be difficult, however, to distinguish between an active and an inactive bone lesion without serial biopsies, which are not performed in most cases for obvious ethical reasons. Treatment of severe or chronic disease, relying on cytotoxic chemotherapy, continues to be controversial and, in many cases, ineffectual. Although LCH has been proposed to be a clonal disorder,10,11 its cause remains unknown, despite an extensive search for evidence of consistent cytogenetic abnormalities or gene rearrangements. Whether LCH is reactive or neoplastic is even debated, and several features provide seemingly contradictory evidence on this point (spontaneous resolution of disease on the one hand and clonality of lesional LCH on the other). Similarly, the pathogenesis of the disease is enigmatic, although the altered expression of cytokines and cellular adhesion molecules, important for migration and homing of the normal Langerhans cell (LC), may play an important role.4,12-18 It has been suggested that LCH cells may be in an arrested state of activation and/or differentiation of LCs. Apparently, contrasting studies have reported that LCH cells may be activated, based on phenotypic data,17,19 whereas others have failed to detect alloantigen-presenting activity.20 Although no immune defect has been identified in affected children, some T lymphocyte phenotype abnormalities that suggest alterations in antigen-driven activation processes have been reported.21
Recent progress in the field of dendritic cell (DC) biology has led us to revisit the phenotype and function of LCH cells in an attempt to better understand the pathophysiology of this disease and to explain why an accumulation of antigen-presenting cells may develop in the apparent absence of an efficient immune response. We first investigated the differentiation stages of LCH cells in the distinct clinical forms of the disease by combining phenotypic and functional studies. We concluded that LCH cells are functionally immature DCs in the chronic form of the disease. We then investigated whether immature LCH cells may be induced to become mature DCs, and we found that CD40 triggering induced their differentiation as efficiently as for normal DCs. To investigate why LCH cells remain immature in vivo, we examined their microenvironment. We found that non-Langerhans cells (macrophages) within bone and chronic lesions produced interleukin-10 (IL-10) in vivo. On the contrary, we have observed that LCH cells in isolated or healing skin lesions have a more mature phenotype and that IL-10–producing cells were absent from these lesions. The presence of IL-10 may contribute to the immaturity of LCH cells in bone/chronic lesions. The results of our study therefore indicate that LCH cells, in the bone/chronic form of the disease, are maintained immature in vivo, most probably by extrinsic signals. These data shed some light on the pathogenesis of LCH and may be useful for designing therapeutic strategies in this disease.
Patients, materials, and methods
Patients
Biopsy samples from 25 patients, referred to Necker Enfants Malades Hospital and diagnosed as having Langerhans cell histiocytosis, on the basis of their clinical history and the expression of CD1a by histiocytic cells, were examined by immunohistochemistry and confocal microscopy in this study. For 6 patients (numbers 7459, 10331, 10337, 9980, 9387, 3774), fresh tissues were available at the time of frozen examination and were studied by flow cytometry and/or functional assays. Patients were evaluated as having various clinical forms of the disease (self-healing Hashimoto-Pritzker disease n = 4, cutaneous histiocytosis n = 4, eosinophilic granuloma n = 12, and multisystem disease n = 5). Twenty-nine biopsy samples from various anatomic sites were examined (Table 1).
In situ immunophenotype of Langerhans cell histiocytosis cells in 25 patients
| Patient number . | Age/sex . | Clinical stage . | Biopsy site . | Immunostaining (LCH cells) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Langerin . | CD14 . | CD68 . | CD40 . | CD80 . | CD86 . | CD83 . | ||||
| 7459 | 7 y/M | EG | Bone | ++ | ++ | ++ | ++ | + | ± | − |
| 3774 | 2 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | ± | ± |
| 10391 | 3 y/M | EG | Bone | ++ | ++ | ++ | ++ | ± | ± | − |
| 9980 | 11 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ± | − |
| 10337 | 6 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ± | ± |
| 2558 | 8 y/M | EG | Bone | ++ | ++ | ++ | ++ | + | ± | ± |
| 7553 | 6 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | − | − |
| 3389 | 10 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ++ | ± |
| 5259 | 3 y/M | EG | Bone | ++ | ++ | ++ | + | ++ | − | − |
| 4188 | 8 y/M | EG | Bone | ++ | ++ | ++ | ++ | nd | − | − |
| 5149 | 6 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | − | − |
| 9387 | 8 y/F | EG | Bone | ++ | nd | nd | nd | nd | nd | nd |
| 6423 | 1 mo/M | Skin (HP) | Skin | ++ | ± | ++ | ++ | + | + | + |
| 3807 | 15 d/F | Skin (HP) | Skin | ++ | ± | + | ++ | ++ | + | ± |
| 5828 | 7 d/F | Skin (HP) | Skin | ++ | + | ++ | ++ | ++ | + | ± |
| 5464 | 3 d/M | Skin (HP) | Skin | ++ | nd | nd | nd | nd | nd | nd |
| 3771 | 4 mo/F | Skin | Skin | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 7333 | 7 mo/F | Skin | Skin | ++ | + | ++ | ++ | ± | + | ± |
| 7089 | 1 mo/M | Skin | Skin | ++ | ± | ++ | ++ | − | nd | − |
| 4968 | 8 mo/M | Skin | Skin | ++ | ± | ++ | ++ | ++ | + | ± |
| 6193 | 1 y/M | MS | Skin | ++ | + | + | ++ | + | − | − |
| LN | ++ | + | + | ++ | ++ | ± | ± | |||
| 6146 | 1 y/M | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
| Bone | ++ | + | + | + | − | − | − | |||
| LN | ++ | + | + | ++ | + | + | + | |||
| 6277 | 3 mo/M | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
| LN | ++ | ++ | ++ | ++ | nd | ± | ± | |||
| 8718 | 2 mo/F | MS | Skin | ++ | + | ++ | ++ | + | ± | ± |
| 564 | 2 mo/F | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
| Patient number . | Age/sex . | Clinical stage . | Biopsy site . | Immunostaining (LCH cells) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Langerin . | CD14 . | CD68 . | CD40 . | CD80 . | CD86 . | CD83 . | ||||
| 7459 | 7 y/M | EG | Bone | ++ | ++ | ++ | ++ | + | ± | − |
| 3774 | 2 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | ± | ± |
| 10391 | 3 y/M | EG | Bone | ++ | ++ | ++ | ++ | ± | ± | − |
| 9980 | 11 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ± | − |
| 10337 | 6 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ± | ± |
| 2558 | 8 y/M | EG | Bone | ++ | ++ | ++ | ++ | + | ± | ± |
| 7553 | 6 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | − | − |
| 3389 | 10 y/M | EG | Bone | ++ | ++ | ++ | ++ | ++ | ++ | ± |
| 5259 | 3 y/M | EG | Bone | ++ | ++ | ++ | + | ++ | − | − |
| 4188 | 8 y/M | EG | Bone | ++ | ++ | ++ | ++ | nd | − | − |
| 5149 | 6 y/F | EG | Bone | ++ | ++ | ++ | ++ | + | − | − |
| 9387 | 8 y/F | EG | Bone | ++ | nd | nd | nd | nd | nd | nd |
| 6423 | 1 mo/M | Skin (HP) | Skin | ++ | ± | ++ | ++ | + | + | + |
| 3807 | 15 d/F | Skin (HP) | Skin | ++ | ± | + | ++ | ++ | + | ± |
| 5828 | 7 d/F | Skin (HP) | Skin | ++ | + | ++ | ++ | ++ | + | ± |
| 5464 | 3 d/M | Skin (HP) | Skin | ++ | nd | nd | nd | nd | nd | nd |
| 3771 | 4 mo/F | Skin | Skin | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 7333 | 7 mo/F | Skin | Skin | ++ | + | ++ | ++ | ± | + | ± |
| 7089 | 1 mo/M | Skin | Skin | ++ | ± | ++ | ++ | − | nd | − |
| 4968 | 8 mo/M | Skin | Skin | ++ | ± | ++ | ++ | ++ | + | ± |
| 6193 | 1 y/M | MS | Skin | ++ | + | + | ++ | + | − | − |
| LN | ++ | + | + | ++ | ++ | ± | ± | |||
| 6146 | 1 y/M | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
| Bone | ++ | + | + | + | − | − | − | |||
| LN | ++ | + | + | ++ | + | + | + | |||
| 6277 | 3 mo/M | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
| LN | ++ | ++ | ++ | ++ | nd | ± | ± | |||
| 8718 | 2 mo/F | MS | Skin | ++ | + | ++ | ++ | + | ± | ± |
| 564 | 2 mo/F | MS | Skin | ++ | nd | nd | nd | nd | nd | nd |
LCH cells in biopsy samples were positive for CD1a in all 25 patients. Staining with Langerin, CD14, CD68, CD40, CD80, CD86, and CD83 was evaluated on serial sections.
LCH indicates Langerhans cell histiocytosis; EG, eosinophilic granuloma; HP, Hashimoto-Pritzker disease; MS, multisystem disease; LN, lymph node. Most (++) denotes positivity of most (more than 75%) of cells; numerous (+), 25% to 75%; rare (±), less than 25%; negative (−), less than 5%; nd, not available.
Cells
Only samples from eosinophilic granuloma met the conditions for separation of LCH cells (sufficient numbers of lesional cells and availability of fresh tissue at the time of diagnosis). After frozen section examination, sterile tissue from eosinophilic granuloma was harvested in RPMI 1640 supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum (FCS) myoclone (all from GIBCO BRL, Gaithersburg, MD), referred to below as complete medium. Tissues were immediately gently dissociated through a nylon mesh. The cell suspension was washed 3 times and incubated with human IgG to block Fc receptor, and anti-CD1a antibody (BL6; Immunotech, Marseille, France). The cells were washed twice, incubated with antimouse microbeads (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 minutes at 4°C. Cells were washed again, and then CD1a+ and CD1a− LCH cells were separated by positive immunomagnetic selection by using a magnetic cell separator (MACS) according to the manufacturer's instructions. Between two 105 and six 105 CD1a+ cells were recovered from each sample. Purity of CD1a+ and CD1a− sorted fraction was 80% or greater and mortality 10% or less.
LC-type dendritic cells were prepared as previously described.22 23 Briefly, fresh CD14+ monocytes were isolated from peripheral blood mononuclear cells (PBMCs) of healthy volunteers obtained by the standard Ficoll-Hypaque method and immediately separated by negative magnetic depletion by using hapten-conjugated CD3, CD7, CD19, CD45RA, CD56, and anti-IgE antibodies (MACS; Miltenyi Biotec) and a MACS according to the manufacturer's instructions, routinely resulting in more than 95% purity of the CD14+ cells. Cells were cultured in flasks or in 6- or 24-well tissue culture plates (Costar Corp, Cambridge, MA) for 5 to 7 days in complete medium supplemented with 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 10 ng/mL IL-4, and 10 ng /mL transforming growth factor beta 1(TGFβ1).
Murine fibroblast cell lines transfected with human CD40L (LcCD40L) or CD32 (LcCD32) were kindly provided by Dr J. Banchereau and Dr F. Briére (Schering-Plough, Dardilly, France).24
T cells were isolated from the PBMCs of healthy volunteers by the standard Ficoll-Hypaque method, followed by magnetic depletion of non-T cells (MACS; Miltenyi Biotec).
Antibodies
Uncoupled antibody to CD1a (clone BL6) used for cell separation and immunochemistry on tissue sections was purchased from Immunotech (Marseille, France). Fluoroscein isothiocyanate (FITC)–conjugated anti-CD1a (clone VVM-35) used for flow cytometry was purchased from TEBU (Le Perray en Yvelines, France). Unconjugated antibodies to HLA-DR (clone B8.12.2), CD80 (MAB 104) IgG1, CD83 (HB15A), DC-Lamp, and CD40 (mAb89) were obtained from Immunotech. Antibodies to CD86 (IT2.2) and CD14 (Leu-M3) were obtained from Becton Dickinson (Le Pont de Claix, France). Phycoerythrin (PE)–conjugated anti-CD1a, CD14, and CD86 and FITC-conjugated HLA-DR were obtained from the same company as the unconjugated counterparts. Biotinylated rat antihuman IL-10 (JES3–12G8) was purchased from Pharmingen (Becton Dickinson). Monoclonal antibody DCGM4 to Langerin has been described.25 26
Immunohistochemistry
Serial cryostat sections of biopsy specimens were stained with CD1a, Langerin, CD14, CD40, CD80, CD83, DC-Lamp, or CD86 mouse primary antibodies at the appropriate concentration as determined by titration, and then labeled with a goat antimouse alkaline phosphatase-conjugated antibody. Fast red (Sigma, St Louis, MO) was used as a substrate for alkaline phosphatase. Isotype-matched antibodies were used as negative control. Internal positive controls were always observed. Mounted slides were evaluated on serial sections for the expression of Langerin, CD14, CD40, CD80, CD83, or CD86 by CD1a+ LCH cells. The results were scored as follows: ++ staining of most (more than 75%) LCH cells ; + staining of numerous (25%-75%) LCH cells ; ± staining of few LCH cells (less than 25%); and − absence of staining of LCH cells (less than 5%).
Flow cytometry
For 2-color flow cytometry, 5 × 104 to 1 × 105 cells were incubated in 96-well plates (Becton Dickinson) for 15 minutes at 4°C in phosphate-buffered saline (PBS), 2% human AB serum, and 0.01 M NaN3, with FITC- and PE-conjugated monoclonal antibodies (mAbs) at the appropriate concentration, or with control isotype-matched irrelevant mAbs at the same concentration (Becton Dickinson). After washing, 104 events were analyzed with a FACScalibur (Becton Dickinson) using CellQuest software (Becton Dickinson).
Confocal microscopy
Cells were washed in Ca++/Mg++-free PBS and centrifuged onto glass slides by using Cytospins (Shandon, Pittsburgh, PA), dried for 1 hour at room temperature, fixed in acetone for 10 minutes, and stored at −20°C. Frozen tissue section (5 μM thick) were also fixed in acetone for 10 minutes and stored at −20°C. For staining, slides were rehydrated for 5 minutes in PBS with 2% pooled normal human serum AB (staining medium), and then incubated for 1 hour at room temperature with mouse antihuman Langerin, followed by goat antimouse Cy3 or FITC-conjugated mAb to Langerin, FITC-conjugated mAb to HLA-DR, PE-conjugated mAb to CD1a, CD14, CD86, and CD68, biotinylated rat antihuman IL-10 and streptavidin Cy3 and Cy5 (Jackson Laboratories, Bar Harbor, ME). Slides were mounted with Fluoprep (Biomerieux SA, Marcy l'Etoile, France) and analyzed with a confocal laser microscope system attached to a microscope.
Allogeneic lymphocyte proliferation
Patient CD1a+ cells and LC-type DCs were resuspended in 24-well tissue culture plates at a concentration of 5.105 cell/mL in complete medium supplemented with 100 ng/mL GM-CSF and 10 ng/mL IL-4 for stimulation. Fibroblastic L-cells transfected with either CD40L, or CD32 as control, were irradiated at 80 Gy and added to the culture wells at a proportion of 25%. Cells were collected after 40 hours of stimulation, washed 3 times in PBS, resuspended in RPMI with 10% human AB serum, and added in triplicate at various concentrations to 105 autologous T cells per well in 96-well tissue culture plates (Falcon, Amersham, Freiburg, Germany). [3H]Thymidine (Amersham Life Science, Buckinghamshire, United Kingdom) incorporation was measured in newly synthesized DNA over 18 hours, by using pulses initiated at day 5 of the culture with 0.037 MBq (1 μCi) per well of [3H]thymidine. Cells were then harvested with a 96-well Harvester (Pharmacia, St. Quentin, France), collected on glass-fiber filter (Pharmacia), and the incorporation of thymidine was measured with a beta-plate microscintillation counter (LKB, Pharmacia).
Results
Patients
As shown in Table 1, 12 patients presented with bone disease (eosinophilic granuloma), either unifocal or multifocal, 8 patients presented with LCH restricted to the skin, and among them 4 had self-healing cutaneous histiocytosis (Hashimoto-Pritzker syndrome) diagnosed. Five patients presented with multifocal LCH involving more than 2 organs. In patients with skin LCH, lesional cells constituted an homogenous dermoepidermal infiltrate of CD1a+ cells (Figure1) also characterized by their round shape, admixed with various amounts of small lymphocytes. A few polymorphonuclear eosinophils were present in some patients' biopsy specimens, and CD1a− macrophages were absent or very rare in all cases. In the bone biopsy specimens (eosinophilic granuloma), round-shaped CD1a+ LCH cells were admixed with relatively numerous CD68+ CD1a− macrophages (Figures 1,4), occasional multinucleated giant cells, eosinophilic polymorphonuclear cells, and scattered lymphocytes. Lymph node biopsy specimens revealed the presence of CD1a+ cells within the sinuses and the T-cell areas, and the presence of CD1a−, CD68+ macrophages. These features fitted with previously described characteristics of cutaneous, bone, and lymph node lesions of LCH.1 6-8
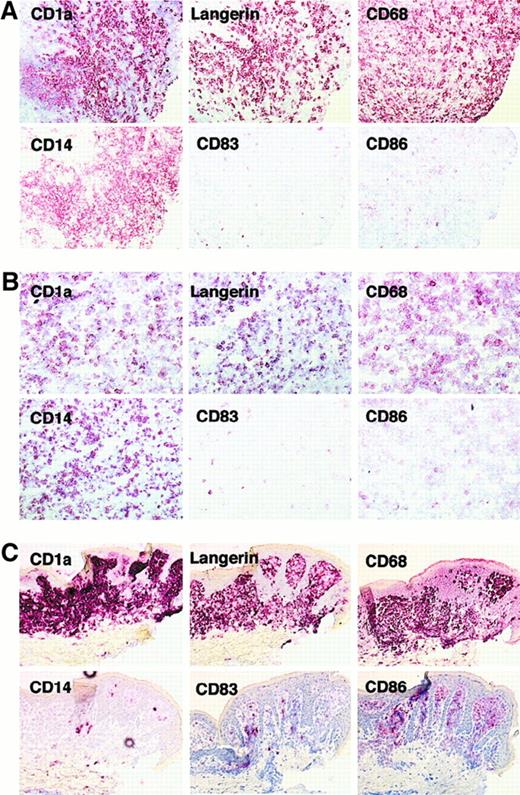
Expression of CD1a, Langerin, CD68, CD14, CD83, and CD86 on LCH cells.
Serial sections from bone lesion of patient 10337 (eosinophilic granuloma) (A), lymph node from patient 6277 (multisystem disease) (B), and skin from patient 4968 (cutaneous histiocytosis) (C) were stained with CD1a, Langerin, CD68, CD14, CD86, and CD83. CD1a+cells from bone (A) and lymph node (B) lesions expressed Langerin, CD14, CD68, and did not express CD86 or CD83. CD1a+ skin lesion cells rarely expressed CD14 but expressed CD86 and some cells were also CD83+. Note that in bone and lymph node lesions CD68 and CD14 stained more cells than did CD1a and Langerin, but this was not the case in skin lesion.
Expression of CD1a, Langerin, CD68, CD14, CD83, and CD86 on LCH cells.
Serial sections from bone lesion of patient 10337 (eosinophilic granuloma) (A), lymph node from patient 6277 (multisystem disease) (B), and skin from patient 4968 (cutaneous histiocytosis) (C) were stained with CD1a, Langerin, CD68, CD14, CD86, and CD83. CD1a+cells from bone (A) and lymph node (B) lesions expressed Langerin, CD14, CD68, and did not express CD86 or CD83. CD1a+ skin lesion cells rarely expressed CD14 but expressed CD86 and some cells were also CD83+. Note that in bone and lymph node lesions CD68 and CD14 stained more cells than did CD1a and Langerin, but this was not the case in skin lesion.
Langerhans cell histiocytosis cells express Langerin, CD14, and CD68
In all 25 patients, more than 75% CD1a positive cells stained for Langerin in serial sections (Figure 1; Table 1). Confocal microscopy study on eosinophilic granuloma samples (n = 3) further demonstrated that antibodies against CD1a and Langerin labeled the same cells (Figure 2A). Langerin expression was a constant feature of CD1a+ LCH cells, whatever the site of the biopsy (skin, bone, or lymph node), or the stage or clinical form of the disease. We found that CD1a+ cells frequently coexpressed CD14 in situ on serial sections in all patients with extracutaneous disease (Figure 1A,B; Table 1). To exclude that the CD14 positive staining may be solely due to expression of CD14 by macrophages that are present in LCH lesions, flow cytometry analysis was performed in 3 patients and confirmed the expression of various levels of CD14 by up to 70% of the CD1a+ cells (Figure3A). In addition, confocal microscopy (Figure 2B) showed that besides Langerin− CD14+ macrophages, both Langerin+ CD14− and Langerin+CD14+ Langerhans cells were observed. CD14 expression appeared to depend on the clinical form of the disease. Numerous CD1a+ cells were stained in bone lesions (eosinophilic granuloma, a chronic lesion) (11 patients) and numerous LCH cells were also CD14+ on serial sections from involved lymph nodes (n = 3, Figure 1B). However, many fewer cells were stained in skin samples (Figure 1C), and CD14+ cells were numerous in only 3 of 7 tested patients with pure cutaneous disease (Table 1). In addition, in all patients, most CD1a+ Langerin+cells coexpressed CD68, although at a lower level than do macrophages (Table 1, Figure 1; also Figure 5B). This is in accordance with previous studies.27 28 CD68 is a lysosomal antigen expressed (at high levels) in monocyte/macrophages, and (at low levels) in immature skin LCs, and down-regulated on maturation. In skin lesions, CD68, CD1a, and Langerin staining patterns were very similar on serial sections. In bone and lymph node lesions, similarly to what was observed for CD14 staining, some CD1a− Langerin− cells were CD68+, indicating the presence of macrophages admixed with Langerhans cells (Figure 5B). Altogether, these data suggested that LCH cells have features of immature LCs, and we therefore investigated the expression of costimulatory molecules and the cellular localization of major histocompatibility complex (MHC) class II molecules in these cells.
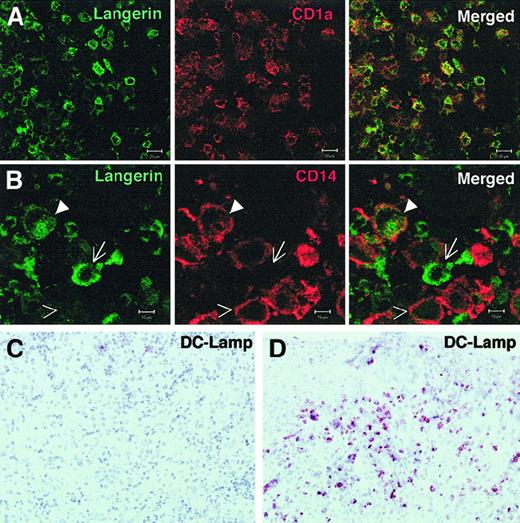
CD1a+ Langerin+ cells may express CD14 but not DC-Lamp in eosinophilic granuloma.
(A, B) Frozen sections from eosinophilic granuloma of the bone (patient 7459) were fixed in acetone and stained with anti-Langerin FITC and either CD1a-PE (A), or CD14-PE (B) and analyzed by confocal microscopy. (A): antibodies against CD1a and Langerin labeled the same cells. (B): Langerin+CD14− (arrow), Langerin+, CD14+(closed arrowhead), and Langerin− CD14+ (open arrowhead) are observed. Similar results were obtained in 3 different patients. A representative experiment is shown. Bar represents 20 μM in panel A and 10 μM in panel B. (C, D) Sections from a bone lesion (C), and a skin lesion (D) were stained with anti–DC-Lamp antibody. Although numerous positive cells are seen in the skin lesion, they are very rare in the bone lesions. Similar results were obtained in 3 different patients in each group.
CD1a+ Langerin+ cells may express CD14 but not DC-Lamp in eosinophilic granuloma.
(A, B) Frozen sections from eosinophilic granuloma of the bone (patient 7459) were fixed in acetone and stained with anti-Langerin FITC and either CD1a-PE (A), or CD14-PE (B) and analyzed by confocal microscopy. (A): antibodies against CD1a and Langerin labeled the same cells. (B): Langerin+CD14− (arrow), Langerin+, CD14+(closed arrowhead), and Langerin− CD14+ (open arrowhead) are observed. Similar results were obtained in 3 different patients. A representative experiment is shown. Bar represents 20 μM in panel A and 10 μM in panel B. (C, D) Sections from a bone lesion (C), and a skin lesion (D) were stained with anti–DC-Lamp antibody. Although numerous positive cells are seen in the skin lesion, they are very rare in the bone lesions. Similar results were obtained in 3 different patients in each group.
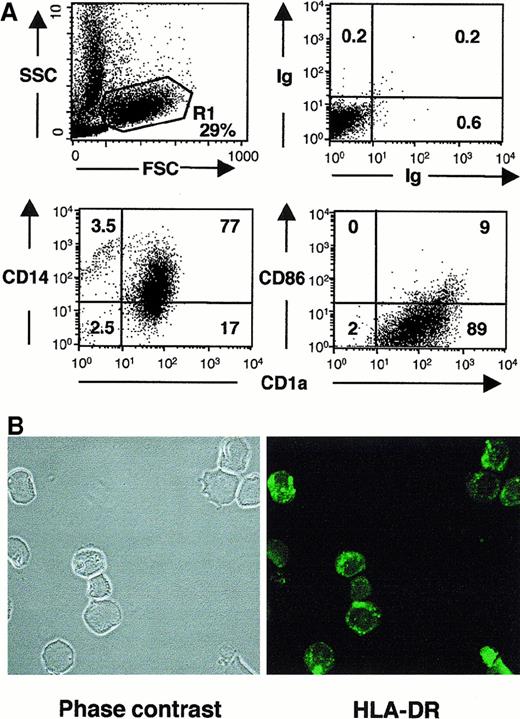
LCH cells from eosinophilic granuloma coexpress CD1a and CD14 but not CD86 at the cell surface, and MHC class II within intracellular vesicular compartments.
(A) Fresh tissue from eosinophilic granuloma (patient 5149) was gently dissociated, and the cell suspension was washed 3 times and incubated with FITC-conjugated anti-CD1a and PE-conjugated CD14 or CD86, or with FITC and PE isotypic-matched controls. Dot plots represent the phenotype and percentages of labeled cells in the monocytic gate (R1, 29% of total events). Similar results were obtained in 2 other patients. (B) Confocal microscopy analysis of CD1a+ cells sorted from eosinophilic granuloma stained with FITC-conjugated anti–HLA-DR. Note the vesicular, intracytoplasmic staining.
LCH cells from eosinophilic granuloma coexpress CD1a and CD14 but not CD86 at the cell surface, and MHC class II within intracellular vesicular compartments.
(A) Fresh tissue from eosinophilic granuloma (patient 5149) was gently dissociated, and the cell suspension was washed 3 times and incubated with FITC-conjugated anti-CD1a and PE-conjugated CD14 or CD86, or with FITC and PE isotypic-matched controls. Dot plots represent the phenotype and percentages of labeled cells in the monocytic gate (R1, 29% of total events). Similar results were obtained in 2 other patients. (B) Confocal microscopy analysis of CD1a+ cells sorted from eosinophilic granuloma stained with FITC-conjugated anti–HLA-DR. Note the vesicular, intracytoplasmic staining.
Major histocompatibility complex class II and costimulatory molecules
Although CD80 was frequently detected (Table 1), CD86 (B7-2) expression was undetectable on most LCH cells in the majority of bone lesions (10 of 11), in 2 of 3 cases of lymphadenopathy, and in skin lesions from patients with multisystem disease (Table 1, Figure 1, Figure 3A). CD83, a marker of mature DCs, was expressed only by scattered cells in all these samples, except for one case of lymphadenopathy (Table 1, Figure 1). In accordance, DC-Lamp, another molecule selectively expressed by mature DCs,29 was only expressed by scattered cells (Figure 2C). Moreover, although it has been reported that LCH cells express MHC class II molecules,27 30 we showed by confocal microscopy on sorted LCH cells from bone lesions that most class II resides within intracellular vesicular compartments (Figure 3B), as observed in immature LCs. In contrast, among patients with pure cutaneous disease, including patients with Hashimoto-Pritzker syndrome, CD86 was expressed by the majority of LCH cells in skin lesions of all tested patients (6 of 6) (Table 1, Figure 1C) and CD83 was expressed by a majority of cells in 2 of 7. Moreover, DC-Lamp was also expressed by the majority of cells in 3 of 3 patients tested (Figure 2D). This confirms that the phenotype of LCH cells differs between cases of isolated skin involvement and bone/disseminated diseases, being more immature in the latter. This suggests that LCH cells, although most frequently immature, may become mature in some circumstances. This may possibly occur through CD40/CD40L interaction, because in all patients, LCH cells expressed CD40 at an even higher level than did normal epidermal Langerhans cells (Table 1, Figure 4A). We therefore investigated the functional properties of LCH cells.
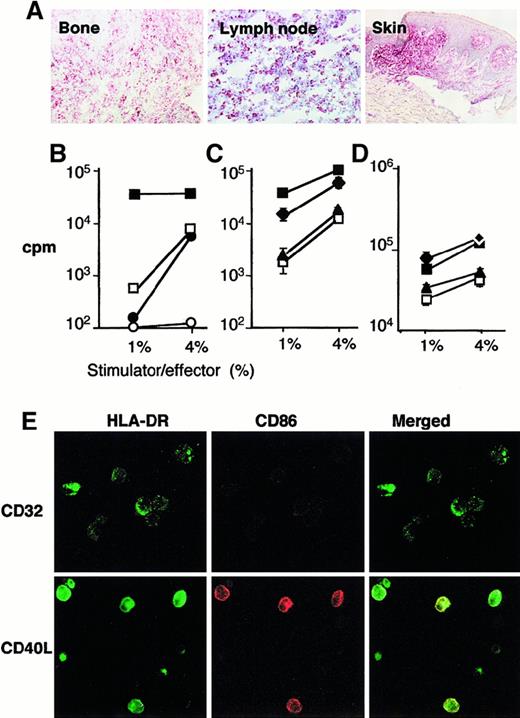
LCH cells from eosinophilic granuloma are immature DCs but may be induced to express membrane class II and C86 and to stimulate allogenous lymphocytes on stimulation with CD40L.
(A) Sections from bone lesion of patient 10337 (eosinophilic granuloma), lymph node from patient 6277 (multisystem disease), and skin from patient 4968 (cutaneous histiocytosis) were stained with antibody to CD40. (B) Control immature (□) and mature (■) (CD40L treated) monocyte-derived DCs and CD1a+ (●) and CD1a− (○) cells sorted from fresh eosinophilic granuloma tissue from patient 7459 were cultured with 105 allogenous lymphocytes at 1% or 4% stimulator to effector ratio. (C) sorted CD1a+ cells from eosinophilic granuloma from patient 10391 (▴, ⧫) and control monocyte-derived DC (□, ■) were either cultured with LcCD32 (▴, □) or with LcCD40L (⧫, ■) for 2 days, and then cultured with 105 allogenous lymphocytes at 1% or 4% stimulator to effector ratio. Note that LCH CD1a+cells, either fresh or cultured with LcCD32 stimulate T-cell proliferation in a similar manner to that of control immature monocyte-derived DCs, whereas LCH CD1a+ cells cultured with LcCD40L stimulate T-cell proliferation in a similar manner to that of control mature monocyte-derived DCs cultured with LcCD40L. (D) The same experiment was performed with fresh tissue from eosinophilic granuloma from patient 3774. (E) Sorted CD1a+ cells from eosinophilic granuloma from patient 10391 cultured with LcCD32 or with LcCD40L for 2 days were also analyzed by confocal microscopy for expression of HLA-DR (green) and CD86 (red). Note that green DR staining increased and is present at the cell surface, in cells cultured with LcCD40L, and that red CD86 staining, absent on cells cultured with LcCD32, is induced on cells cultured with LcCD40L.
LCH cells from eosinophilic granuloma are immature DCs but may be induced to express membrane class II and C86 and to stimulate allogenous lymphocytes on stimulation with CD40L.
(A) Sections from bone lesion of patient 10337 (eosinophilic granuloma), lymph node from patient 6277 (multisystem disease), and skin from patient 4968 (cutaneous histiocytosis) were stained with antibody to CD40. (B) Control immature (□) and mature (■) (CD40L treated) monocyte-derived DCs and CD1a+ (●) and CD1a− (○) cells sorted from fresh eosinophilic granuloma tissue from patient 7459 were cultured with 105 allogenous lymphocytes at 1% or 4% stimulator to effector ratio. (C) sorted CD1a+ cells from eosinophilic granuloma from patient 10391 (▴, ⧫) and control monocyte-derived DC (□, ■) were either cultured with LcCD32 (▴, □) or with LcCD40L (⧫, ■) for 2 days, and then cultured with 105 allogenous lymphocytes at 1% or 4% stimulator to effector ratio. Note that LCH CD1a+cells, either fresh or cultured with LcCD32 stimulate T-cell proliferation in a similar manner to that of control immature monocyte-derived DCs, whereas LCH CD1a+ cells cultured with LcCD40L stimulate T-cell proliferation in a similar manner to that of control mature monocyte-derived DCs cultured with LcCD40L. (D) The same experiment was performed with fresh tissue from eosinophilic granuloma from patient 3774. (E) Sorted CD1a+ cells from eosinophilic granuloma from patient 10391 cultured with LcCD32 or with LcCD40L for 2 days were also analyzed by confocal microscopy for expression of HLA-DR (green) and CD86 (red). Note that green DR staining increased and is present at the cell surface, in cells cultured with LcCD40L, and that red CD86 staining, absent on cells cultured with LcCD32, is induced on cells cultured with LcCD40L.
LCH cells from bone lesions are functionally immature, but can mature after CD40 triggering
Sorted CD1a+ cells from the bone lesion (eosinophilic granuloma) of 3 patients (7459, 10391, 3774) were studied for their ability to trigger allogeneic lymphocyte proliferation. In situ, cells from these patients were CD1a+, Langerin+, CD40+, CD14+, CD68+. CD80 cells were rare (10391) or numerous (3774, 7459), and CD86 and CD83 cells were rare in all patients. Sorted cells exhibited the same phenotype (Figure 4E; data not shown). In patient 7459 (Figure 4B), the CD1a+ and CD1a− fractions were purified as described in the “Materials and methods” section, and cocultured with sorted allogenous T lymphocytes. As a control, immature DCs (Langerhans cell type) and CD40L-treated mature DCs, generated as described,23 were also cultured in the same experiment with allogeneic T lymphocytes from the same donor. Patients' CD1a+ cells and control cultured immature DCs had comparable effects on T lymphocytes and failed to induce significant thymidine incorporation at a 1% stimulator/effector ratio. This is clearly different from the vigourous T-cell proliferation induced by mature DCs (Figure 4B). In further experiments (Figure 4C,D, patients 10391 and 3774, respectively), sorted CD1a+ LCH cells and control immature DCs were cultured either with CD40L or with CD32 transfected fibroblasts for 2 days before being added to allogeneic lymphocytes. Strikingly, although both LCH cells and control immature DCs cultured with CD32 transfected cells equally (poorly) stimulated lymphocyte proliferation, both LCH cells and control immature DCs stimulated via CD40 showed a strong increase in their capacity to stimulate lymphocytes at low stimulator/effector ratio. Confocal microcopy examination of patients' CD1a+ sorted cells after the 2-day culture with transfected fibroblasts substantiated this finding, showing that, although cells cocultured with control fibroblasts still had an immature (intracellular MHC class II and CD86−) phenotype, cells cultured with CD40L-transfected fibroblasts expressed high-membrane MHC class II and CD86 (Figure 4E). It is, however, noticeable that, in these experiments, LCH cells remained round shaped (Figure 4E) and frequently did not acquire a “dendritic” morphology. Therefore, LCH cells from eosinophilic granuloma displayed both an immature phenotype and function, but could be induced to express CD86 and membrane class II and to become potent stimulators of an allogeneic response in vitro.
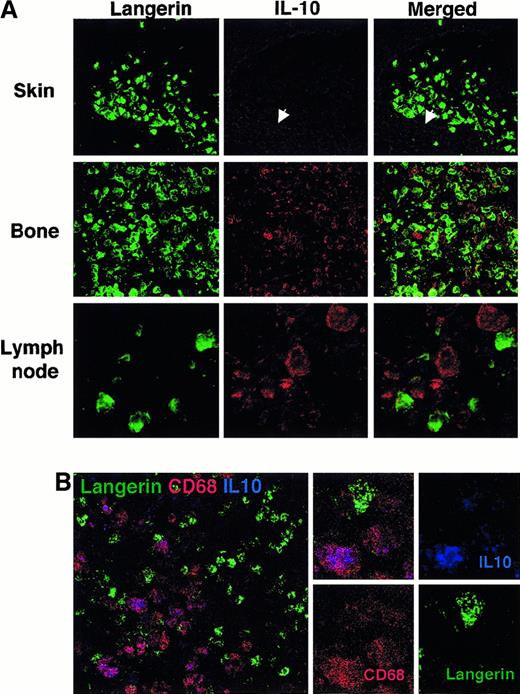
Macrophages produce IL-10 in eosinophilic granuloma lesions and involved lymph nodes
The above results suggested that the immature phenotype of LCH cells in bone lesions did not result from an intrinsic maturation blockade. We investigated whether inhibitory signals may be found in the vicinity of LCH cells within lesions. With the use of confocal microscopy examination of patients' tissue sections, no IL-10 was detected within LCH lesions from patients with localized cutaneous disease (Figure 5A, n = 3). In contrast, relatively numerous IL-10–expressing cells were detected within bone and lymph node lesions (Figure 5A, n = 4). Interestingly, the cells that expressed IL-10 were found to be very large sized, did not express CD1a or Langerin (Figure 5A,B), and were also CD3 negative (data not shown) but strongly expressed CD68 (Figure 5B), and therefore were identified as macrophages. These IL-10–expressing macrophages were found to be frequently in close contact with LCH Langerin+ cells (Figure 5A,B) and T lymphocytes (data not shown). Examination of control reactive lymph nodes did not reveal the presence of these IL-10–expressing cells (data not shown). These observations suggest that Langerin+ LCH cells and infiltrating T cells are exposed to IL-10, mainly produced by surrounding macrophages.
Presence of IL-10–expressing macrophages in the vicinity of LCH cells in bone and lymph node lesions but not in isolated skin lesions.
(A) Frozen sections from skin lesion (patient 6423), eosinophilic granuloma of the bone (patient 7553), and a lesional lymph node (patient 6193) were fixed in acetone and stained with anti-Langerin (mouse IgG1) revealed with a goat F(ab)2 antimouse IgG and with anti–IL-10 (rat IgG) and streptavidin-Cy3 and examined by confocal microscopy. Only faint background staining with anti–IL-10 was detectable in the epidermis (arrow) and dermis of the skin lesion (upper panel, magnification × 40). Similar results were observed in 3 patients with isolated skin disease. At the same magnification, relatively numerous cells stained with anti–IL-10 were readily detectable (in red) within eosinophilic granuloma (middle panel). Although faint IL-10 staining may be observed in some Langerin-stained cells, most frequently, IL-10 and Langerin staining did not colocalize. Similar results were observed in 3 patients with bone disease. A similar pattern is also observed in lymph node section (lower panel), shown at a higher magnification (× 100) to illustrate that Langerin positive cells display virtually no staining for IL-10, whereas the Langerin− large-sized cells in their close vicinity strongly express IL-10. (B) Frozen sections from eosinophilic granuloma of the bone (patient 10337) were stained with FITC-conjugated anti-Langerin (DCGM-5, mouse IgG1), PE-conjugated CD68 (Pharmingen) and with anti–IL-10 (rat IgG) and streptavidin-Cy5 and examined by confocal microscopy. Cells that stained strongly for IL-10 (blue) also stained strongly for CD68 (red) and appeared purple, whereas Langerin-stained cells (green) display weaker CD68 expression and no or very weak IL-10 staining.
Presence of IL-10–expressing macrophages in the vicinity of LCH cells in bone and lymph node lesions but not in isolated skin lesions.
(A) Frozen sections from skin lesion (patient 6423), eosinophilic granuloma of the bone (patient 7553), and a lesional lymph node (patient 6193) were fixed in acetone and stained with anti-Langerin (mouse IgG1) revealed with a goat F(ab)2 antimouse IgG and with anti–IL-10 (rat IgG) and streptavidin-Cy3 and examined by confocal microscopy. Only faint background staining with anti–IL-10 was detectable in the epidermis (arrow) and dermis of the skin lesion (upper panel, magnification × 40). Similar results were observed in 3 patients with isolated skin disease. At the same magnification, relatively numerous cells stained with anti–IL-10 were readily detectable (in red) within eosinophilic granuloma (middle panel). Although faint IL-10 staining may be observed in some Langerin-stained cells, most frequently, IL-10 and Langerin staining did not colocalize. Similar results were observed in 3 patients with bone disease. A similar pattern is also observed in lymph node section (lower panel), shown at a higher magnification (× 100) to illustrate that Langerin positive cells display virtually no staining for IL-10, whereas the Langerin− large-sized cells in their close vicinity strongly express IL-10. (B) Frozen sections from eosinophilic granuloma of the bone (patient 10337) were stained with FITC-conjugated anti-Langerin (DCGM-5, mouse IgG1), PE-conjugated CD68 (Pharmingen) and with anti–IL-10 (rat IgG) and streptavidin-Cy5 and examined by confocal microscopy. Cells that stained strongly for IL-10 (blue) also stained strongly for CD68 (red) and appeared purple, whereas Langerin-stained cells (green) display weaker CD68 expression and no or very weak IL-10 staining.
Discussion
This study aimed to define phenotypic and functional characteristics of LCH cells that may account for the pathogenesis of the disease, and in particular, their ability to induce an immune response. We have studied the in situ phenotype of LCH cells in a large series of patients, relative to the low incidence, that represents the various clinical courses of the disease. However, flow cytometry analysis and functional studies could be performed only in a smaller number of patients, due to the difficulty to obtain fresh lesional tissue at the time of diagnosis. Despite the widely accepted use of CD1a antibodies to confirm the diagnosis of LCH,31-35 CD1a expression is not restricted to Langerhans cells, and accurate diagnosis of Langerhans cell histiocytosis, required in such a study, may be questionable in the absence of electron microscopy. The results presented here are reinforced by the 100% concordance observed between positivity of Langerin and of CD1a stainings in the 25 patients who had various clinical forms and stages of LCH diagnosed, at various biopsy sites. Assuming that Langerin is a specific LC marker25associated with Birbeck granules at the ultrastructural level,26 this indicates that all studied patients indeed presented with “Langerhans cell” histiocytosis. Our findings also establish Langerin as a useful marker for diagnosis of LCH.
It appears clearly from our results that LCH cells express CD14 at least in bone and lymph node involvement. CD14 expression may depend on the clinical form of the disease because fewer cells are stained in skin lesions. Earlier studies have reported that LCH cells may,30 or may not,27 express CD14. Such a phenotype, CD1a+, Langerin+, CD14+, is unusual because normal Langerhans cells do not, or very poorly, express CD14.36 It is, however, reminiscent of the phenotype of recent immigrant Langerhans cells within the epidermis after a bone marrow graft as described by Murphy et al.37It is noteworthy that (i) during monocyte to DC differentiation in vitro, CD14 and CD68 are down-regulated, whereas CD1a is induced, due to the effect of IL-4 (or IL-13) and TGFβ1, and that (ii) Langerhans cells, markedly diminished in patient skin after allogeneic bone marrow transplantation, are replenished with epidermal dendritic cells exhibiting coexpression of monocyte/macrophage and Langerhans cell surface antigens during the first 4 weeks after this intervention.37 It is thus conceivable that the expression of CD14 by LCH cells reflects their immature stage of differentiation, and therefore their CD14+ origin, although it can be also argued that CD14 expression might have been acquired by lesional cells, or delineate distinct cellular origin or differentiation pathways. However, the heterogeneous level of CD14 expression by CD1a+ cells present in one single lesion (Figures 2B, 3A) argues against the latter hypothesis.
The major finding in this study is that LCH cells are in an immature stage of differentiation in the bone/chronic forms of the disease, but are able to trigger an immune response if they receive a maturation signal such as CD40L in vitro. Although only 3 patients could be studied in functional assay, the results were clear and consistent with phenotypic studies. Both in situ and ex vivo results presented here argue against the hypothesis of an intrinsic maturation blockade of LCH cells, and indicate that LCH cells can be induced to elicit an immune response. Indeed, in the spontaneously regressive form of the disease, LCH cells do frequently exhibit CD86 and DC-Lamp expression in situ, suggesting that they represent more mature DCs. Functional studies in the latter form, however, could not be performed due to the lack of sufficient material, and in addition would have been difficult to interpret because of the probable contamination by normal epidermal LCs. In contrast, CD1a+ LCH cells sorted from bone lesions do not express membrane MHC class II or costimulatory CD86, and poorly stimulate T cells. In vivo, and even in vitro after a 2-day culture with fibroblasts, these cells remain in an immunologically immature stage. Strikingly, however, CD1a+ LCH cells differentiate toward mature DCs on CD40 triggering in vitro.
This is somewhat surprising, because patients with LCH do not present with CD40L deficiency, and because, in addition to the presence of T cells within the lesions, LCH lesions, especially eosinophilic granuloma, abundantly express inflammatory cytokines such as TNFα18 and Il-1β,12 that are believed to induce LC migration and maturation. However, such cytokines may not be sufficient stimuli to induce a functional maturation, and other cytokines such as TGFβ1 or IL-10 may prevent LC maturation.23,38,39 In particular, IL-10 is a cytokine capable of down-regulating the expression of B7 molecules and class II antigens by DC/LC in vitro.38,39 The presence of active TGFβ1 within LCH lesions is likely, because Langerhans cell differentiation requires TGFβ1. However, latent TGFβ1 is abundantly produced by many cell types, and it is not possible to quantitate the presence of active TGFβ1 within tissues. We were able to detect IL-10 in the vicinity of LCH cells in bone and lymph node lesions by confocal microscopy. Interestingly, IL-10–expressing cells in eosinophilic granuloma were most predominantly large-sized CD3−, Langerin−, CD68+ cells, and therefore were neither LCH cells themselves nor T cells, but macrophages. In contrast, within skin lesions from patients with limited or self-healing disease, macrophages are very rare, and there are consistently no IL-10+ cells. These results are consistent with those of 2 recent studies that investigated the presence of numerous cytokines within LCH lesions by immunohistochemistry. In one study, IL-10 was detected in the vicinity of LCH cells in 9 of 11 biopsy specimens studied (all from eosinophilic granuloma or lymph nodes).18 In contrast, in the other study IL-10 was not detected in 5 of 5 LCH lesions restricted to the lung in adults patients who expressed CD86.17 It is possible that pulmonary LCH of the adult, which is a clinically distinct disease, may have a distinct pathophysiology. It is also possible that the same mechanism that could be responsible for the healing of skin lesions, ie, maturation of LCH cells, may be responsible for the pathogenesis of lung lesions because inflammation and subsequent fibrosis, but not tumoral involvement, are unique features of pulmonary LCH of the adult.17
Altogether our findings in this functional study attempting to draw a picture of the pathogenesis of LCH may account for a maturation blockade of LCH cells due to extrinsic signals and reconcile contrasting studies on a few cases that either reported that LCH cells may be activated or mature on phenotypic data17,19 or failed to detect alloantigen-presenting activity by LCH cells.20
In our study, LCH cells from bone/chronic lesions are undoubtedly immature Langerhans-type dendritic cells that express higher levels of CD68 and CD14 than normal LCs, intracellular MHC class II, are frequently negative for CD86 and DC-Lamp and have the same allostimulatory activity as immature normal DCs. It is, however, clear that LCH cells are not by themselves “frozen” in an arrested state of activation/differentiation because we show that LCH cells may become activated in vitro in response to CD40 triggering. Moreover, in some cases in vivo, especially, and interestingly, in self-healing cutaneous lesions, a more mature phenotype can be observed and LCH cells appear to down-regulate CD14 and up-regulate CD86 and DC-Lamp. Although a direct role of IL-10 cannot be demonstrated here, IL-10 produced by CD1a− macrophages may contribute to the maintenance of LCH CD1a+ cells in an immature stage of differentiation.
LCH has been advocated to be a malignancy or a viral disease; however, both the search for a viral cause and for molecular abnormalities are still unsuccessful.10,40 41 Several viruses have been shown to interfere with DC functions, and it is conceivable that an inadequate response to a viral challenge may result in the LCH features described: local recruitment of immature LCs or their precursors, their abnormal homing, and their persistence in the absence of efficient maturation.
Finally, our results may contribute to explain the paradox of an “antigen presenting-cell tumor” that does not induce its own rejection by the immune system. In bone/chronic forms, LCH cells are maintained in an immature stage by factors from their environment. This may open the way for new strategies in the treatment of LCH. Whether drugs that enhance in vivo the ability of LCH cells to become mature may lead to their killing by activated CTL and may be beneficial to some patients should be investigated. Alternatively, pharmacologically induced death of immature DCs may also be considered.
We are grateful to the French Histiocytosis Study Group and to Pr F. Jaubert for support, to Dr Aucouturier for critical reading of the manuscript, and to Mr Y. Goureau for help with confocal microscopy.
Supported by the Université Paris V and the CNRS, and by grants from the Histiocytosis Association of America and the Association pour la Recherche contre le Cancer. J.V. was supported by Fondation Merieux.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frédéric Geissmann, Service d'Anatomie Pathologique and UMR 8603 CNRS-Université Paris V, Hopital Necker-Enfants Malades, 161 rue de Sevres, 75743 Paris Cedex 15, France; e-mail: geissman@necker.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal